International Journal of Organic Chemistry
Vol.4 No.1(2014), Article ID:43829,7 pages DOI:10.4236/ijoc.2014.41007
Synthesis, Resolution and Absolute Configuration of 2,3-Dihydro-2-TertButyl-3-N-Benzylquinazolin-4-One: A Possible Chiral Auxiliary for Synthesis of (-Amino Cyclohexancarboxylic Acid
Fanny A. Cabrera-Rivera, Jaime Escalante*
Centro de Investigaciones Químicas, Universidad Autónoma del Estado de Morelos, Cuernavaca, Mexico
Email: *jaime@ciq.uaem.mx
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 9 December 2013; revised 15 January 2014; accepted 23 January 2014
ABSTRACT
3-Benzyl-2-(tert-butyl)-2,3-dihydroquinazolin-4(1H)-one rac-11 was resolved via the preparation of diastereomers with N-phthalyl-L-alanine chloride and its absolute configuration was determined by X-ray crystallographic analysis. This heterocycle has potential as a substrate chiral in asymmetric induction due to the steric effects of its tert-butyl group.
Keywords:Chiral Auxiliary; 2-Tert-Butyl-Quinazolin-4-One

1. Introduction
2,3-Dihydro-4(1H)-quinazolinones form an important class of bioactive compounds and these can easily be oxidized to their quinazolin-4(3H)-one analogues [1] . In general, the derivatives of the quinazolinones are considered as important building blocks [2] [3] for a large number of diverse alkaloids [4] [5] and present a wide range of biological and pharmaceutical activities [6] -[9] .
On the other hand, recently an efficient method for the conversion of anhydride isatoic into 4(3H)-quinazolinone 1 was described using (S)-a-methylbenzylamine as chiral auxiliary. Enantiomerically pure quinazolinone 1 was reduced diastereoselectively by hydrogenation with PtO2, resulting in octahydroquinazolinone diastereomers. Both cis-annelated derivatives (2 and 3) could be epimerized in the presence of t-BuO−K+, giving the corresponding trans-fused derivatives (4 and 5) respectively in good yields (Scheme 1) [10] -[12] .
Subsequently, the hydrolysis with HCl 6N of the four adducts (2-5) affords all four enantiomers of cisand trans-2-aminocyclohexanecarboxylic acid (6-9) in good yields (Scheme 2).
The present paper describes the synthesis and resolution of 2,3-dihydro-2-tert-butyl-3-N-benzylquinazolin- 4-one rac-11 as a possible precursor of cisand trans-2-aminocyclohexanecarboxylic acids. In this compound, it is important to mention that the tert-butyl group at C(2) adopts a pseudoaxial position, as shown by analysis of X-ray diffraction [10] -[12] , and we would expect higher induction in asymmetric hydrogenation reaction: the addition of the hydrogen on the syn face, leading to the exclusive formation of the only one diastereomer.
2. Results and Discussion
Synthesis of (±)-2,3-dihydroquinazolin-4(1H)-one rac-11.
Our research was focused in the preparation of starting material following the methodology previously reported by our group [11] -[13] in which a reaction between isatoic anhydride and benzylamine in ethyl acetate at 40˚C results in the corresponding aminobenzamide 10 with 90% yield. Next, cyclocondensation of 10 with pivalaldehyde in dichloromethane and p-toluenesulfonic acid monohydrate gives (±)-2,3,dihydro-4(1H)-quinazolinone rac-11 at 86% yield (Scheme 3).
It is noteworthy that was necessary to protect the reaction from light source since this would suffer photoinduced elimination and hence reduces the yield of compound 11 [11] .
The resolution was achieved by the preparation of the diastereomers 13a and 13b via condensation between the quinazolinone anion, formed with NaHMDS at −78˚C, and N-phthalyl-L-alanine chloride (S)-12 as the resoluting agent [14] . Separation of the diastereomers was accomplished by flash chromatography from hexane/ AcOEt (Scheme 4).
The assignment of the absolute configuration of the main products was achieved by X-ray diffraction analysis with the diastereomer 13a (Figure 1). In this way, we were able to determine the relative configuration S at C(2) in the quinazolinone system for diastereomer 13a, and consequently the opposite configuration for diastereomer 13b.
It is important to mention that X-ray crystal-structure determinations used to elucidate the stereochemical outcome of 13a revealed a pseudoaxial disposition of the tert-butyl group at C(2) (consequence of a powerful A1,3 effect) [15] -[21] , which could directs higher induction in addition toward the face opposite to this group in the hydrogenation reaction, leading to the exclusive formation of a single diastereomer.
Finally, as shown in Scheme 5, conversion of diastereoisomers 13a and 13b to the enantiomerically pure quinazolinones (R)-11 and (S)-11, was completed by hydrolysis with Bu4N+−OH in 75 and 67% yield respectively.
3. Conclusion
In conclusion, we present a new method for the preparation of enantiomerically pure quinazolinones (R)-11 and (S)-11. The interest for these quinazolinones as intermediaries is given by their potential use in the formation of
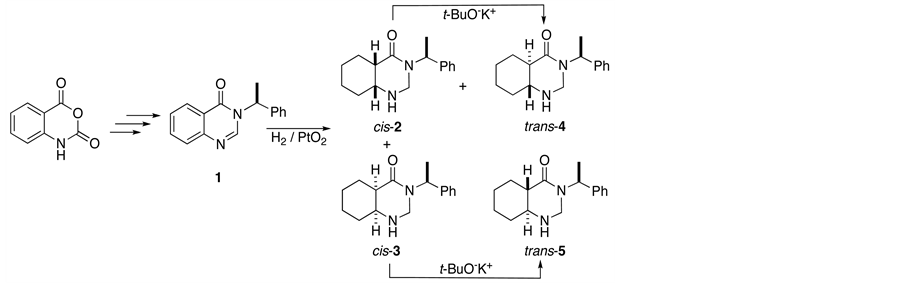
Scheme 1. Synthesis of octahydroquinazolinone diastereoisomers.
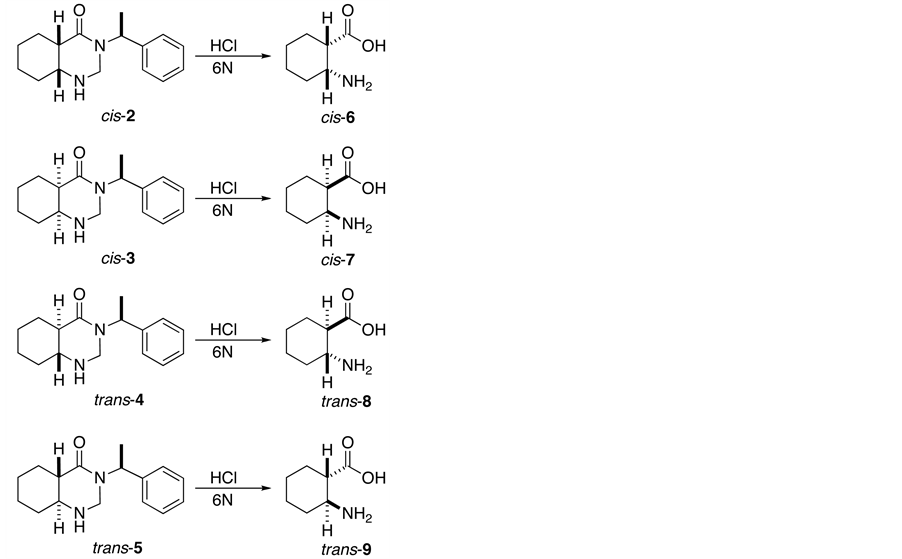
Scheme 2. Synthesis of cisand trans-2-aminocyclohexanecarboxylic acid enantiomers.
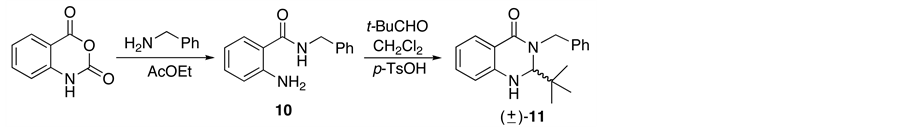
Scheme 3. Synthesis of (±)-2,3.dihydro-4(1H)-quinazolinone rac-11.
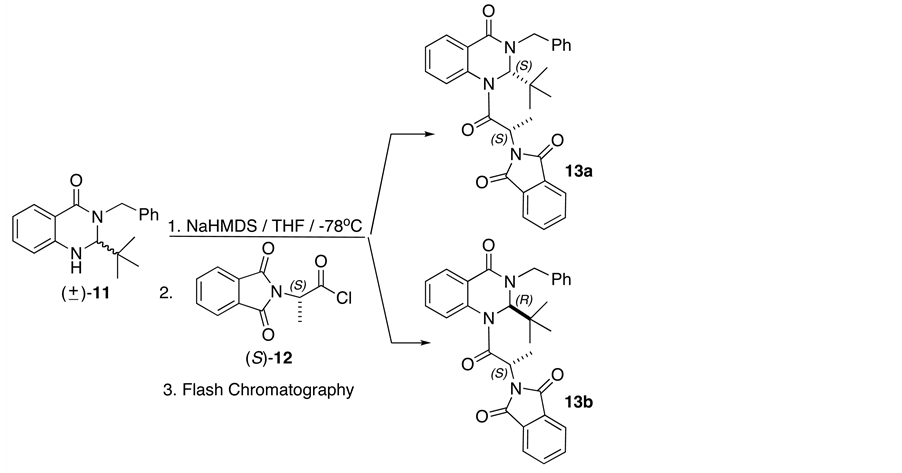
Scheme 4. Synthesis of (S,S)- and (R,S)-diastereomers of quinazolinone (±)-11.
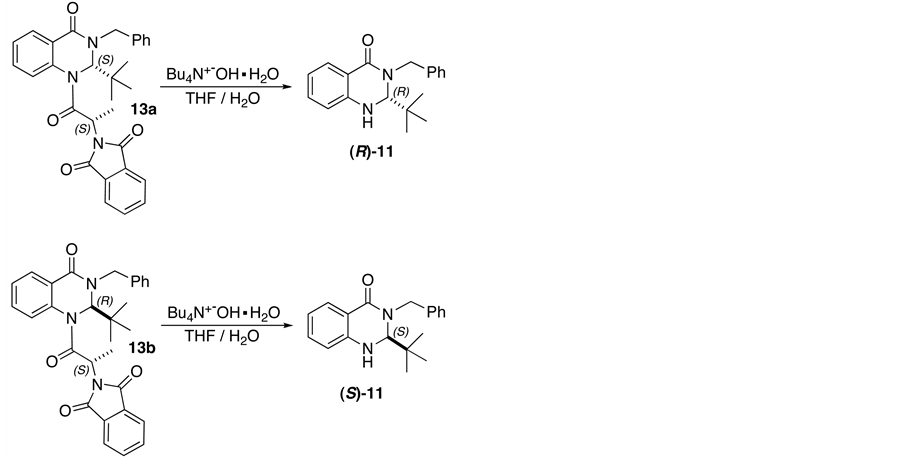
Scheme 5. Synthesis of enantiomerically pure quinazo linone (R)-11 and (S)-11.
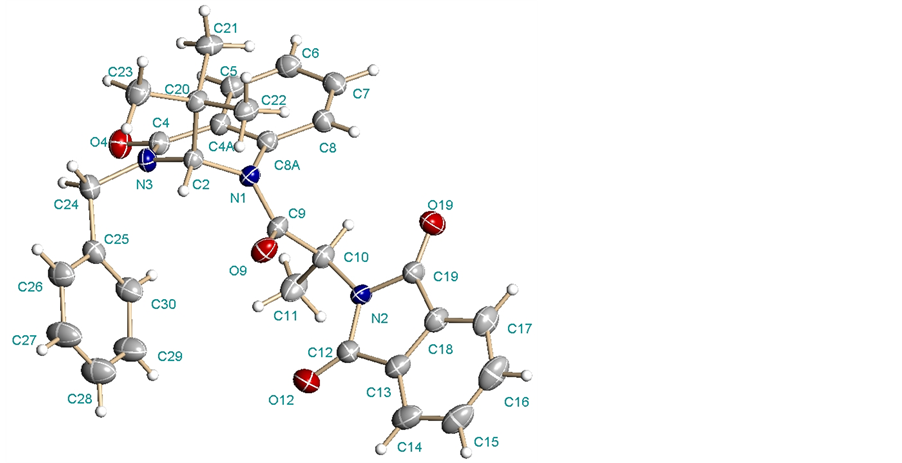
Figure 1. Structure and solid-state confirmation for 13a.
b-amino cyclohexancarboxylic acids. Further studies to explore these quinazolinones as new chiral auxiliaries in the synthesis of b-amino acids are in progress.
4. Experimental
TLC: Merck-DC-F254 plates; detection by UV light. Flash column chromatography [22] : Merck silica gel (0.040 - 0.063 mm). Mp: Mel-Temp apparatus; not corrected. Optical rotations were determined in a Perkin-Elmer 241 polarimeter at the sodium D-line. 1H NMR spectra: Varian Oxford 400 MHz, 13C NMR spectra: Varian Oxford 100 MHz. Chemical shifts (d) in ppm downfield from the integral TMS reference; the coupling constants (J) in Hz. X-Ray: APEX-Bruker diffractometer. The structures were solved by direct methods using the program SHELXS [23] . Flasks, stirring bars and hypodermic needles used for the generation and reactions of organolithiums were dried for 12 h at 120˚C and allowed to cool in a desiccator over anhydrous CaSO4. Anhydrous solvents were obtained by distillation from benzophenone ketyl.
4.1. Synthesis of 2-Amino-N-Benzylbenzamide (10)
A suspension of isatoic anhydride (10.0 g, 62 mmol) was treated with 1.0 equiv of benzylamine (6.7 mL, 62 mmol) in ethyl acetate according to published procedures [11] -[13] . The reaction mixture was warmed to 40˚C during 1 h. The solution was then concentrated under reduced pressure. The crude product was purified by flash chromatography. The benzamide 10 was produced 12.7 g (90%) as a white solid: mp 121˚C - 123˚C (Lit. [10] [12] mp 124˚C - 125˚C), 1H NMR (CDCl3, 400 MHz): δ (ppm): 4.55 (d, J = 5.6 Hz, 2H, CH2), 5.26 (br s, 2H, NH2), 6.50 (br, 1H, NH), 6.59 (t, Jortho = 7.4 Hz, 1H, C5-H), 6.65 (d, Jortho = 8.4 Hz, 1H, C3-H), 7.18 (t, Jortho = 7.8 Hz, 1H, C4-H), 7.24 - 7.35 (m, 6H, C6-H, Ph); 13C NMR (CDCl3, 100 MHz): δ (ppm) 43.8, 115.8, 116.7, 117.4, 127.2, 127.5, 128.8, 132.4, 138.3, 148.8, 169.2. Anal. Calcd. for C14H14N2O: C, 74.29; H, 6.24; N, 12.38. Found: C, 74.28; H, 6.21; N, 12.38.
4.2. Synthesis of (()-2,3-Dihydro-2-Tert-Butyl-3-Benzyl-4(1H)-Quinazolinone (Rac-11)
A solution of aminobenzamide 10 (6 g, 26.5 mmol) in 100 mL of CH2Cl2 was added 3.2 mL (31.8 mmol) of pivalaldehyde and then was added 0.12 g (2% by weight) of p-TsOH. The colorless solution was refluxed for 5 h. The reaction was monitored by TLC (hexane:ethyl acetate 7:3). The straw yellow solution was concentrated and the crude was purified by FC eluting with hexane:ethyl acetate 9:1 - 6:4 to afford 6.7 g (86%) of 11 as white solid. mp 140˚C - 143˚C. 1H NMR (CDCl3, 400 MHz): δ (ppm) 0.87 (s, 9H, t-Bu), 3.92 (d, J = 15.6 Hz, 1H, CH2), 4.24 (d, J = 3.2 Hz, 1H, C2-H), 4.40 (br d, J = 3.2 Hz, 1H, NH-1), 5.81 (d, J = 15.2 Hz, 1H, CH2), 6.49 (d, Jortho = 8 Hz, 1H, C8-H), 6.73 (t, Jortho = 7.5 Hz, 1H, C6-H), 7.20 - 7.34 (m, 6H, C7-H y Ph), 7.85 (dd, Jortho = 7.8 Hz, Jmeta = 1.2 Hz, 1H, C5-H); 13C NMR (CDCl3, 100 MHz): δ (ppm) 26.6, 42.0, 51.4, 75.7, 113.3, 116.6, 118.1, 127.3, 127.4, 128.5, 128.7, 133.6, 137.4, 146.6, 163.8. Anal. Calcd. for C19H22N2O: C, 77.52; H, 7.53; N, 9.52. Found: C, 77.61; H, 7.46; N, 9.30.
4.3. Procedure for the Quinazolinone-11 Resolution
A solution of rac-11 in THF was cooled to −78˚C before slowly adding 1.1 mol equiv. of NaHMDS in hexane (1.0 M). The resulting solution was stirred at −78˚C for 10 min and treated successively with the resolution agent (N-phthalyl-L-alanine chloride) [12] [24] . The mixture was stirred at the same temperature for 1 h and treated with saturated ammonium chloride solution and then with water. The aqueous phase was extracted with CH2Cl2. The combined extracts were dried (Na2SO4), filtered and evaporated to give the crude product. Purification of the crude product was accomplished by flash chromatography (hexane/AcOEt) and then by recrystallization from hexane/AcOEt yielding the corresponding diastereomer.
2-[(S)-1-[(S)-3-Benzyl-2-tert-butyl-4-oxo-3,4-dihydroquinazolin-1(2H)-yl]-1-oxopropan-2-yl]isoindoline-1,3-dione, (S,S)-13a.
44% Yield; mp 226˚C - 227˚C; [a]D25 = + 439.5 (c 1.04, CHCl3); 1H NMR (CDCl3, 400 MHz): δ (ppm) 0.806 (s, 9H, t-Bu), 1.20 (d, J = 7.6 Hz, 3H, CH3), 4.00 (d, J = 14.8 Hz, 1H, CH2), 5.65 (d, J = 14.8 Hz, 1H, CH2), 5.70 (q, J = 8 Hz, 1H, CH), 5.79 (s, 1H, C2-H), 7.34 - 7.42 (m, 6H, C6-H y Ph), 7.60 (dt, Jortho = 7.8 Hz, Jmeta = 1.2 Hz, 1H, C7-H), 7.68 - 7.73 (m, 3H, Ph), 7.83 - 7.85 (m, 2H, Ph), 8.08 (dd, Jortho = 7.6 Hz, Jmeta = 1.6 Hz, 1H, C5-H); 13C NMR (100 MHz, CDCl3) d (ppm) 13.5, 27.0, 39.3, 49.3, 52.1, 74.9, 123.2, 123.5, 125.3, 127.0, 128.2, 128.3, 128.9, 129.1, 132.1, 133.1, 133.3, 134.2, 137.1, 138.5, 162.5, 168.6, 170.2. HRMS: Calcd for C30H29N3O4: 495.2158 found: [M + H]+ C30H30N3O4, 496.2252. X-Ray crystallographic structure in Figure 1 [25] .
2-((S)-1-((R)-3-Benzyl-2-tert-butyl-4-oxo-3,4-dihydroquinazolin-1(2H)-yl)-1-oxopropan-2-yl)isoindoline-1,3-dione, (S,R)-13b.
36% Yield; mp 110˚C - 112˚C; [a]D25 = −224.3 (c 1.0, CHCl3); 1H NMR (CDCl3, 400 MHz): δ (ppm) 0.66 (s, 9H, t-Bu), 1.57 (d, J = 7 Hz, 3H, CH3), 4.07 (d, J = 14.4 Hz, 1H, CH2), 5.10 (d, J = 13.6 Hz, 1H, CH2), 5.24 (b, 1H, CH), 5.81 (s, 1H, C2-H), 6.94 (t, Jortho = 7.4 Hz, 1H, C6-H), 7.13 (d, Jortho = 8 Hz, 1H, C8-H), 7.22 - 7.37 (m, 5H, C7-H Ph), 7.46 (d, Jortho = 6.8 Hz, 1H, C5-H), 7.51 (m, 5H, Ph) 13C NMR (100 MHz, CDCl3) d (ppm) 16.6, 27.1, 38.6, 47.2, 53.6, 76.0, 122.4, 123.2, 124.8, 126.3, 127.5, 128.2, 128.5, 129.0, 131.2, 132.9, 134.1, 137.2, 138.5, 162.7, 166.4, 168.9. HRMS: Calcd for C30H29N3O4: 495.2158 found: [M + H]+ C30H30N3O4, 496.2235.
4.4. Procedure for Remotion of the Resolution Agent
To the appropriate diastereomer in THF was added at 0˚C an excess of Bu4NOH solution under stirring for 12 h. The resulting mixture was concentrated at reduced pressure and purified by column chromatography (hex/AcOEt 50:50_0:10) to give the product as a white solid.
(S)-3-Benzyl-2-tert-butyl-2,3-dihydroquinazolin-4(1H)-one, (S)-11.
67% Yield. White solid, mp 153˚C - 154˚C. [a]D25 = +68.72 (c 1.01, CHCl3). Spectroscopy data were exactly identical to the racemic mixture.
(R)-3-benzyl-2-(tert-butyl)-2,3-dihydroquinazolin-4(1H)-one, (R)-11.
75% Yield. White solid, mp 152˚C - 153˚C. [a]D25 = −68.87 (c 1.07, CHCl3). Spectroscopy data were exactly identical to the racemic mixture.
Acknowledgements
We are grateful to CONACYT for financial support (Project CB2010/151875) and for scholarships to F. A. C.-R.
References
- Zhang, Z., Lu, H., Yang, S. and Gao, J. (2010) Synthesis of 2,3-Dihydroquinazolin-4(1H)-Ones by Three-Component Coupling of Isatoic Anhydride, Amines, and Aldehydes Catalyzed by Magnetic Fe3O4 Nanoparticles in Water. Journal of Combinatorial Chemistry, 12, 643-646. http://dx.doi.org/10.1021/cc100047j
- Wu, H., Xie, X. and Liu, G. (2010) Parallel Solution Phase Synthesis of 3,6,7-4(3H)-Quinazolinones and Evaluation of Their Antitumor Activities against Human Cancer. Journal of Combinatorial Chemistry, 12, 346-355. http://dx.doi.org/10.1021/cc900173s
- Bowman, W., Elsegood, M., Stein, T. and Weaver, G. (2007) Radical Reactions with 3H-Quinazolin-4-Ones: Synthesis of Deoxyvasicinone, Mackinazolinone, Luotonin A, Rutaecarpine and Tryptanthrin. Organic & Biomolecular Chemistry, 5, 103-113. http://dx.doi.org/10.1039/b614075k
- Kshirsagar, U., Puranik, V. and Argade, N. (2010) Total Synthesis of Proposed Auranthine. Journal of Organic Chemistry, 75, 2702-2705. http://dx.doi.org/10.1021/jo100400z
- Mhaske, S. and Argade, N. (2006) The Chemistry of Recently Isolated Naturally Occurring Quinazolinone Alkaloids. Tetrahedron, 62, 9787-9826. http://dx.doi.org/10.1016/j.tet.2006.07.098
- Zeng, F. and Alper, H. (2010) One-Step Synthesis of Quinazolino[3,2-a]Quinazolinones via Palladium-Catalyzed Domino Addition/Carboxamidation Reactions. Organic Letters, 12, 3642-3644.
- Witt, A. and Bergman, J. (2000) Synthesis and Reactions of Some 2-Vinyl-3H-Quinazolin-4-Ones. Tetrahedron, 56, 7245-7253. http://dx.doi.org/10.1016/S0040-4020(00)00595-0
- Larksarp, C. and Alper, H. (2000) Palladium-Catalyzed Cyclocarbonylation of o-Iodoanilines with Heterocumulenes: Regioselective Preparation of 4(3H)-Quinazolinone Derivatives. Journal of Organic Chemistry, 65, 2773-2777. http://dx.doi.org/10.1021/jo991922r
- Wang, M., Dou, G. and Shi, D. (2010) Efficient and Convenient Synthesis of Pyrrolo[1,2-a]Quinazoline Derivatives with the Aid of Tin(II) Chloride. Journal of Combinatorial Chemistry, 12, 582-586. http://dx.doi.org/10.1021/cc100062e
- Priego, J., Flores, P., Ortiz-Nava, C. and Escalante, J. (2004) Synthesis of Enantiopure Cisand Trans-2-Aminocyclohexane-1-Carboxylic Acids from Octahydroquinazolin-4-Ones. Tetrahedron: Asymmetry, 15, 3545-3549. http://dx.doi.org/10.1016/j.tetasy.2004.08.032
- Cabrera-Rivera, F., Ortiz-Nava, C., Escalante, J., Hernandez-Perez, J. and Ho, M. (2012) Photoinduced Elimination in 2,3-Dihydro-2-Tert-Butyl-3-Benzyl-4(1H)-Quinazolinone: Theoretical Calculations and Radical Trapping Using TEMPO Derivatives. Synlett, 23, 1057-1063. http://dx.doi.org/10.1055/s-0031-1290492
- Escalante, J., Flores, P. and Priego, J. (2004) Synthesis of 2,3-Dihydro-4(1H)-Quinazolinones. Heterocycles, 63, 2019- 2032. http://dx.doi.org/10.3987/COM-04-10130
- Escalante, J., Ortiz-Nava, C., Flores, P., Priego, J. and Garcia-Martinez, C. (2007) Synthesis, NMR and Crystallographic Studies of 2-Substituted Dihydroquinazolinones Derived from (S)-Phenylethylamine. Molecules, 12, 173-182. http://dx.doi.org/10.3390/12020173
- Escalante, J. and Gonzalez-Tototzin, M. (2003) Synthesis, Resolution and Absolute Configuration of Trans-4,5-Diphenyl-Pyrrolidin-2-One: A Possible Chiral Auxiliary. Tetrahedron: Asymmetry, 14, 981-985. http://dx.doi.org/10.1016/S0957-4166(03)00097-1
- Seebach, D., Lamatsch, B., Amstutz, R., Beck, A., Dobler, M., Egli, M., Fitzi, R., Gautschi, M., Herradon, B., et al. (1992) Structure and Reactivity of Fiveand Six-Ring N,N-, N,O-, and O,O-Acetals: A Lesson in Allylic 1,3-Strain (A1,3 Strain). Helvetica Chimica Acta, 75, 913-934. http://dx.doi.org/10.1002/hlca.19920750326
- Murer, P., Rheiner, B., Juaristi, E. and Seebach, D. (1994) Enantioselective Synthesis of β-Amino Acids. 5. Stereoselective Reaction of Chiral Pyrimidinone Enolates with Aldehydes. Heterocycles, 39, 319-344. http://dx.doi.org/10.3987/COM-94-S(B)35
- Seebach, D., Boog, A. and Schweizer, W.B. (1999) EPC-Synthesis of B-Amino Acid Derivatives through Lithiades Hydropyrimidines. European Journal of Organic Chemistry, 1999, 335-360.
- Ramirez-Quiros, Y., Balderas, M., Escalante, J., Quintana, D., Gallardo, It., Madrigal, D., Molins, E. and Juaristi, E. (1999) X-Ray Crystallographic Study of Substituted Perhydropyrimidinones. Extreme Changes in Ring Conformation. Journal of Organic Chemistry, 64, 8668-8680. http://dx.doi.org/10.1021/jo991297q
- Johnson, F. (1968) Allylic Strain in Six-Membered Rings. Chemical Reviews, 68, 375-413. http://dx.doi.org/10.1021/cr60254a001
- Hoffmann, R. (1989) Allylic 1,3-Strain as a Controlling Factor in Stereoselective Transformations. Chemical Reviews, 89, 1841-1860. http://dx.doi.org/10.1021/cr00098a009
- Broeker, J., Hoffmann, R. and Houk, K. N. (1991) Conformational Analysis of Chiral Alkenes and Oxonium Ions: Ab Initio Molecular Orbital Calculations and an Improved MM2 Force Field. Journal of the American Chemical Society, 113, 5006-5017. http://dx.doi.org/10.1021/ja00013a041
- Still, W., Kahn, M. and Mitra, A. (1978) Rapid Chromatographic Technique for Preparative Separations with Moderate Resolution. Journal of Organic Chemistry, 43, 2923-2925. http://dx.doi.org/10.1021/jo00408a041
- Sheldrick, G.M. (1998) SHELX97, Programs for Crystal Structure Analysis, Release 97-2. Institute for Inorganic Chemistry der Universität, Göttingen.
- Camps, P., Perez, F., Soldevilla, N. and Borrego, M. (1999) (R)- and (S)-3-Hydroxy-4,4-Dimethyl-1-Phenyl-2-Pyrrolidinone as Chiral Auxiliaries in the Enantioselective Preparation of α-Amino Acids. Tetrahedron: Asymmetry, 10, 493-509. http://dx.doi.org/10.1016/S0957-4166(99)00018-X
NOTES

*Corresponding author.

