International Journal of Organic Chemistry
Vol.3 No.2(2013), Article ID:33046,3 pages DOI:10.4236/ijoc.2013.32017
One-Pot Three-Component Synthesis of N-Arylmethyl-4-(7-cyclohepta-1,3,5-trienyl)anilines
Perm State Agricultural Academy, Perm, Russia
Email: yunnikova@yahoo.com
Copyright © 2013 Lidia P. Yunnikova et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 2, 2013; revised April 30, 2013; accepted May 3, 2013
Keywords: Imines; Tropylium Tetrafluoroborate; Sodium Tetrahydroborate; N-Arylmethyl-4-(7-cyclohepta-1,3,5-trienyl)anilines
ABSTRACT
We report a one-pot three-component synthesis of N-arylmethyl-4-(7-cyclohepta-1,3,5-trienyl)anilines by using various aromatic imines, tropylium tetrafluoroborate, and sodium tetrahydroborate in the presence of imidazole as activator.
1. Introduction
Nitrogen-containing compounds with the 1,3,5-cycloheptatriene fragment have a marked biological action [1, 2]. The use of tropylium salts (tetrafluoroborate or perchlorate) is a method to introduce the 1,3,5-cycloheptatriene cycle into aniline molecules and into its substituted molecules. Anion in a tropylium salt influences the N-[3] or C-[1] tropylation process of aniline, of arylamines or of N-substituted anilines, and a yield of final products.
Interaction between tropylium tetrafluoroborate and aniline or arylamines proceeds as N-tropylation of amino-group with subsequent transformation to 8-aryl-8- azaheptafulvenes [3]. Reaction with N,N,-dimethylaniline proceeds as C-tropylation; however, the expected 7-(4- N,N-dimethylaminophenyl)-1,3,5-cycloheptatriene was not educed. Its isomer, namely 3-(4-N,N-dimethylaminophenyl)-1,3,5-cycloheptatriene (18% yield), was identified from a mixture of reaction products [4].
A good result was obtained after replacement of tetrafluoroborate with tropylium perchlorate to react with N, N-dimethylaniline or with aniline; this reaction is known to proceed as C-tropylization and enables educing 7- (4-N,N-dimethylaminophenyl)-1,3,5-cycloheptatriene (up to 76% yield), or 7-(4-aminophenyl)-1,3,5-cycloheptatriene (up to 82% yield). The latter compound has a marked antimycobacterial action against Staphylococcus aureus, Staphylococcus epidermis, Staphylococcus saprophyticus bacteria, and Candida albicans fungi [1].
Earlier [5], we had reported the reducing reaction of imine hydrotropylation in the “tropylium perchlorate— sodium tetrahydroborate” system resulting in unstable tertiary amines, namely N-phenyl-N-arylmethyl-N-(7- cyclohepta-1,3,5-trienyl)anilines. However, direction of this reaction changes after addition of imidazole to the reaction mass. Imidazole promotes a shift of the tropylium moiety from the nitrogen atom to para-position of the aniline ring and, thus, leads to formation of such stable products as N-arylmethyl-4-(7-cyclohepta-1,3,5- trienyl)anilines. The educed products contain two important biogenic moieties, namely tropilidene cycle and NH-group. The NH-group—in accordance with investigations in modeling and in predicting properties of active heterocyclic compounds based on the “structure-actiontoxicity” link [6]—promotes an increase in pharmacological action and a decrease in toxicity of compounds, so important for investigations in growth control of crops.
Thus, the use of tropylium perchlorate enabled educing para-tropylated aniline [1] which had been inaccessible earlier [7], as well as secondary tropylated amines by using the ionic imine-hydrotropylation reaction [5]. A disadvantage of these methods is the use of explosive tropylium perchlorate.
2. Results and Discussion
Our investigation aims at the one-pot three-component synthesis of N-arylmethyl-4-(7-cyclohepta-1,3,5-trienyl) anilines by using the method [5] with tropylium tetrafluoroborate instead of explosive tropylium perchlorate.
The conducted investigation has shown that ionic hydrotropylation of imines in the “tropylium perchlorate— sodium tetrahydroborate” system in the presence of imidazole as activator leads to formation of N-arylmethyl- 4-(7-cyclohepta-1,3,5-trienyl)anilines (3a-e) with the 23.3% - 51.4% yield (Scheme 1). It is worth noting that, in the absence of activator, the yield of tropylation products (3a-e) is only 1.5% - 8%, N-arylmethylanilines (4a-e) being the main reaction products; these results correspond with the data in [5].
Optimal ratio of reagents, namely the Schiff base: Tropylium tetrafluoroborate: Sodium tetrahydroborate: Imidazole = 1:1:1:0.5, is ascertained. The reaction proceeds at room temperature, with tetrahydrofuran as a solvent.
A role of the activator is, probably, to form the B complex promoting a shift of the cycloheptatriene cycle to the para-position of the aniline fragment of the A intermediate (Scheme 2).
3. Experimental Part
The FTIR spectra were registered by using the IR Fourier spectrometer (Bruker, Germany), registration condition: suspension in vaseline oil. The 1H NMR spectra were registered by using the Mercury 300 device (300 MHz), with hexamethyldisiloxane (HMDSO) as internal standard. Chromatograph mass spectrum was recorded by using the Agilent Technologies 689ON/597B device, the HP-5ms column (30 × 0.25 mm, helium as carrier gas, ionization by 70 eV electron shock, 100˚C temperature of the column’s thermostat, 250˚C, 270˚C temperature of evaporator); CHNS-932 (Carbon, Hydrogen, Nitrogen and Sulfur Determinator), LECO Corporation (USA).
General Preparation Procedure for the 3a-e Compounds
Imine (1 mmol) is dissolved in 5 ml of tetrahydrofuran, whereupon tropylium tetrafluoroborate (1 mmol), sodium tetrahydroborate (1 mmol), and imidazole (0.5 mmol) are added at one go. The reaction mass is then stirred for 2.5 h at room temperature, diluted with water, and neutralized to pH 7.
1) N-Benzyl-4-(7-cyclohepta-1,3,5-trienyl)aniline (3a)
The yield of the 3a compound: 0.07 g (25%), appearance: white crystals with m.p. 69˚C - 70˚C (hexane), (ref. 69˚C - 70˚C [5]). IR (vas): νNH 3391, δNH 1611, 820 cm−1. 1H-NMR (CDCl3), δ, ppm, (J, Hz): 2.58 (1 H, t, J 5.4, C7H in C7H7); 4.05 (1 H, br.s, NH); 4.34 (2 H, s, CH2); 5.36 - 5.41 (2 H, dd, J 5.4, C1,6H in C7H7); 6.19 - 6.24 (2 H, m, C2,5H in C7H7); 6.65 (2 H, d, J 8.7, ortho-C6H4-NH); 6.71 - 6.73 (2 H, m, C3,4H in C7H7); 7.17 (2 H, d, J 8.4, meta-C6H4-NH); 7.27 - 7.40 (5 H, m, Ph). MS (EI)+ : m/z 273 (100) [M]+. Anal. Calcd for C20H19N (273,38): C, 87.87; H, 7.00; N, 5.12. Found: C, 87.58; H, 6.95; N, 5.10.
2) N-(4-Bromphenylmethyl)-41-(7-cyclohepta-1,3,5- trienyl)aniline (3b)
The yield of the 3c compound: 0.26 g (74.3%), appearance: white crystals with m.p. 107˚C - 108˚C (hexane). 1H-NMR (CDCl3), δ, ppm, (J, Hz): 2.59 (1 Н, t, J1,2 5.1, J2,3 5.4, С7Н in С7Н7); 4.06 (1 Н, br.s, NН); 4.29 (2 Н, s, СН2); 5.34 - 5.39 (2 Н, dd, J 5.4, С1,6Н in С7Н7); 6.19 - 6.23 (2 Н, m, С2,5 Н in С7Н7); 6.65 (2 Н, d, J 8.1, оrthо-С6Н4-NH); 6.70 - 6.72 (2 H, m, С3,4Н in С7Н7); 7.16 (2 Н, d, J 8.4, mеtа-С6Н4-NH); 7,25 (2 Н, d, J 8.1 оrthо-С6Н4-СH2); 7.45 (2 Н, d, J 8.4, meta-С6Н4-СH2). MS (EI)+: m/z 352 (32) [М]+. Anal. Calcd for C20H18NBr (352,27): C, 67.96; H, 5.05; N, 3.83. Found: C, 68.16; H, 5.15; N, 3.97.
3) N-(4-Сhlorphenylmethyl)-41-(7-cyclohepta-1,3,5- trienyl)aniline (3c)
The yield of the 3b compound is 0.25 g (83.3%), appearance: white crystals with m.p. 94˚C - 95˚C (hexane), (ref. 94˚C - 95˚C [5] ). 1H-NMR (CDCl3), δ, ppm, (J, Hz): 2.59 (1 H, t, J 5.4, C7H in C7H7); 4.07 (1 H, br.s, NH); 4.30 (2 H, s, CH2); 5.34 - 5.39 (2 H, dd, J 5.4, C1,6H in C7H7); 6.19 - 6.22 (2 H, m, C2,5H in C7H7); 6.63 (2 H, d, J 8.4, ortho-C6H4-NH); 6.70 - 6.72 (2 H, m, C3,4H in C7H7); 7.16 (2 Н, d, J 8.4, meta-С6Н4-NH); 7,23 - 7.30 (4 Н, m,
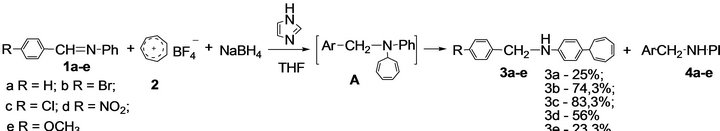
Scheme 1. One-pot synthesis of N-arylmethyl-4-(7-cyclohepta-1,3,5-trienyl)anilines (3а-е) in the presence of imidazole.

Scheme 2. The role of the B complex in the reducing imine hydrotropylation process.
Ar). MS (EI)+: m/z 307 (100) [M]+. Calcd for C20H18NCl (307,82): C, 78.03; H, 5.89; N, 4.55. Found: C, 78.63; H, 6.00; N, 3.44.
4) N-(4-Nitrоphenylmethyl)-41-(7-cyclohepta-1,3,5- trienyl)aniline (3d).
The yield of the 3d compound is 0.18 g (56%), appearance: white crystals with m.p. 128˚C - 130˚C (hexane). 1H-NMR (CDCl3), δ, ppm, (J, Hz): 2.59 (1 Н, t, J 5.1, С7Н in С7Н7); 4.25 (1 Н, br.s, NН); 4.48 (2 Н, s, СН2); 5.34 - 5.38 (2 Н, dd, J1,2 5.4, J3,4 5.7, С1,6Н in С7Н7); 6.20 - 6.23 (2 Н, m, С2,5Н in С7Н7); 6.62 (2 Н, d, J 8.7, оrthо-С6Н4-NH); 6.69 - 6.72 (2 Н, m, С3,4Н in Н С7Н7); 7.16 (2 Н, d, J 8.4, mеtа-С6Н4-NH); 7.55 (2 Н, d, J 8.4, оrthо-С6Н4-СH2); 8.19 (2 Н, d, J 9.0, mеtа- С6Н4-СH2). MS (EI)+: m/z 318 (100) [М]+. Calcd for C20H18N2O2 (318,37): C, 75.45; H, 5.69; N, 8.79. Found: C, 75.15; H, 5.95; N, 8.68.
5) N-(4-Methoxyphenylmethyl)-41-(7-cyclohepta-1,3,5- trienyl)aniline (3e)
The yield of the 3e compound is 0.07 g (23.3%), appearance: white crystals with m.p. 73˚C - 74˚C (hexane). 1H-NMR (CDCl3), δ, ppm, (J, Hz): 2.60 (1 H, t, J1,2 5.4, J3,4 5.7, C7H in C7H7); 3.79 (3 H, s, OCH3), 3.81 (1 H, br.s, NH); 4.26 (2 H, s, CH2); 5.35 - 5.40 (2 H, dd, J1,2 5.4, J3,4 5.7, C1,6H in C7H7); 6.20 - 6.23 (2 H, m, C2,5H in C7H7); 6.65 (2 H, d, J 8.4, ortho-C6H4-NH); 6.71 - 6.73 (2 H, m, C3,4H in C7H7); 6.88 (2 H, d, J 8.7, metaC6H4-CH2); 7.16 (2 H, d, J 8.4, meta-C6H4-NH), 7.29 (2 Н, d, J 9.0, ortho-С6Н4-СH2). MS (EI)+: m/z 303 (24) [М]+; Anal. Calcd for C21H21NO (303.40): C, 80.73; H, 6.57; N, 4.44. Found: C, 81.13; H, 6.97; N, 4.62.
4. Conclusions
Thus, the one-stage synthesis route of n-arylmethyl-4- (7-cyclohepta-1,3,5-trienyl)anilines through ionic hydrotropylation of imines in the “tropylium perchlorate—sodium tetrahydroborate” system in the presence of imidazole as activator has been designed.
The advantage of this method is the use of safe-tohandle tropylium tetrafluoroborate (instead of tropylium perchlorate), and the increased yield of the 3b-d compounds.
The advantage of the use of tropylium tetrafluoroborate is that higher yields for the 3b-d compounds are achieved as compared with tropylium perchlorate [5].
The educed compounds contain two biogenic moieties, namely NH group and tropilidene cycle.
5. Acknowledgements
This work was financially supported by the Ministry of Education of Perm Region Administration (Russian Federation).
REFERENCES
- L. P. Yunnikova, T. A. Akentieva, T. V. Makhova and G. A. Aleksandrova, “4-(7-Cyclohepta-1,3,5-trienyl)aniline and Derivatives Featured by Antimycobacterial Action,” Butlerov Communications, Vol. 32, No. 10, 2012, pp. 22-26.
- L. P. Yunnikova, T. A. Akentieva, T. V. Makhova and G. A. Aleksandrova, “Synthesis and Antimicrobial Activity of Amines and Imines with a Cycloheptatriene Fragment,” Pharmaceutical Chemistry Journal, Vol. 46, No. 12, 2012, pp. 106-108.
- K. Sanechika, S. Kajigaeshi and S. Kanemasa, “Azafulvenes; 51. A. Facile Synthesis of 8-Azaheptafulvenes,” Synthesis, Vol. 3, 1977, pp. 202-204. doi:10.1055/s-1977-24325
- J. J. Looker, “Alkylation of N,N-Dialkylarylamines with Tropylium Fiuoroborate,” Journal of Organical Chemistry, Vol. 30, No. 12, 1965, pp. 4180-4183. doi:10.1021/jo01023a046
- L. P. Yunnikova and T. A. Akentieva, “Synthesis of NArylmethyl-4-(1-cyclohepta-2,4,6-trienyl)anilines,” Natural and Technical Sciences, Vol. 6, No. 50, 2010, pp. 86-90.
- S. A. Kirlan, E. A. Kantor, A. S. Dimoglo and M. K. Vovdenko, “Regularities of the ‘structure-action-toxicity’ Link,” Bashkirian Chemical Journal, Vol. 18, No. 2, 2011, pp. 30-34.
- K. Takahashi, S. Takenaka and T. Nozoe, “Cyclic CrossConjugated Hydrocarbons Having Inserted p-Quinoid Ring-I,” Tetrahedron, Vol. 30, 1974, pp. 2191-2195. doi:10.1016/S0040-4020(01)97357-0

