Journal of Analytical Sciences, Methods and Instrumentation
Vol.2 No.1(2012), Article ID:17801,4 pages DOI:10.4236/jasmi.2012.21007
Liquid Scintillation Spectroscopy of 227Ac and Daughters
![]()
1Institute for Energy Technology, Kjeller, Norway; 2Algeta ASA, Oslo, Norway.
Email: d.o.eriksen@kjemi.uio.no
Received September 13th, 2011; revised October 22nd, 2011; accepted November 24th, 2011
Keywords: Dipex; LSC; Ra-223; Ac-227; Spectroscopy; Quantulus
ABSTRACT
In order to find a fast and reliable method for measurements of organic and aqueous liquid phases containing α- and β-emitters in mixtures we developed a method based on Liquid Scintillation Counting (LSC) using Quantulus Low Level Spectrometer. The use of low level LSC instead of γ-spectroscopy allows reduced sample activity, shorter count rates and use of sample changer. This is of great advantage when many samples have to be measured during a short time period. The main nuclides of interest were 227Ac and 223Ra. 227Ac does not have any appropriate γ-radiation, therefore measurements of the nuclei of interest must be based on α- and β-spectroscopy. In this study, it is shown that analyses of the radionuclidic purity of the liquid phases could be determined by α- and β-spectroscopy with Quantulus.
1. Introduction
In radiopharmacy the purity of the species involved is extreemly high both concerning the radioactive and the chemical compounds. Thus, in therapeutic injection fluids there are almost no radioactive or chemical contaminants. As a part of their work on developing new anticancer therapeutic agents, Algeta ASA is extracting radium from actinium. Radium grows in as a daughter nuclide from actinium (through thorium) [1]. It is well known that such separation can be achieved by both ion-exchange [2,3] and solvent extraction [4]. References 1 and 2 represent prior art of separation of actinides and of radium from their decay products and also from most other elements. This work is a study of di-phosphonate extractant Dipex® supplied by Eichrom as a separation agent for radium and actinium. The main nuclides of interest were 227Ac, 227Th and 223Ra. The series of daughter nuclides is shown in Figure 1 [5], where also the 1.38%—branch [4] of α-radiation creating 223Fr is shown separately. 227Ac does not have any appropriate γ-radiation, therefore direct measurements must be based on its β-particles whereas the daughter nuclide 227Th could be measured by its α-particles. 227Ac can be discriminated from the β-emitting daughter nuclides by the energy. Both 211Pb and 207Tl have β-particles with maximum energy >1 MeV [5]. The time of measurements is important to make proper corrections for the decay of the chain of daughters. In order to find a fast and reliable method for measurements of the liquid phases containing α- and β-emitters in mixtures it was decided to develop a method based on Liquid Scintillation Counting (LSC) using Quantulus Low Level Spectrometer. The use of low level LSC instead of γ-spectroscopy allows reduced sample activity, shorter count rates and use of sample changer. This is of great advantage when many samples have to be measured during a short time period.
2. Experimental
In all samples the liquid scintillator cocktail UltimaGold AB made by Packard was used. Features of Quantulus were utilised, comprising Peak Shape Analysis (PSA) for separating α- and β-events, divisions of spectrum in low and high energy windows, and Spectral Quench Parameter (SQP) for control of quenching. γ-spectroscopy was used for qualitative calibration.
Working with α-activity it is an advantage to keep the volumes as small as possible. Thus, most of the extraction tests were performed with small volumes, i.e. 0.5 - 5 mL, of both aqueous and organic phases. To ensure that
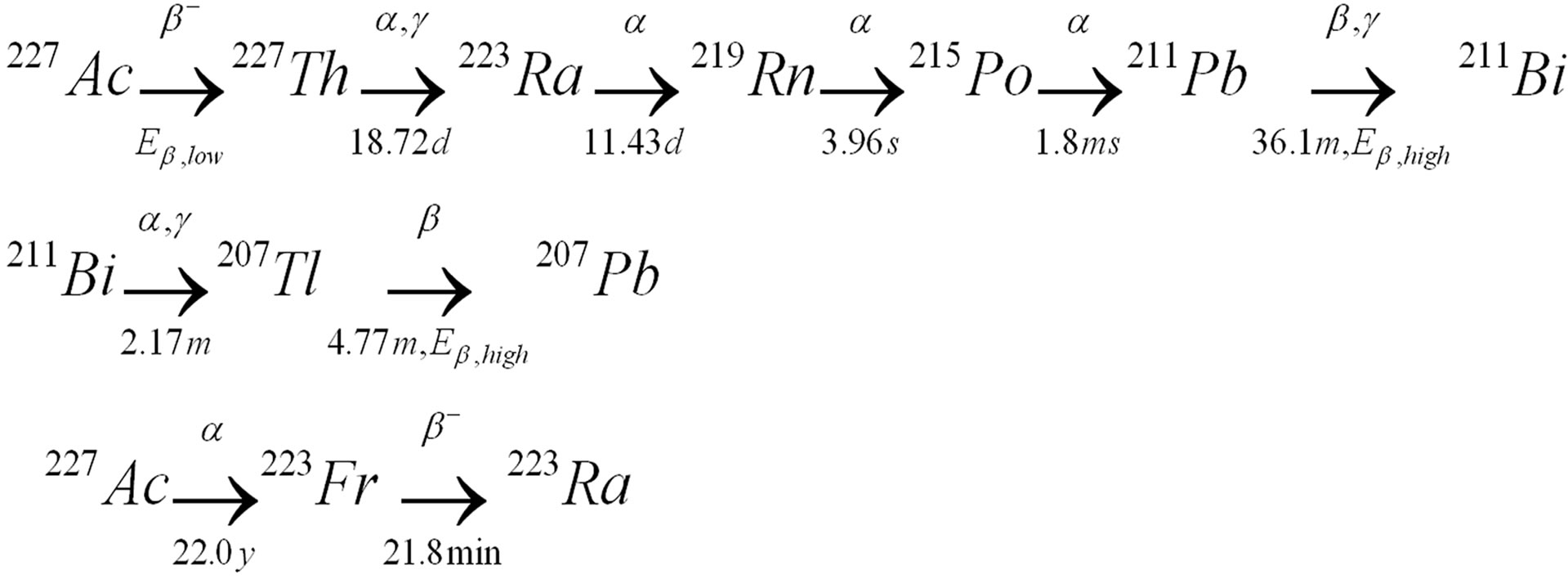
Figure 1. 227Ac and the series of daughter nuclides. Also shown is the low abundant (1.38%) 227Ac a-transformation to 223Fr.
all tests had equal and comparable mixing a rod stirrer in a small cylindrical vial, e.g. counting vial, was used since extraction funnels and manual shaking showed to be inaccurate. The two phases were stirred for three minutes before the sample was set to rest a few minutes until the two phases were separated. As chemical equilibrium was shown to be quickly obtained, three minutes was chosen as mixing time in all extraction and back-extraction (stripping) tests.
3. Results and Discussion
To enable α- and β-separation the PSA-parameter must be set manually. In Figure 2, the relative α- and β-activities as functions of the PSA-value is shown. It was a definite minimum of α’s in β’s and vice versa for PSA = 60. This value was therefore used throughout the tests. Also, the SQP versus counting efficiency was determined. However, all samples had SQP-values > 850 which was a region where the efficiency was constant. There was therefore no need for corrections to compare the results. It must be noted, however, that the SQP is a relative measure of the maximum energy of an induced Compton electron spectrum from an external γ-source, and is thus only a measure of the quenching of the β-spectra, not the α-spectra.
In Figure 3 γ-spectra of the aqueous and organic phases after extraction are shown. The organic phase is containing 227Th, whereas the aqueous phase is containing 223Ra and its daughter nuclides. As is seen, 227Ac has no appropriate γ-energies and cannot be determined. Included in the figure are two β-spectra showing 227Ac in the low energy region (organic phase) and 223Ra and its daughter nuclides in the high energy region (aqueous phase).
Figure 4 shows LSC-spectra after extraction with three different concentrations of Dipex in hexane, 0.008%, 0.2%, and 2.15% by volume, respectively. The aqueous phase was 3 M HCl in all cases. These spectra
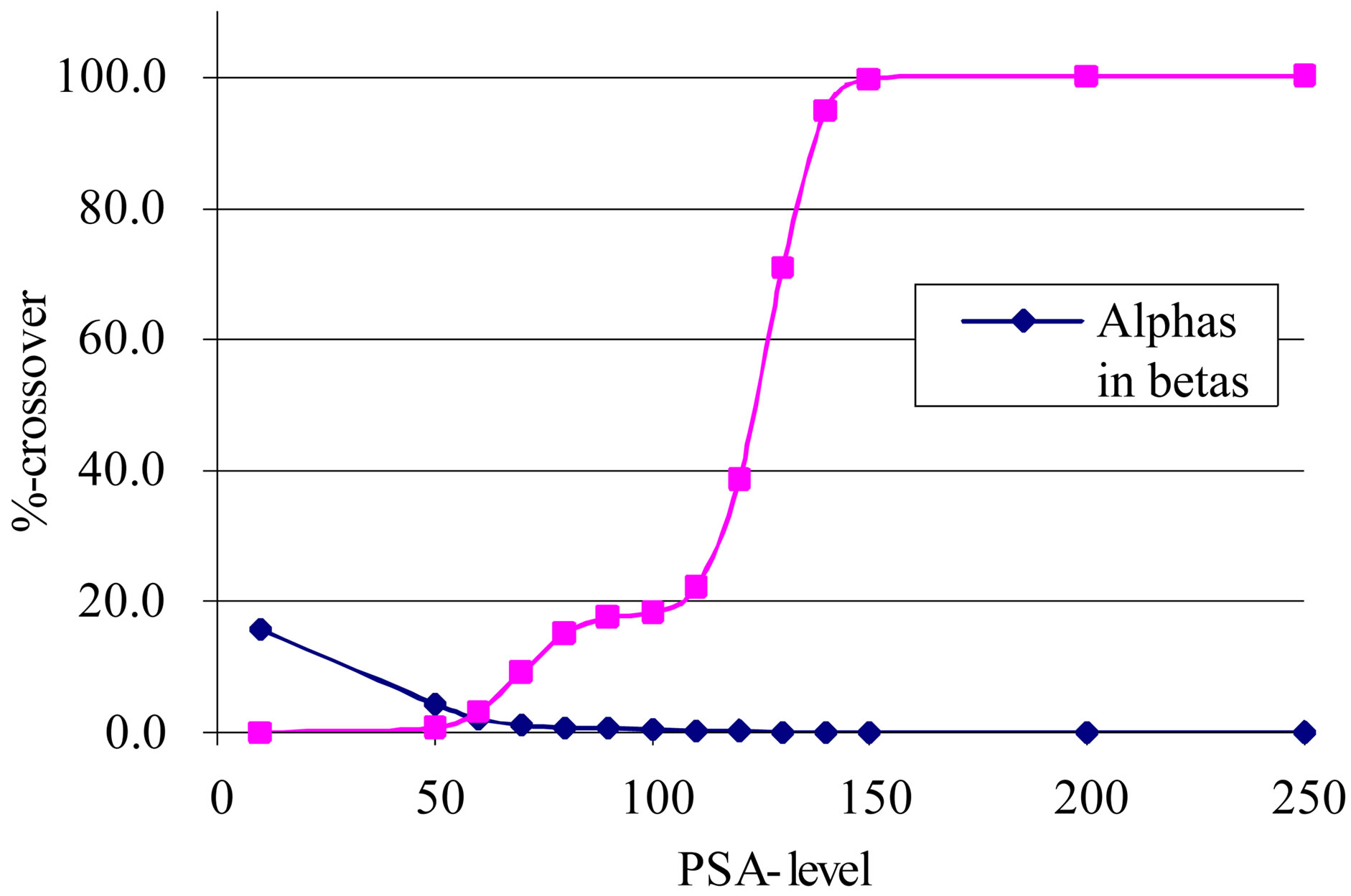
Figure 2. The relative aand b-activities as functions of the PSA-value.
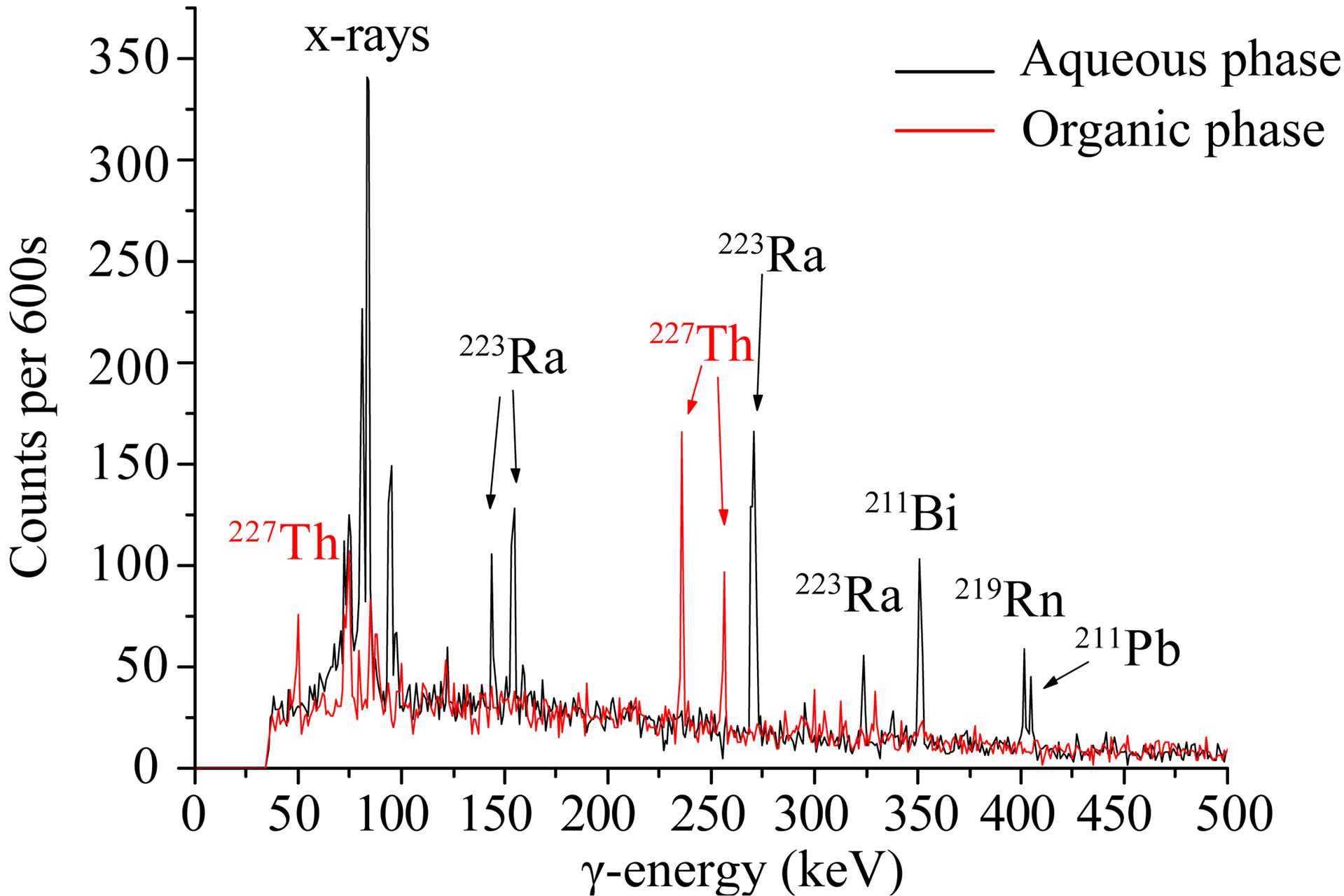
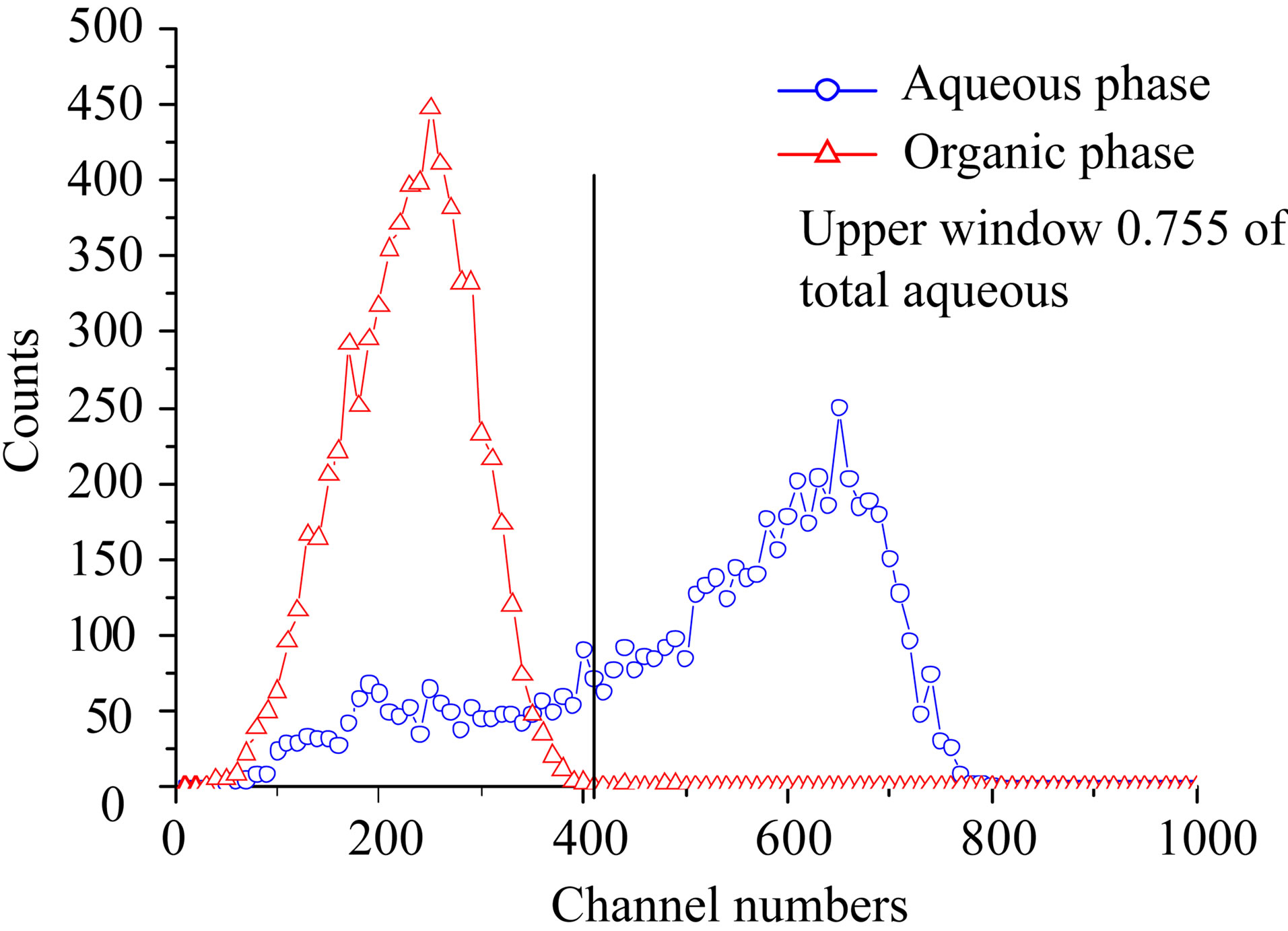
Figure 3. γ-spectra of the aqueous and organic phases after extraction. The organic phase contains 227Th, whereas the aqueous phase contains 223Ra and its daughter nuclides. Included are two b-spectra measured by LSC showing 227Ac in the low energy region (organic phase) and 223Ra and its daughter nuclides in the high energy region (aqueous phase).
confirm that separation of Ac and Ra may be obtained with solvent extraction. The lower portion of the figure (2.15% Dipex) shows an organic phase containing solely 227Ac, while the high energy β-emitting daughter nuclei of 223Ra, i.e. 211Pb and 207Tl, are present in the 3 M HCl aqueous phase. It also shows that the small peak in the low energy part of the β-spectrum in Figure 3 is contribution from 227Ac in the aqueous phase. A strip with pure water of an organic solution containing 227Ac gave the spectrum in Figure 5. This cannot be any of the elements in the main chain of daughter nuclides of Ac as they are strongly extracted by Dipex at high pH-values. The only plausible element that would seek a neutral aqueous phase is an alkaline element, i.e. Fr. This may not be in accordance with Mitsugashira et al. [4] who used a buffer solution at pH 2.2 to recover 223Fr from 1M HDEHP-solution in benzene. We have not compared the extraction ability of 1 M (33%) HDEHP with 2% Dipex, but presumably it is much easier to recover Fr from the latter. It
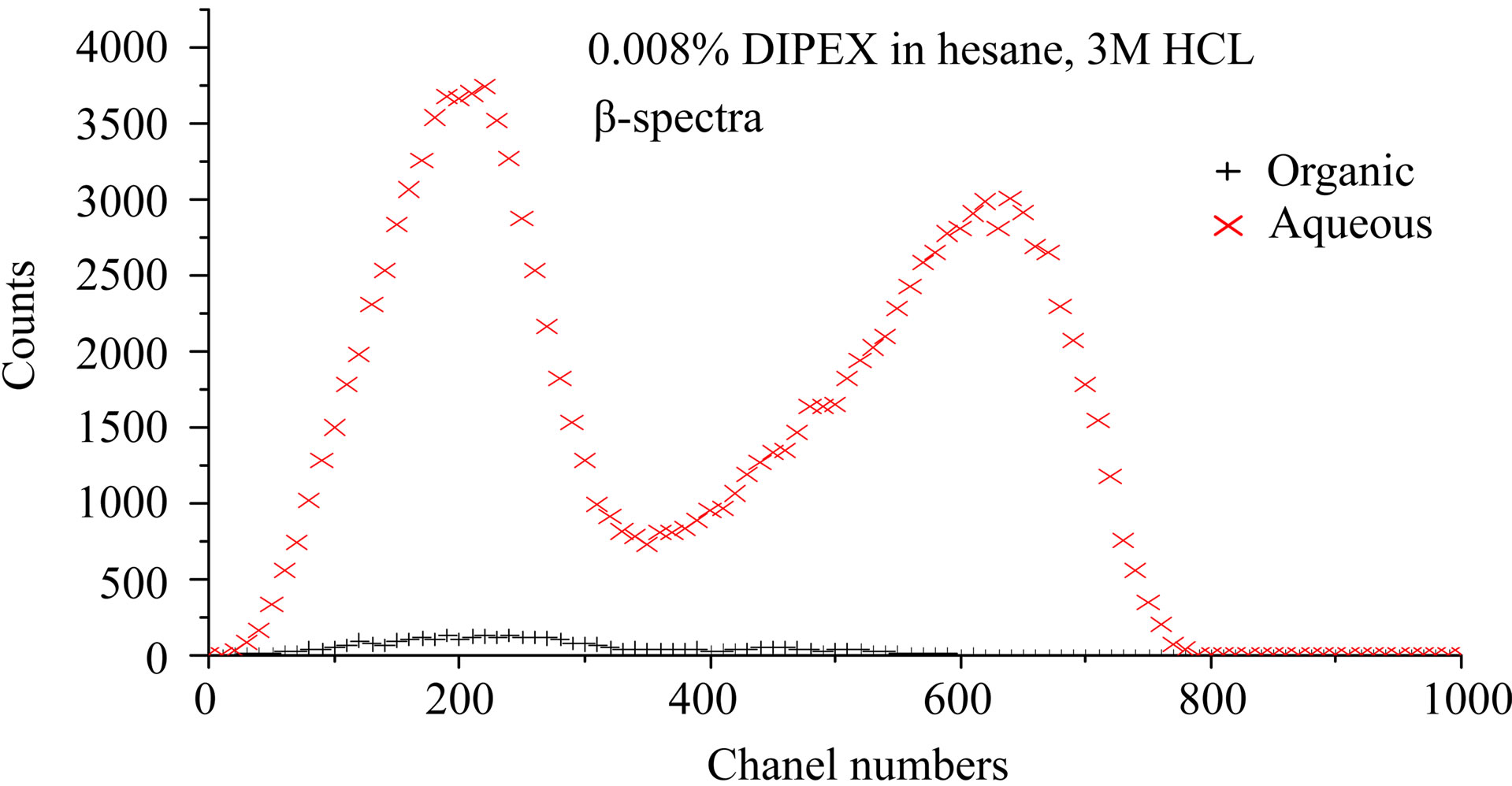

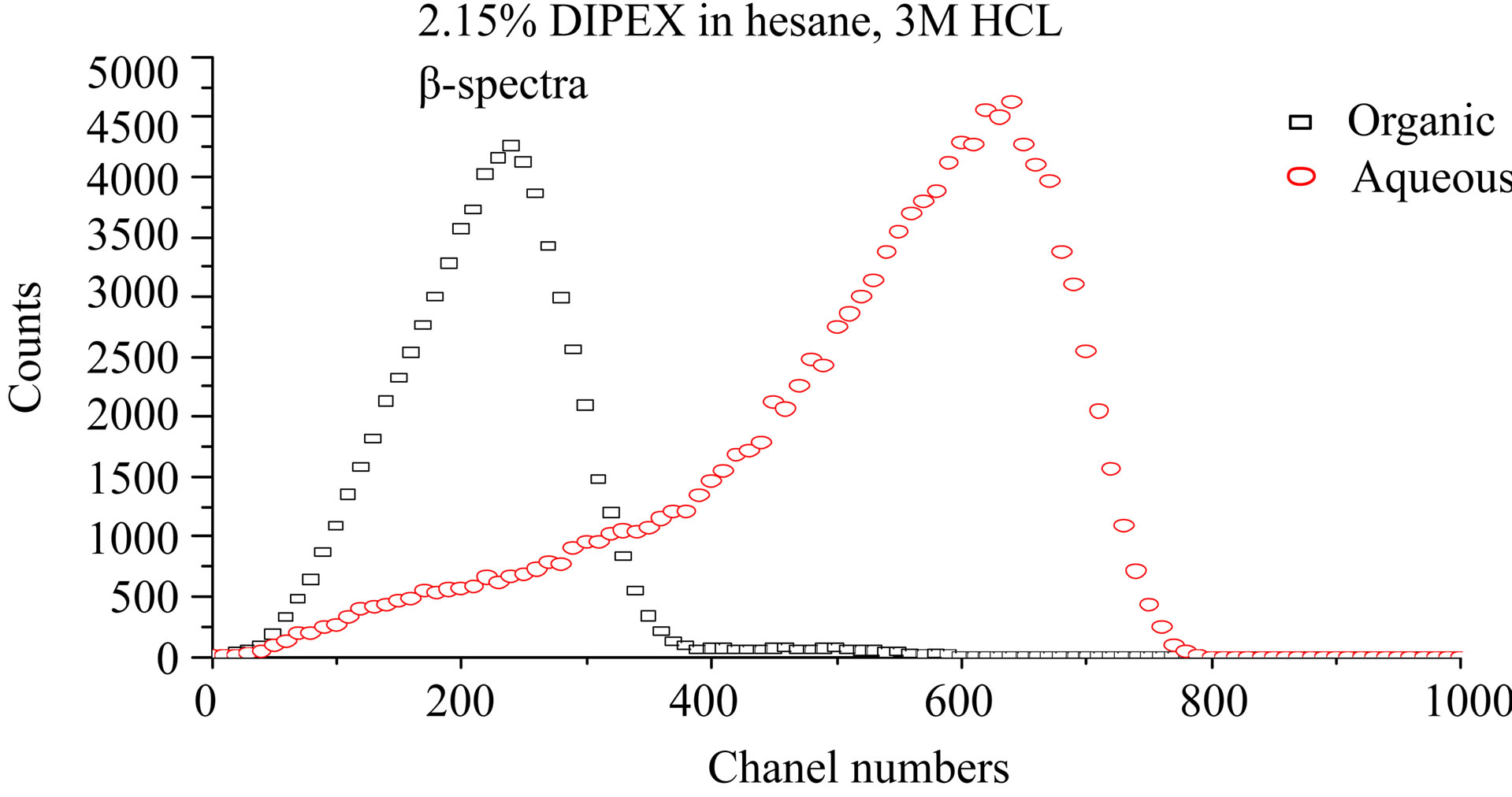
Figure 4. LSC-spectra after extraction with three different concentrations of dipex in hexane, 0.008%, 0.2%, and 2.15% respectively. The aqueous phase was 3 M HCl in all cases.
is noteworthy that the measured β-spectrum (Figure 5) is not in accordance with the decay characteristics reported by Abdul-Hadi et al. [6], but there seems not to be published β-spectra of 223Fr. The closest we have found is a spectrum calculated from data given by Table of Isotopes [7]. Except for 223Fr we have no suggestion for which nuclide the measured β-spectrum belong to. Unfortunately, the half-life was not determined.
4. Conclusion
We have developed a method of using low level LSC for measurements of α-and β-emitting nuclides in mixtures. Features of modern liquid scintillation spectrometers like α- and β-separation (peak shape analysis), quench corrections, etc must be utilised. For the samples of interest in this project there was, however, no need for quenching corrections. The method applies e.g. very well to solvent
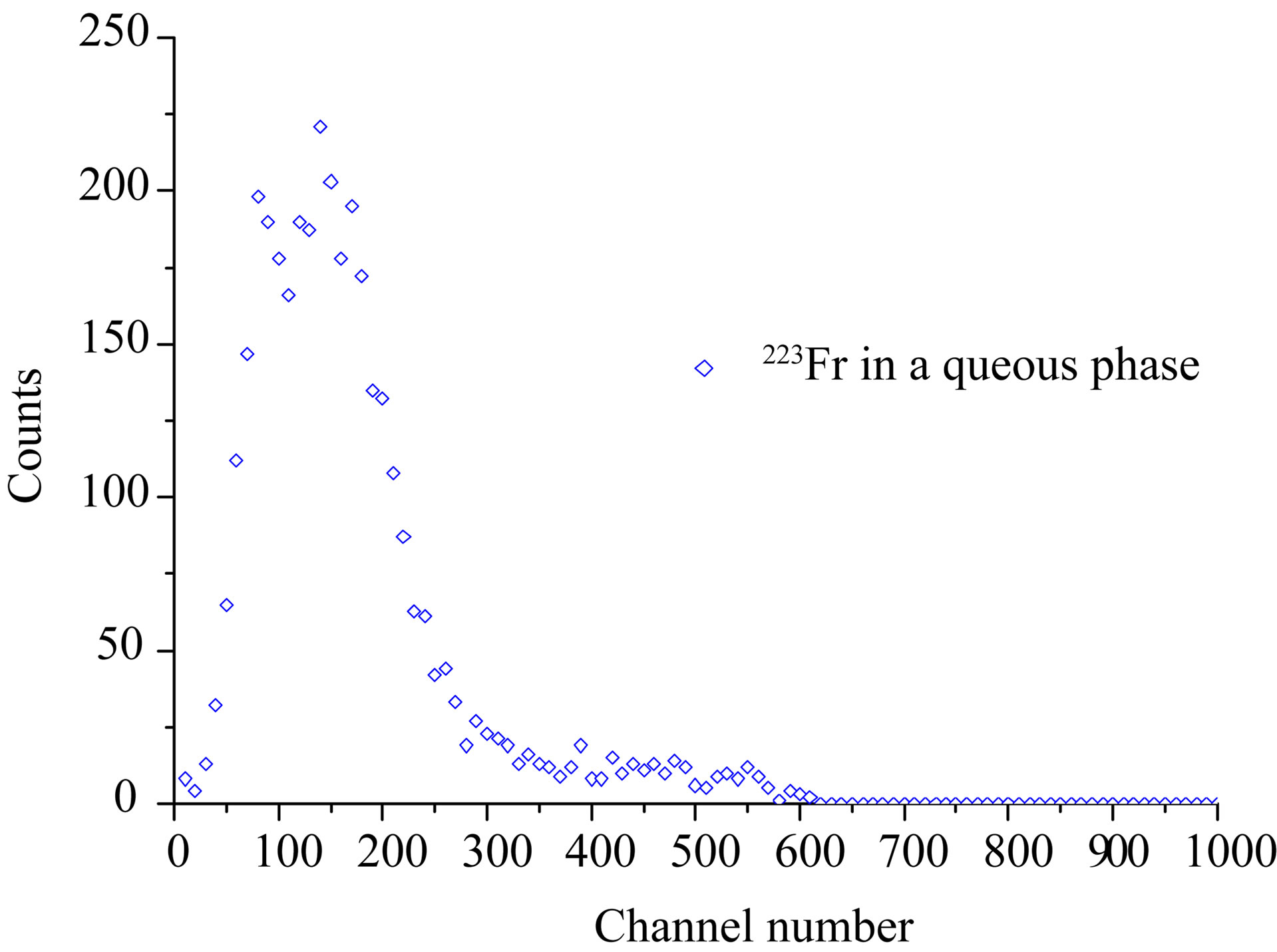
Figure 5. Spectrum obtained from a strip of an organic solution containing 227Ac with pure water. The most probable nuclide is 223Fr based on the chemistry. Its spectrum is however, not in accordance with literature.
extraction samples requiring to be measured within a relatively short period of time after separation and is a relevant alternative to gamma spectroscopy for nuclides with low gamma abundance.
5. Acknowledgements
The authors are grateful to Algeta for allowing publication of this work.
REFERENCES
- G. Henriksen, P. Hoff, J. Alstad and R. H. Larsen, “223Ra for Endoradiotherapeutic Applications Prepared from an Immobilized 227Ac/227Th Source,” Radiochimica Acta, Vol. 89, No. 10, 2001, pp. 661-666. doi:10.1524/ract.2001.89.10.661
- R. Chiarizia and E. P. Horwitz, “Radiolytic Stability of Some Recently Developed Ion Exchange and Extraction Chromatographic Resins Containing Diphosphonic Acid Groups,” Solvent Extraction and Ion Exchange, Vol. 18, No. 1, 2000, pp. 109-132. doi:10.1080/07366290008934675
- E. P. Horwitz, R. Chiarizia and M. L. Dietz, “DIPEX: A New Extraction Chromatographic Material for the Separation and Preconcentration of Actinides from Aqueous Solution,” Reactive & Functional Polymers, Vol. 33, No. 1, 1997, pp. 25-36. doi:10.1016/S1381-5148(97)00013-8
- T. Mitsugashira, H. Yamana and S. Suzuki, “The Mutual Separation of 227Ac, 223Ra, and 223Fr by the Solvent Extraction Technique Using Bis(2-ethylhexyl)phosphoric Acid as an Extractant,” Bulletin of the Chemical Society of Japan, Vol. 50, No. 11, 1977, pp. 2913-2916. doi:10.1246/bcsj.50.2913
- G. Pfennig, H. Klewe-Nebenius and W. Seelmann-Eggebert, “Karlsruher Nuklidkarte,” 6th Edition, Forschungszentrum Karlsruhe, 1995.
- A. Abdul-Hadi, V. Barci, B. Weiss, H. Maria, G. Ardisson, M. Hussonnois and O. Constantinescu, “223Ra Levels Fed in the 223Fr β Decay,” Physical Review C, Vol. 47, No. 1, 1993, pp. 94-109. doi:10.1103/PhysRevC.47.94
- S. Y. F. Chu, R. B. Firestone, L. N. Nguyen, P. Ekström, “Isotope Explorer,” DE-AC03-76SF00098, 1998.

