Paper Menu >>
Journal Menu >>
 Materials Sciences and Applicatio n, 2011, 2, 1121-1126 doi:10.4236/msa.2011.28151 Published Online August 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA 1121 Electronic States in Trans-CoCl2(H2O)4 Complex Hajer Souissi1*, Souha Kammoun1 1Université de Sfax, Laboratoire de Physique Appliquée, Groupe de Physique Théorique, Département de Physique, Faculté des Sciences de Sfax, Sfax, Tunisie. Email: *hajer_souissi_fss@yahoo.fr Received March 7th, 2011; revised March 29th, 2011; accepted June 12th, 2011. ABSTRACT The polarized absorption spectra of cobalt(II) in trans-CoCl2(H2O)4.2H2O provides important information about the electronic structure. Semi-empirical calculation of the crystal-field levels of the cobalt(II) with D4h point group symme- try in CoCl2(H2O)4·2H2O are carried out, leading to a good agreement between the theoretical and experimental energy levels. Keywords: Crystal And Ligand Fields, Transition-Metal Compounds, Cocl2(H2O)4 Chromophore 1. Introduction Several spectroscopic investigations of the transition metal complexes with the trans-dichlorotetraaquo ligand environment MCl2(H2O)4n+ (M: transition metal, M = V3+, Ni2+, Co2+, Cr3+) are reported in literature [1-8]. For these complexes, the bands of the absorption and luminescence spectra are related to the transition metal ions M with 3dn (n = 2, 3, 7, 8) configuration and originate from elec- tronic transition in the unfilled 3d electronic shell. A de- tailed crystal-field studies of the electronic energy levels of trans-NiCl2(H2O)4, trans-VCl2(H2O)4+ and trans- CrCl2(H2O)4+ complexes were presented in a recent work [9-11]. These theoretical studies emphasized the impor- tance of coupling between electronic states in the case of the NiCl2(H2O)4 chromophore and of the Jahn-Teller effect in the case of CrCl2(H2O)4+. Crystals of trans-CoCl2- (H2O)4.2H 2O crystallize as regular monoclinic mauve prisms [8,12]. Crystallographic studies confirm that crystal of CoCl2(H2O)4.2H2O contain the trans- CoCl2(H2O)4 chromophore where the Co2+(3d7) ions oc- cupy a site with D4h symmetry [8]. A spectroscopic stud- ies of the optical characteristics of trans-CoCl2(H2O)4 complex were presented by Bussière et al. [8]. In their publications, the crystal field calculations based on the angular overlap model (AOM) were performed for this complex. In this actual work, we present a detailed crystal-field analysis of the electronic energy levels of trans-CoCl2 (H2O)4 complex. This analysis based on the Racah theory was carried out for the Co2+ centre with a D4h site sym- metry. The objective of this theoretical analysis is to de- termine the Racah and the crystal-field parameters for the CoCl2(H2O)4 chromophore, and, therefore, to deduce the Stark levels which are not observed experimentally. 2. Experimental Support The bases of our theoretical analysis will be the experi- mental energy data obtained from the optical spectra of trans-CoCl2(H2O)4 complex. For this reason, we present briefly in this section the results of the spectroscopic studies carried out by Bussière et al. at low temperature [8,12]. The polarized absorption spectra of trans- CoCl2(H2O)4 is measured at low temperature 5 K (Fig- ure1) [8]. Three quartet bands are expected for Cobalt(II) in octahedral symmetry : the first one is seen in the near-infrared region of the spectrum around 8000 cm–1, involving the 4T2g(4F) state. This band will be split into 4Eg and 4B2g in the D4h point group. The assignment of the other two quartet bands is problematic and there are substantial differences between the assignments given by different authors [8]. For this reason Bussière et al. l de- clare that this assignment is uncertain [8]. For a mixed water and chloride ligand spheres, the 4A2g(4F) and 4T1g (4P) states should be near a crossing point [8]. Bussière et al.l have reported the 4A2g (4F) state to be at 16450 cm–1 (4B1g in D4h symmetry) and the 4T1g (4P) state, to be split into 4Eg and 4A2g (in D4h symmetry) at 18600 and 22250 cm–1 corresponding to the strong bands in Figure 1. The experimental energy levels are listed in Table 1. The angular overlap model (AOM) [13] was used by Bussière et al. in order to obtain the energetic order of quartet and doublet excited states in this tetragonal chromophore. 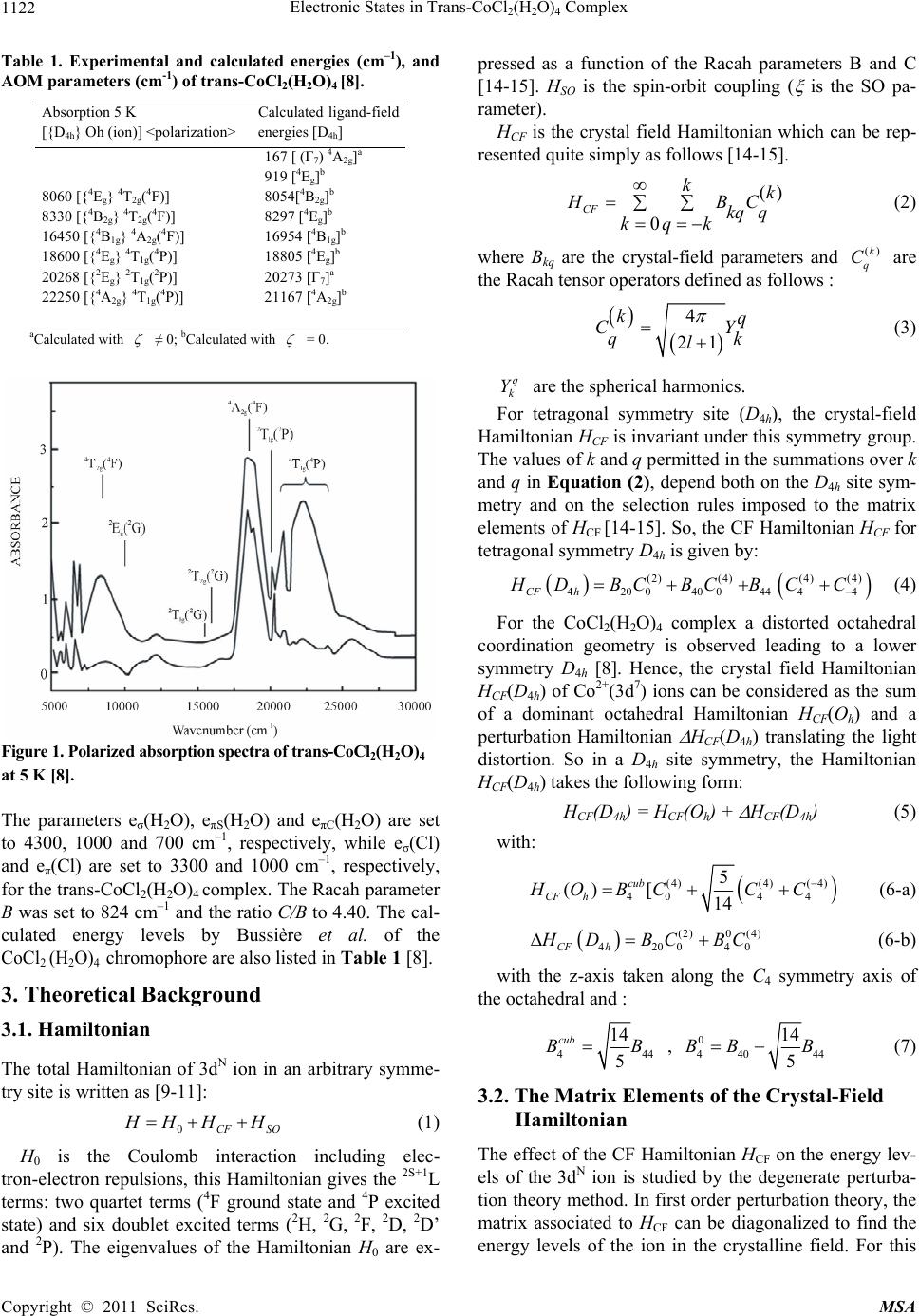 Electronic States in Trans-CoCl(H O) Complex 1122 2 2 4 Table 1. Experimental and calculated energies (cm–1), and AOM parameters (cm-1) of trans-CoCl2(H2O)4 [8]. Absorption 5 K [{D4h} Oh (ion)] <polarization> Calculated ligand-field energies [D4h] 8060 [{4Eg} 4T2g(4F)] 8330 [{4B2g} 4T2g(4F)] 16450 [{4B1g} 4A2g(4F)] 18600 [{4Eg} 4T1g(4P)] 20268 [{2Eg} 2T1g(2P)] 22250 [{4A2g} 4T1g(4P)] 167 [ (Γ7) 4A2g]a 919 [4Eg]b 8054[4B2g]b 8297 [4Eg]b 16954 [4B1g]b 18805 [4Eg]b 20273 [Γ7]a 21167 [4A2g]b aCalculated with ≠ 0; bCalculated with = 0. Figure 1. Polarized absorption spectra of trans-CoCl2(H2O)4 at 5 K [8]. The parameters eσ(H2O), eπS(H2O) and eπC(H2O) are set to 4300, 1000 and 700 cm–1, respectively, while eσ(Cl) and eπ(Cl) are set to 3300 and 1000 cm–1, respectively, for the trans-CoCl2(H2O)4 complex. The Racah parameter B was set to 824 cm–1 and the ratio C/B to 4.40. The cal- culated energy levels by Bussière et al. of the CoCl 2 (H 2 O) 4 chromophore are also listed in Table 1 [8]. 3. Theoretical Background 3.1. Hamiltonian The total Hamiltonian of 3dN ion in an arbitrary symme- try site is written as [9-11]: 0CF SO H HH H (1) H0 is the Coulomb interaction including elec- tron-electron repulsions, this Hamiltonian gives the 2S+1L terms: two quartet terms (4F ground state and 4P excited state) and six doublet excited terms (2H, 2G, 2F, 2D, 2D’ and 2P). The eigenvalues of the Hamiltonian H0 are ex- pressed as a function of the Racah parameters B and C [14-15]. HSO is the spin-orbit coupling ( is the SO pa- rameter). HCF is the crystal field Hamiltonian which can be rep- resented quite simply as follows [14-15]. () 0 CF kk HB kq q kqk C (2) where Bkq are the crystal-field parameters and are the Racah tensor operators defined as follows : ()k q C 4 21 kq C q l Y k (3) q k Y are the spherical harmonics. For tetragonal symmetry site (D4h), the crystal-field Hamiltonian HCF is invariant under this symmetry group. The values of k and q permitted in the summations over k and q in Equation (2), depend both on the D4h site sym- metry and on the selection rules imposed to the matrix elements of HCF [14-15]. So, the CF Hamiltonian HCF for tetragonal symmetry D4h is given by: (2)(4)(4) (4) 42004004444CF h HDBCBC BCC (4) For the CoCl2(H2O)4 complex a distorted octahedral coordination geometry is observed leading to a lower symmetry D4h [8]. Hence, the crystal field Hamiltonian HCF(D4h) of Co2+(3d7) ions can be considered as the sum of a dominant octahedral Hamiltonian HCF(Oh) and a perturbation Hamiltonian HCF(D4h) translating the light distortion. So in a D4h site symmetry, the Hamiltonian HCF(D4h) takes the following form: HCF(D4h) = HCF(Oh) + HCF(D4h) (5) with: (4)(4)( 4) 4044 5 () [14 cub CF h HO BCCC (6-a) (2)0(4) 42004CF h0 H DBCBC (6-b) with the z-axis taken along the C4 symmetry axis of the octahedral and : 444 14 5 cub BB , 0 4404 14 5 BB B 4 (7) 3.2. The Matrix Elements of the Crystal-Field Hamiltonian The effect of the CF Hamiltonian HCF on the energy lev- els of the 3dN ion is studied by the degenerate perturba- tion theory method. In first order perturbation theory, the matrix associated to HCF can be diagonalized to find the energy levels of the ion in the crystalline field. For this Copyright © 2011 SciRes. MSA  Electronic States in Trans-CoCl(H O) Complex1123 2 2 4 reason, we give the method of calculation of the matrix elements of the Hamiltonian HCF. Since for 3dN ions of the first series in crystals the crystal-field is of the inter- mediate strength [14-15], the basis functions N LS dLSMM in the LS-coupling scheme have been adopted in our computer package. The CF Hamiltonian is developed as a function of the Racah tensor . The matrix ele- ments of operators are calculated numerically by using the Racah tensor algebraic methods [14,15]: ()k q C ()k q C () ' ' ' ' (1) ' L k LS qL S LM k LL LSM MCLSMM LL kLSCL S MM q The 3j-symbols ' ' LL LL k MM q carry all the dependence on the labels ML, q and ML’ while the reduced matrix elements ' k LSCLS is independent of these labels, but depends on the normali- zation of the tensor operators. 3.3. A Crystal-Field Analysis Computer Package for 3dN (N = 2, 3, 7, 8) Ions As a part of a larger project we have set to develop a computer package based on Maple software to calculate the energy levels and state vectors for any transition metal ions with the 3dN configuration (N = 2, 3, 7, 8) located at sites with symmetry given by any of the 32 crystallo- graphic point groups. This computer package comprises the following extensions: (1) the calculation of the matrix elements of the crystal-field HCF by considering the 3j-symbols and the reduced matrix elements. (2) diago- nalization of the full Hamiltonian matrices (taking into account H0, HCF and HSO), this diagonalization yields the energy levels as functions of the Racah parameters B and C, crystal-field parameters Bkq and spin-orbit coupling constant ξ. These parameters are regarded as empirical parameters to be determined from the optical spectra. (3) the determination of the Racah, crystal-field and spin-orbit coupling parameters by minimising the gap between the experimental and the theoretical energies. These parame- ters allow us to deduce the calculated energy levels. 4. Results and Discussion In this work, we are interested in the crystalline-field splitting of the energy levels of Co2+(3d7) ions in CoCl2(H2O)4 chromophore. The energetic contribution of the Hamiltonian H0 + HCF(Oh) is dominant compared to that of HCF(D4h) translating the light distortion. So, we firstly determine the Racah parameter B and the octahe- dral crystal-field parameter 4 from the mean values of the observed spin-allowed bands in Oh symmetry (<4T2g(4F)> = 8195 cm–1, <4A2g(4F) > = 18600 cm–1 and <4T1g(4P) > = 22250 cm–1). These parameters are de- duced from the eigenvalues obtained by diagonalising the matrix 10 × 10 associated to the Hamiltonian H0 + HCF(Oh) in the free-ion eigenstates cub B , L LM within 4F and 4P terms. According to Sugano et al. [16], the (10-N)-electron system can be regarded as the N-hole sys- tem. So, one can obtain the d7 matrix by changing the signs of only the crystal-field matrix elements within the complete energy matrix for the d3 configuration. The Ra- cah parameter C is deduced from the observed spin-forbidden bands in Oh symmetry and the eigenvalues obtained by diagonalising the matrix 40 × 40 associated to H0 + HCF(Oh) in the free-ion eigenstates , L LM within 2H, 2G, 2F, 2D, 2D’ and 2P terms. The values of the Racah and crystal-field parameters B, C and B4 cub obtained are listed in Table 2. The calculated Oh energies for CoCl2(H2O)4 chromo- phore are listed in Table 3. Then, the matrix elements of the Hamiltonian perturbation HCF(D4h) are calculated within the basis formed by the octahedral eigenstates. The remaining crystal-field parameters B20 and were calculated from the eigenvalues of the matrix associated to HCF(D4h) and the observed bands at 8060 and 8330 cm–1 corresponding respectively to transitions from 4Eg (4T1g(4F)) ground state to 4Eg(4T2g(4F)) and 4B2g(4T2g(4F)) excited states. The crystal-field parameters B20 and obtained are respectively –540 cm–1 and –405 cm–1. From equation 7, we deduce the values of the crys- tal-field parameters B44 and B40. The spin-orbit coupling constant λ (λ = /3) considered in this work is similar to that of reference [8]. The values of the crystal-field pa- rameters B20, B40 and B44 and the spin-orbit coupling constant λ are listed in Table 2. By using these parame- ters, the diagonalization of the 120 × 120 matrix within the quartet and doublet states associated to the Hamil- tonien HCF(D4h) of equation 4 lead to the calculated en- ergies for CoCl2(H2O)4 chromophore listed in Table 4. 0 4 B 0 4 B Table 2. Racah and Crystal-field parameter values (cm–1) for CoCl2(H2O)4 chromophore. B C B4cub B20 B40 B44 λ 948 3460 -19710 -540 -20115 -11778 -171 Copyright © 2011 SciRes. MSA  Electronic States in Trans-CoCl2(H2O)4 Complex Copyright © 2011 SciRes. MSA 1124 Table 3. Computed energies (cm–1) in Oh symmetry for CoCl2(H2O)4 chromophore. 4T1g(4F) 0 4T2g (4F) 2Eg (2G) 8195 9788 2T1g(2G) 15786 2T2g (2G) 16135 4A2g(4F) 17580 2T1g (2P) 20006 4T1g (4P) 21223 2A1g (2G) 21308 2A2g(2F) 40268 Table 4. Calculated energies for Co2+ centres with D4h symmetry in CoCl2(H2O)4 chromophore. Oh D 4h Ecalculated (cm-1) Spin-orbit coupling 0 320 369 4Eg 0 904 946 4T1g(4F) 4A2g 90 978 8766 8810 8846 4 Eg 8270 8951 9057 4T2g (4F) 4 B2g 8441 9169 2 A1g 8880 9170 2Eg(2G) 2 A2g 9187 9476 15094 15639 2T1g(2G) 2 Eg 15692 2 A2g 15232 15806 2B2g 15801 16310 16418 2T2g (2G) 2Eg 15931 16756 18463 4A2g(4F) 4 B1g 17826 18464 20588 2T1g(2P) 2Eg 20103 20618 2 A2g 20174 20932 21549 4 A2g 21086 21587 21837 22084 22207 4T1g (4P) 4 Eg 21486 22385 2A1g(2G) 2 A1g 22228 22817 2A2g(2F) 2 A2g 41196 41764 Our calculated energies are in agreement with the ob- served ones. The discrepancy between the theoretical energies of this work and the experimental energies is explained by the existence of a recovery between the d orbitals of the central metallic ion and the orbitals of the ligands. The crystal field theory provides a way of determining, by simple electrostatic considerations, how the energies of the d central metal ion orbitals will be affected by the set of the surrounding ligands. So, the electrostatic crys- tal field theory deals only with the d orbitals. The mo- lecular orbitals d are not pure metal d orbitals, these or- bitals are influenced by the interactions with the σ and π orbitals of the ligands. The theoretical results give values of B = 948 cm–1, C = 3460 cm–1, Dq = 938.57 cm–1 ( = –19710 cm–1), Dq/B = 0.99 and C/B= 3.65. The Racah parameters B and C are reduced compared to the free ion values (Bfree ion = 1038 cm–1 and Cfree ion = 4366 cm–1 [14,16]) due to covalency effects. With βB=Bcomplex ion/Bfree ion and βC=Ccomplex ion/Cfree ion it follows: βB = 0.91 and βC = 0.80. The Dq/B ratio for CoCl2(H2O)4 is lower than the ratio of 1.2 reported for Co(H2O)62+ [7]. This comparison shows that the crystal field strength for CoCl2(H2O)4 is slightly lower than in [Co(H2O)6]2+. This can be explained by the dependence of crystal field strength parameter Dq on the distance between Co2+ ion and ligands. Effectively, the bond length Co-Cl (2.488 Å [17]) is higher than the bond length Co-O (1.896 to 1.916 Å [17]). So, the crystal-field strength Dq is reduced by increasing the number of cobalt ligator atoms. Co2+ oc- cupy an Oh site symmetry in [Co(H2O)6]2+ [7]. The Ra- cah parameters B and C were set respectively to 829 cm–1 and 3316 cm–1 for Co(H2O)62+ [7]. As the values of B and C are higher for the case of CoCl2(H2O)4, the nephe- lauxetic effect is more important in our complex. The comparison with Cr3+ ion in CrCl2(H2O)4 (β = 0.63) fits well into the sequence β(Cr3+) < β(Co2+). Also, the crys- tal field parameter Dq fits into the expected sequence Dq(Cr3+) = 1638 cm–1 [11] > Dq(Co2+) = 938.57 cm–1. cub B4 In Figure 2, the Tanabe-Sugano diagram for d7 ions in octahedral coordination is shown with the crystal field strength of the Co2+ ion in CoCl2(H2O)4 indicated by a vertical line. The set of calculated parameters given in Table 2 re- produce well the energies of the observed spin-allowed absorption band of Figure 1. Moreover, we have de- duced the energy levels which are not observed experi- mentally in D4h symmetry taking into account the spin-orbit coupling. From Tanabe-Sugano diagram, we remark that the 4T2g(4F) quartet state and the 2Eg(2G) doublet state are nearly for the calculated parameters Dq, B and C. This shows a strong coupling between the elec- tronic states of different multiplicities. Tetragonal D4h symmetry offers two doublet states 2A1g(2Eg(2G)) and 2A2g(2Eg(2G)) interacting with tow quartet states 4Eg(4T2g(4F)) and 4B2g(4T2g(4F)) (Figure 3). All these ex- 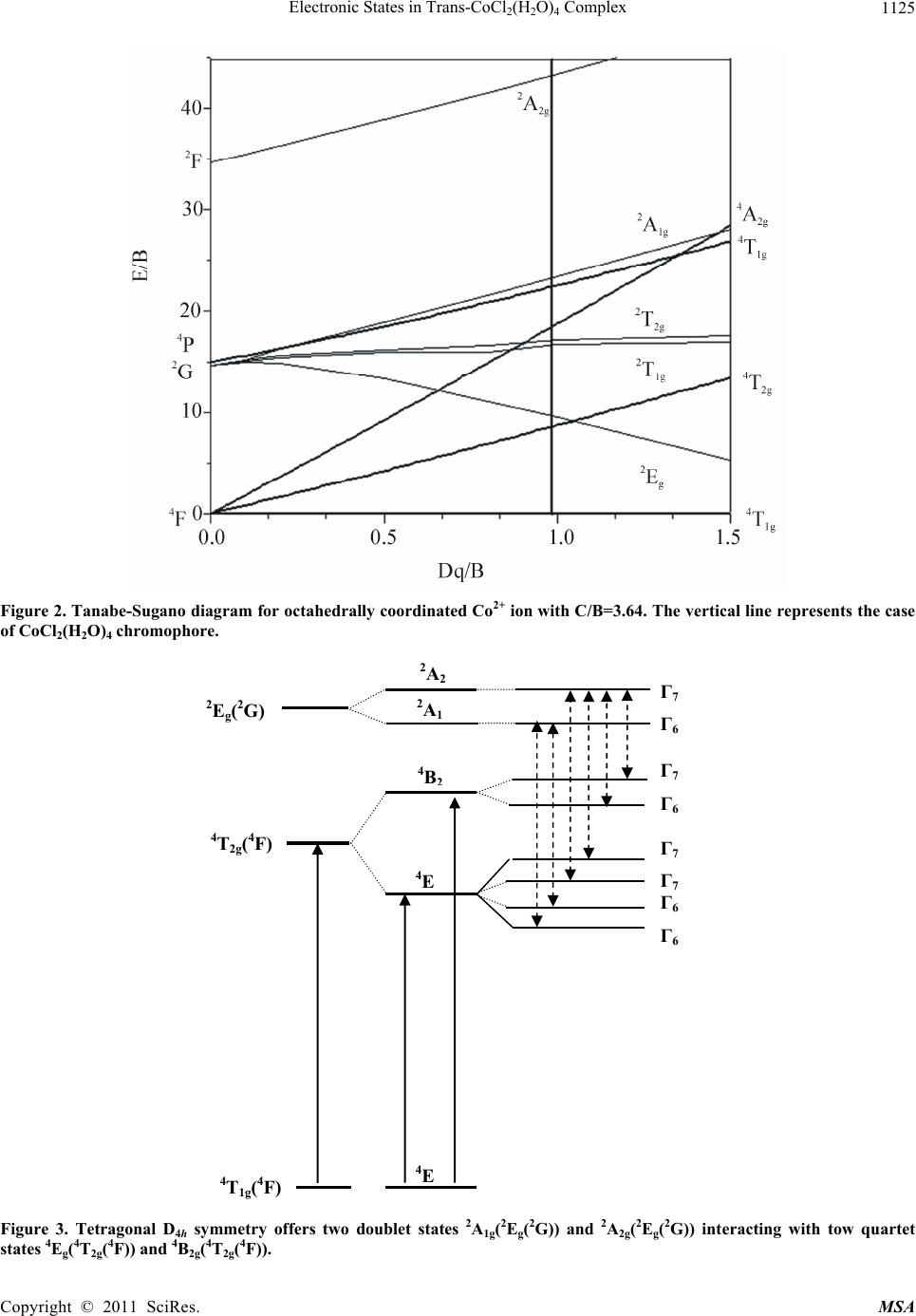 Electronic States in Trans-CoCl(H O) Complex 1125 2 2 4 Figure 2. Tanabe-Sugano diagram for octahedrally coordinated Co2+ ion with C/B=3.64. The vertical line represents the case of CoCl2(H2O)4 chromophore. Figure 3. Tetragonal D4h symmetry offers two doublet states 2A1g(2Eg(2G)) and 2A2g(2Eg(2G)) interacting with tow quartet tates 4Eg(4T2g(4F)) and 4B2g(4T2g(4F)). s 4E 4T2g(4F) 4B 2 Γ7 Γ7 Γ6 Γ6 Γ7 Γ6 2A 2 Γ7 2Eg(2G) 2A 1 Γ6 4E 4T1g(4F) Copyright © 2011 SciRes. MSA  Electronic States in Trans-CoCl(H O) Complex 1126 2 2 4 cited states are subdivided into doublet Kramer’s states Γ6 and Γ7 by spin-orbit coupling. The interaction between the same symmetry levels Γ6 and Γ7 deriving from electronic states of different multiplicities leads to the observed spin-forbidden transition 4T1g(4F) → 2Eg(2G) (Oh symmetry) with lower intensity compared to a spin-allowed transition (Figure 3). The same explanation is also applied for the 4A2g(4F) quartet state and the 2T1g(2G) and 2T2g(2G) doublet states. 5. Conclusions A theoretical crystal-field analysis, based on the Racah theory, was carried out for the electronic energy levels of CoCl2(H2O)4. The observed crystalline-field splittings of the Co2+ levels were accounted by using a D4h symmetric Hamiltonian. As a result, Racah and crystal field parame- ters have been reliably obtained. Calculated crystalline field levels are in agreement with the observed ones. The theoretical study confirms the charge state Co2+ of cobalt and the site occupied D4h. The assignment of the first quartet band 4T2g(4F) seen in the near-infrared region is in agreement with the literature. Whereas, we find that the assignment of the two other quartet bands are respectively 4A2g(4F) and 4T1g(4P). This assignment is uncertain in the literature. REFERENCES [1] C. Reber, “Absorption and Luminescence Spectroscopy of Transition Metal Compounds: from Coordination Geome- tries to Exited-State Properties,” Canadian Journal of Ana- lytical Sciences and Spectroscopy, Vol. 53, 2008, pp. 91-101. [2] E. González, A. Rodrigue-Witchel and C. Reber, “Absorp- tion Spectroscopy of Octahedral Nickel(II) Complexes: A Case Study of Interactions between Multiple Electronic Ex- cited States,” Coordination Chemistry Reviews, Vol. 251, 2007, p. 351. doi:10.1016/j.ccr.2006.08.011 [3] R. Beaulac and C. Reber, “Spectroscopic Effects of Ex- cited-State Coupling in a Tetragonal Chromonium (III) Complex,” Inorganic Chemistry, Vol. 47, No. 12, 2008, pp. 5048-5054. doi:10.1021/ic702281k [4] G. Bussière, C. Reber, D. Neuhauser, D. A. Walter and J. I. Zink, “Molecular Properties Obtained by Analysis of Elec- tronic Spectra Containing Interference Dips. Comparisons of Analytical Equations and Exact Models Based on Cou- pled Potential Energy Surfaces,” Journal of Physics Chem- istry A, Vol. 107, 2003, pp. 1258-1265. doi:10.1021/jp0218490 [5] P. L. W. Tregenna-Pigott, S. P. Best, H. U. Gudel, H. Weihe and C. C. Wilson, “Influence of the Mode of Water Coordination on the Electronic Structure of the [V(OH2)6]3+ Cation,” Journal of Solid State Chemistry, Vol. 145, 1999, p. 460. doi:10.1006/jssc.1999.8154 [6] R. Meier, M. Boddin, S. Mitzenheim, V. Schmid and T. Schonherr, “Elucidation of V(III) Complex Coordination Numbers by Electronic Spectra,” Journal of Inorganic Biochemistry, Vol. 69, No. 4, 1998, pp. 249-252. doi:10.1016/S0162-0134(97)10031-9 [7] T. Schonherr, V. Schmid and R. Meier, “Study on Coordi- nation Numbers Six and Seven in V(III) Complexes with Aquo, Cyanide and Aminopolycarboxylic Ligands by Means of the Angular Overlap Model,” Spectrochimica Acta Part A, Vol. 54, No. 11, 1998, pp. 1659-1669. doi:10.1016/S1386-1425(98)00094-8 [8] G. Bussière, R. Beaulac, B. Cardinal-David and C. Reber, “Interacting Electronic States in Trans-MCl2(H2O)4n+ Com- plexes (M: V3+, Cr3+, Ni2+, Co2+),” Coordination Chemistry Reviews, Vol. 219-221, 2001, pp. 509-543. [9] S. Kammoun, M. Dammak, R. Maalej and M. Kamoun, “Crystal-Field Analysis for D8 ions at D4h Symmetry Sites: Electronic States in Trans-NiCl2(H2O)4 Complex,” Journal of Luminescence, Vol. 124, No. 2, 2007, pp. 316-320. doi:10.1016/j.jlumin.2006.04.006 [10] S. Kammoun, M. Dammak, R. Maalej, M. Kamoun. “Elec- tronic States of Vanadium(III) in Trans-VCl2(H2O)4+ Chro- mophore,” Journal of Alloys and Compounds, Vol. 453, No. 1-2, 2008, pp. 64-68. doi:10.1016/j.jallcom.2006.11.180 [11] H. Souissi and S. Kammoun, “Theoretical Studyoftheelec- Tronicstructureofa Tetragonal chromium(III)complex,” Jour- nal of Luminescence, Vol. 131, No. 12, 2011, pp. 2515- 2520. doi:10.1016/j.jlumin.2011.05.044 [12] G. Bussière, C. Reber, “Coupled Excited States in Nickel(II) Complexes Probed by Polarized Absorption Spectroscopy,” Journal of American Chemistry Society, Vol, 120, No. 25, 1998, pp. 6306. doi:10.1021/ja9740733 [13] M. A. Hitchman, R. G. McDonald, P. W. Smith and R. Stranger, “Electronic Spectrum and Metal-Ligand Bonding Parameters of the V(H2O)63+ Ion,” Journal of Chemistry Socience Dalton Transition, 1988, pp. 1393-1395. doi:10.1039/dt9880001393 [14] R. C. Powell, “Physics of Solid-State Laser Materials,” Springer-Verlag, New York, 1998. [15] D. J. Newman and B. Ng, “Crystal Field Handbook,” Cam- bridge University Press, Cambridge, 2000. [16] S. Sugano, Y. Tanabe and H. Kamimura, “Multiplets of Transition-Metal Ions in Crystals,” Academic Press, New York, 1970. [17] F. A. Cotton, C. A. Murillo and X. Wang, “Linear Tricobalt Compounds with Di-(2-pyridyl)amide (dpa) Ligands: stud- ies of the paramagnetic Compound Co3(dpa)4Cl2 in Solu- tion,” Inorganic Chemistry, Vol. 38, 1999, pp. 6294-6297. doi:10.1021/ic990944t Copyright © 2011 SciRes. MSA |

