Open Access Library Journal
Vol.03 No.05(2016), Article ID:69302,6 pages
10.4236/oalib.1102646
Structure of Aldoses Condensation Products with SH-Containing Hydrazides
Andrei Yu Ershov1*, Igor V. Lagoda2,
1Institute of Macromolecular Compounds, Russian
2Scientific Research Test Center (Medical and Biological Protection), Institute of Military Medicine, Saint Petersburg, Russia
3Saint Petersburg State University, Saint Petersburg, Russia
4I.P.

Copyright © 2016 by authors and OALib.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 12 April 2016; accepted 14 May 2016; published 23 May 2016

ABSTRACT
The structure of the condensation products of thiobenzohydrazide, 2-sulfanylacetohydrazide, 3-sulfanylpropiohydrazide, and 2-sulfanylbenzohydrazide with a series of aldoses (L-arabinose, D-ribose, L-rhamnose, D-galactose, D-glucose, and D-mannose) was studied by 1H- and 13C-NMR spectroscopy.
Keywords:
Aldoses SH-Acylhydrazones, 1,3,4-Thiadiazolines, 1,3,4-Thiadiazines, 1,3,4-Thiadiazepines, Ring-Chain-Ring Tautomerism
Subject Areas: Organic Chemistry

1. Introduction
Condensation products of aldoses with acylhydrazines attracted attention due to their high biological activity. Some of them exhibited an antimicrobial [1] and antifungal [2] activity. Among aldoses acylhydrazones, containing in their structure, a functional sulfohydryl group is known only thiobenzohydrazones of arabinose, glucose and mannose [3] , as well as 2-sulfonylbenzohydrazones of arabinose and glucose [4] , the structure of which is not proved. The presence of a functional nucleophilic SH-group in the aldosohydrazone fragment could give rise in appearance of new structural possibilities in further transformations. Intermolecular nucleophilic attacks of SH-fragments at the C=N polar bond contained in the linear structure can lead to repeated cyclization with the formation of new cyclic forms.
The aim of the present work was to study of the structure of the condensation products of aldoses with hydrazides of thiobenzoic (PhCSNHNH2), sulfanylacetic (HSCH2CONHNH2), 3-sulfanylpropionic (HSCH2CH2CONHNH2), and 2-sulfanylbenzoic (2-HSC6H4CONHNH2) acids by 1H- and 13C-NMR spectroscopy methods (Figure 1, Figure 2, Figure 3 and Figure 4).
2. Results and Discussion
Compounds 1-4 were synthesized in yields 55% - 90% by heating equimolar amounts of the corresponding aldose (L-arabinose, D-xylose, D-ribose, L-rhamnose, D-galactose, D-glucose, D-mannose) and corresponding sulfur-containig hydrazide (thiobenzohydrazide, 2-sulfanylacetohydrazide, 3-sulfanylpropiohydrazide, 2-sulfa- nylbenzohydrazide) in boiling methanol for a short time (Table 1, Table 2, Table 3, and Table 4).
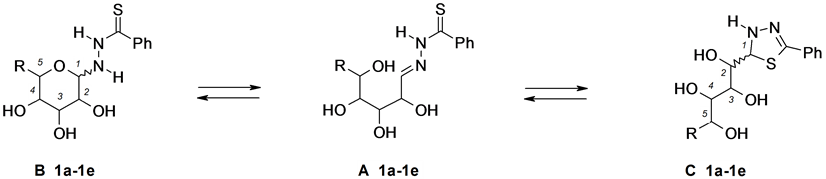
Figure 1. Aldoses thiobenzoylhydrazones 1a-1e.
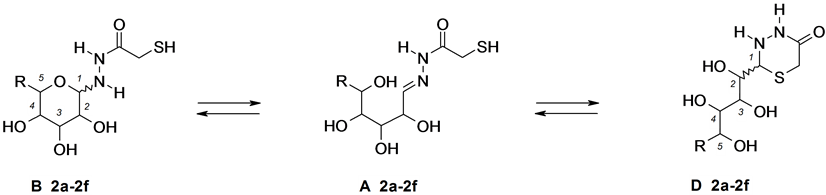
Figure 2. Aldoses 2-sulfanylacetylhydrazones 2a-2f.
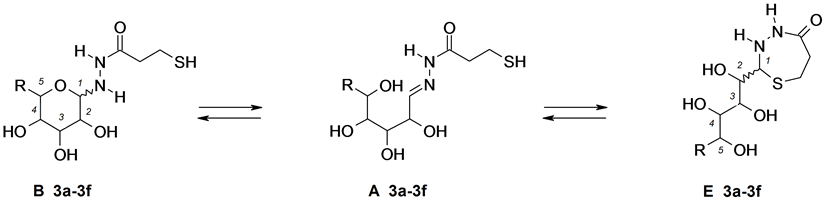
Figure 3. Aldoses 3-sulfanypropionyhydrazones 3a-3f.
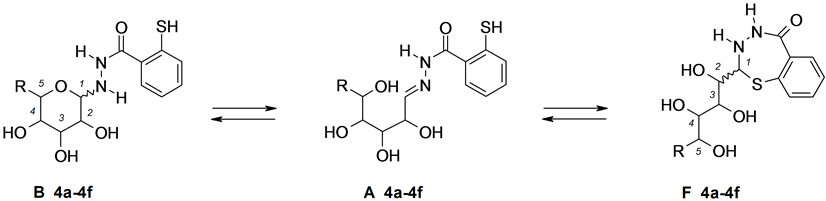
Figure 4. Aldoses 2-sulfanylbenzoylhydrazones 4a-4f.
Table 1. Tautomeric composition of aldoses thiobenzoylhydrazones 1a-1e (72 h after dissolution).
Table 2. Tautomeric composition of aldoses 2-sulfanylacetylhydrazones 2a-2f (72 h after dissolution).
Table 3. Tautomeric composition of aldoses 3-sulfanypropionyhydrazones 3a-3f (72 h after dissolution).
Table 4. Tautomeric composition of aldoses 2-sulfanylbenzoylhydrazones 4a-4f (72 h after dissolution).
In all experiments, the 1H- and 13C-NMR spectra were recorded at definite time intervals starting from the moment of dissolution until the end of transformations. In addition, the structure of the compounds under study in the crystalline state was confirmed by solid-phase high-resolution 13C-NMR spectroscopy (CPMAS). For example, pyranose form B was expected to give a signal from the anomeric C-1 atom at δ 85 - 90 ppm; analogous signals from five-membered 1,3,4-thiadiazoline C form, six-membered 1,3,4-thiadiazine D form, or seven- membered 1,3,4-thiadiazepine E and F forms should appear in a stronger field, at δ 70 - 75 ppm. It is typical of sp3-hybridized carbon atom for saturated ring systems, located between sulfur and nitrogen atoms [5] [6] . Hydrazone structure A should give rise to a downfield signal at δ 145 - 155 ppm (C=N) in the 13C-NMR spectrum.
One set of signals belonging to 1,3,4-thiadiazoline form C is observed in 1H- and 13C-NMR spectra of the products of the condensation of aldoses with thiobenzohydrazide 1a-1e (Table 1). This finding suggests that compounds 1a-1e has the same structure in the crystalline state. Solid-phase 13C-NMR spectrum was recorded for arabinose condensation product 1a. When the spectral patterns of solutions of compounds 1a-1e to in DMSOd6 no longer changed indicating the achievement of an equilibrium state (72 h after the dissolution), signals corresponding to linear hydrazone structure A were fixed. In the case of glucose tiobenzoylhydrazone 1d about 10% of the pyranose form B can be detected.
In the 1H- and 13C-NMR spectra of solutions of aldoses sulfanylacetylhydrazones 2a, 2b, 2d, and 2f in D2O (other solvents proved to be unsuitable due to the low solubility) the observed signals corresponded to cyclic tautomeric forms B and D, and mainly each of these forms was present as two stereoisomers: α,β-B and (2R,S)-D or (D and D′) (Table 2). The relative intensity of signals belonging to forms B and D in the 13C-NMR spectra changed with time; after 72 h these variations finished indicating the attainment of the equilibrium state. Therewith in the 13C NMR spectra of compounds 2a, 2b, 2d, and 2f taken just after the dissolution the intensity of signals belonging to the thiadiazine form D was significantly higher than in the spectra registered after the establishment of the equilibrium. This fact suggests that in the crystalline state compounds 2a, 2b, 2d, and 2f exist in the thiadiazine structure D, and in solution they are partially converted in the pyranose form B. On the contrary, at recording of the 13C-NMR spectra of the glucose derivative 2e the intensity of signals of pyranose form B decreased with time and the intensity of the signals of thiadiazine form D grew, suggesting that in the crystalline state this compound had the pyranose structure A. Finally, in the 13C-NMR spectra of rhamnose derivative 2c both just after dissolution and 72 h later only the signals of pyranose form B were observed. In neither example we could detect the hydrazone form A; consequently the name “sulfanylacetylhydrazone” could be applied to these systems only tentatively.
Compounds 3a-3f were expected to exhibit complicated tautomeric behavior due to their ability to undergo cyclization with formation of both six-membered pyranose form B and seven-membered 1,3,4-thiadiazepin form E (Table 3). According to 13C-NMR spectra taken off in solid phase, the condensation products of 3-sulfanylpropionylhydrazine with arabinose, xylose, and ribose (compounds 3a-3c respectively) exist in crystalline state as thiadiazepins E. Two sets of the signals belonging to the configurational isomers of thiadiazepin forms E and E′ appeared in the solid phase 13C-NMR spectrum of compound 3a. It was impossible to determine the 2R- or 2S-configuration of these forms. Unlike pentose derivatives 3a-3c, the condensation products 3d-f derived from 3-sulfanylpropionylhydrazine and hexoses do not give rise to cyclic thiadiazepin form E in crystals. Compounds 3d-3f in the crystalline state has cyclic structure B. The 1H-NMR spectra of solutions of 3-sulfanylpropanoylhydrazones 3a-3f in D2O, recorded immediately after dissolution, contained signals assignable to pyranose structure B. After 72 h, i.e., when the spectral patterns of solutions of 3a-3f in D2O no longer changed (equilibrium was attained), signals corresponding to both cyclic tautomers B and E and linear hydrazone structure A were present, and each cyclic tautomer was a mixture of two stereoisomers: α,β-B and (2R,S)-E or (E and E′). The equilibrium position varies over a wide range: from В:А:E ratio 85:5:10 for glucose derivative 3e to 50:10:40 for xylose derivative 3b respectively.
A different situation was observed with the condensation products obtained from aldoses and 2-sulfanylben- zohydrazide (Table 4). Judging by variation of their 1H- and 13C-NMR spectra, compounds 4a- 4f in the crystalline state have cyclic 1,3,4-benzothiadiazepin structure F. Solid-phase 13C-NMR spectrum was recorded for glucose condensation product 4e. With the lapse of time, signals belonging to the second configurational isomer of benzothiadiazepin form F′ appeared in the 1H- and 13C-NMR spectra of solutions of 4a-4f in DMSOd6, but it was impossible to assign these signals to particular (2R)- or (2S)-isomer. The 1H- and 13C-NMR spectra of condensation products 4a, 4b, and 4d derived from arabinose, ribose, and galactose no longer change in 15 - 20 days, indicating the absence of linear structure A and cyclic form B in the equilibrium mixture. Compounds 4c and 4e obtained by condensation of 2-sulfanylbenzohydrazide with rhamnose and glucose behaved differently. After keeping their solutions in DMSOd6 for 72 h, the 1H- and 13C-NMR data indicated formation of cyclic pyranose form B (two sets of signals were observed due to the presence of several stereoisomers). Up to 20% of linear hydrazone tautomer A was detected for rhamnose derivative 4c. After 30 days, the 1H- and 13C-NMR spectra of compounds 4c and 4e contained only signals belonging to pyranose form B. We succeeded in isolating tautomer B of compound 4c in the crystalline state, and its structure was confirmed by the solid-phase 13C-NMR spectrum. In other words, compounds 4c and 4e demonstrated irreversible transformation of benzo-1,3,4-thiadiazepin tautomer F into tetrahydropyran structure B, which can be observed spectrally. The 1H- and 13C-NMR spectra of the condensation product of 2-sulfanylbenzohydrazide with mannose (compound 4f ) finished to change in 72 h. The equilibrium mixture consisted of 50% of benzothiadiazepin tautomer F, 15% of linear structure A, and 35% of pyranose B form; in addition, each cyclic tautomer was a mixture of two stereoisomers.
3. Conclusion
Thus, the study noted the general tendency of the condensation products of aldoses with thiobenzoic, sulfanylacetic, 3-sulfanylpropionic, and 2-sulfanylbenzoic acids hydrazides to undergo ring-chain-ring tautomeric transformation involving two different cyclic structures via intramolecular nucleophilic addition of the SH group at the hydrazone C=N bond. The results of the present study may be interesting from the practical viewpoint, e.g., for the design of new radioprotective agents as well as of polymeric materials for techniques, medicine, and biology. The condensation products of SH-acylhydrazides with aldoses may also be applied for complexing the colloid species of the noble metals controlling their structure and the size of the forming nanoparticles and thus governing the process of their self-organization into supramolecular structures [7] [8] . This will be the subject of our future investigations.
4. Experimental Part
1Н- and 13С-NMR spectra were registered on a spectrometer Bruker AV-400 at operating frequencies 400 and 100 MHz respectively (internal reference hexamethyldisiloxane). The solid-phase 13C-NMR spectra were obtained on a Bruker AM-500 spectrometer (125 MHz) using a standard procedure utilizing cross polarization and magic angle spinning (CPMAS) technique (frequency 4.5 kHz; internal reference hexamethylbenzene). The tautomeric composition of obtained compounds was estimated by the integration of the appropriate signals in the 1Н NMR spectra. Elemental analysis of previously unknown compounds was carried out on a CHN Analyzer Hewlett Packard 185B. The purity of prepared compounds was checked by TLC on Silufol UV-254 plates, eluent butanol-water-acetone, 8:1:1.
Synthesis of aldoses SH-acylhydrazones (1-4)
To a solution of 0.01 mol of SH-containing hydrazide in 25 ml of methanol 0.01 mol of an appropriate aldose was added, and the mixture was boiled for a period of 1-3 h. The solvent was removed at a reduced pressure, and the residue was washed with ether (3 × 50 ml), and the colorless crystalline substance was filtered off on a glass filter funnel (40 - 100 μm), dried and stored in a desiccator over P2O5.
L-Arabinose thiobenzoylhydrazone (1a)
Yield 70%, m.p. 161˚C - 162˚C (lit. [3] m.p. 163˚C - 164˚C). 13C-NMR spectrum (solid phase): δ = form C(100%): 64.23 (C-5), 70.21 (C-2), 71.15 (C-3), 73.11 (C-4), 76.07 (C-1), 118.96, 122.78, 128.11, 130.90 (Ar), 134.05 (ArC-S), 143.18 (C=N) ppm. 13C-NMR spectrum (DMSOd6): δ = form A(40%): 63.91 (C-5), 70.87 (C-2), 71.22 (C-3), 72.29 (C-4), 131.81 (ArC-S), 144.50 (C-1), 183.48 (C=S); form C(60%): 63.62 (C-5), 69.90 (C-2), 71.08 (C-3), 72.73 (C-4), 75.91 (C-1), 131.54 (ArC-S), 142.69 (C=N), 121.27 - 131.41 (Ar in A and C) ppm. Found, %: C 50.73; H 5.61; N 9.79. C12H16N2O4S. Calculated, %: C 50.69; H 5.67; N 9.85.
L-Arabinose 2-sulfanylacetylhydrazone (2a)
Yield 75%, m.p. 120 °C - 121 °C . 13C-NMR spectrum (D2O): δ = α-B(10%): 25.52 (CH2SH), 62.38 (C-5), 67.31 (C-4), 68.09 (C-2), 72.71 (C-3), 90.40 (C-1), 172.36 (C=O); form D(55%): 26.90 (CH2S), 63.02 (C-5), 65.58 (C-1), 69.60 (C-2), 70.57 (C-3), 71.12 (C-4), 177.19 (C=O); form D′(35%): 27.04 (CH2S), 62.88 (C-5), 64.40 (C-1), 69.72 (C-2), 70.04 (C-3), 70.87 (C-4), 176.63 (C=O) ppm. Found, %: C 35.34; H 5.88; N 11.80. C7H14N2O5S. Calculated, %: C 35.29; H 5.92; N 11.76.
L-Arabinose 3-sulfanylpropionylhydrazone (3a)
Yield 55%, m.p. 146˚C - 148˚C. 13C-NMR spectrum (solid phase): δ = forms E, E′(100%): 23.74 and 30.14 (CH2S), 35.95 and 39.48 (CH2CO), 63.23 and 64.70 (C-5), 69.78 (C-2), 70.79 (C-3), 71.17 (C-4), 77.34 (C-1), 170.81 (C=O) ppm. 13C-NMR spectrum (D2O): δ = form A(5%): 152.03 (C-1), 169.78 (C=O); forms E, E′(30%): 24.48 and 25.04 (CH2S), 33.41 and 34.16 (CH2CO), 63.70 (C-5), 67.48 (C-1), 69.57 (C-4), 70.23 (C-3), 70.49 (C-2), 172.11 (C=O); α-B(10%): 19.32 (CH2SH), 36.77 (CH2CO), 61.82 (C-5), 68.24 (C-2), 69.07 (C-3), 70.03 (C-4), 85.98 (C-1), 172.63 (C=O); β-B(55%): 19.34 (CH2S), 37.05 (CH2CO), 62.46 (C-5), 68.83 (C-4), 70.29 (C-2), 72.04 (C-3), 89.83 (C-1), 172.57 (C=O) ppm. Found, %: C 38.03; H 6.44; N 11.06. C8H16N2O5S. Calculated, %: C 38.09; H 6.39; N 11.10.
L-Arabinose 2-sulfanylbenzoylhydrazone (4a)
Yield 60%, m.p. 177˚C - 178˚C (lit. [4] m.p. 175˚C - 176˚C). 1H-NMR spectrum (DMSOd6): δ = form F(75%): 9.24 (br.s, NHCO); form F′(25%): 9.24 (br.s, NHCO) ppm. 13C-NMR spectrum (DMSOd6): δ = form F: 63.37 (C-5), 69.40 (C-2), 70.08 (C-3), 71.51 (C-4), 75.40 (C-1), 140.13 (ArC-S), 175.40 (C=O); form F′: 63.61 (C-5), 69.40 (C-2), 70.38 (C-3), 71.01 (C-4), 74.24 (C-1), 140.13 (ArC-S), 172.75 (C=O), 127.90 - 133.47 (Ar in F and F′) ppm. Found, %: C 48.06; H 5.29; N 9.41. C12H16N2O5S. Calculated, %: C 47.99; H 5.37; N 9.33.
Spectral characteristics of compounds 1b-1e, 2b-2f, 3b-2f, and 4b-4f were described previously [9] - [12] .
Acknowledgements
This work received financial support from the Ministry of Education and Science of Russian Federation (contract 14.574.21.0002, No. RFMEFI57414X0002).
Cite this paper
Andrei Yu Ershov,Igor V. Lagoda,Stanislav I. Yakimovich,Lyudmila Yu Kuleshova,Marina Yu Vasileva,Irina S. Korovina,Valery V. Shamanin, (2016) Structure of Aldoses Condensation Products with SH-Containing Hydrazides. Open Access Library Journal,03,1-6. doi: 10.4236/oalib.1102646
References
- 1. Katz, L. (1956) Bactericidal and Fungicidal Compounds. US Patent No. 2767173.
- 2. Kuleshova, L.Y., Frolova, M.A., Konopleva, V.I., Alekseev, V.V. and Ershov, A.Y. (2012) 2-Mercaptobenzoyl Hydrazones of Monose, Having Antimicrobial and Antifungal Activity. RF Patent No. 2454423.
- 3. Holmberg, B. (1955) Zur kenntnis des thiobenzhydrazinen. Arkiv för Kemi, 9, 47-64.
- 4. Katz, L., Karger, L.S., Schroeder, W. and Cohen, M.S. (1953) Hydrazine Derivatives. I. Benzalthio- and Bisbenzaldi-Thiosalicylhydrazides. Journal of Organic Chemistry, 18, 1380-1402.
http://dx.doi.org/10.1021/jo50016a019 - 5. Ershov, A.Y., Lagoda, I.V., Yakimovich, S.I., Pakalnis, V.V., Zerova, I.V., Dobrodumov, A.V. and Shamanin, V.V. (2009) Tautomerism and Conformational Isomerism of Mercaptoacetylhydrazones of Aliphatic and Aromatic Aldehydes. Russian Journal of Organic Chemistry, 45, 660-666.
http://dx.doi.org/10.1134/S1070428009050030 - 6. Ershov, A.Y., Lagoda, I.V., Mokeev, M.V., Yakimovich, S.I., Zerova, I.V., Pakalnis, V.V. and Shamanin, V.V. (2008) Thiosalicyloylhydrazones of Alyphatic Aldehydes and Their Cyclization into 1,3,4-Benzothiadiazepine Derivatives. Chemistry of Heterocyclic Compounds, 44, 356-359.
http://dx.doi.org/10.1007/s10593-008-0052-2 - 7. García, I., Gallo, J., Marradi, M. and Penadés, S. (2011) Glyconanoparticles: New Nanomaterials for Biological Application. In: Narain, R., Ed., Engineered Carbohydrate-Based Materials for Biomedical Applications: Polymers, Surfaces, Dendrimers, Nanoparticles, and Hydrogels, John Wiley & Sons, Inc., Hoboken, 213-259.
http://dx.doi.org/10.1002/9780470944349 - 8. Chuang, Y.-J., Zhou, X., Pan, Z. and Turchi, C. (2009) A Convenient Method for Synthesis of Glyconanoparticles for Colorimetric Measuring Carbohydrate-Protein Interactions. Biochemical and Biophysical Research Communications, 389, 22-27.
http://dx.doi.org/10.1016/j.bbrc.2009.08.079 - 9. Zelenin, K.N., Alekseev, V.V., Kuznetsova, O.B., Terentev, P.B., Lashin, V.V., Ovcharenko, V.V. and Khorseeva, L.A. (1993) Thiosemicarbazones, Thiobenzoylhydrazones and Thiocarbonohydrazones of Monoses. Russian Journal of Organic Chemistry, 29, 278-286.
- 10. Ershov, A.Y., Lagoda, I.V., Yakimovich, S.I., Zerova, I.V., Pakalnis, V.V., Mokeev, M.V. and Shamanin, V.V. (2009) Structure of Products of Aldoses Condensation with Thioglycolic Acid Hydrazide. Russian Journal of Organic Chemistry, 45, 740-742.
http://dx.doi.org/10.1134/S1070428009050169 - 11. Ershov, A.Y., Lagoda, I.V., Yakimovich, S.I., Zerova, I.V., Pakalnis, V.V. and Shamanin, V.V. (2009) Structure of the Condensation Products of 3-Sulfanylpropionic Acid Hydrazide with Aldehydes, Ketones, and Aldoses. Russian Journal of Organic Chemistry, 45, 1488-1495.
http://dx.doi.org/10.1134/S107042800910011X - 12. Alekseev, V.V., Ershov, A.Y., Chernitsa, B.V., Doroshenko, V.A., Lagoda, I.V., Yakimovich, S.I., Zerova, I.V., Pakalnis, V.V. and Shamanin, V.V. (2010) Structure of Aldoses Condensation Products with 2-Hydroxy- and 2-Sulfanyl-Benzohydrazides. Russian Journal of Organic Chemistry, 46, 860-865.
http://dx.doi.org/10.1134/S1070428010060138
NOTES

*Corresponding author.