Paper Menu >>
Journal Menu >>
 Advances in Biological Chemistry, 2011, 1, 15-23 ABC doi:10.4236/abc.2011.12003 Published Online August 2011 (http://www.SciRP.org/journal/abc/). Published Online August 2011 in SciRes. http://www.scirp.org/journal/ABC The role of vitamin C in alteration of enzymes responsible of energy metabolism induced by administration of tamoxifen to mouse* Zaina Zuhair, Hasan ALamri Girls College of Education, King Abdulazia University, Jeddah, Saudi Arabia. Email: Sala201018@gmail.com Received 29 April 2011; revised 2 June 2011; accepted 13 June 2011. ABSTRACT Tamoxifen is a synthetic non-steroidal ant estrogen. It was suggested to study the role of vitamin C in alte- ration of enzymes responsible of energy metabolism induced by administration of tamoxifen to mouse. The effect of tamoxifen and tamoxifen with vitamin C on some activity of enzymes in the mice represe nti ng g ly- colytic, gluconeogenic and glycogenolytic pathway and also, liver function enzymes represented by aspartate aminotransferase (AST), alanine amino-transferase (ALT), acid phosphatase (ACP) and alkaline phos- phatase (ALP) were studied. The present results showed that a significant (p < 0.001) increase in glycolytic enzymes (HK, PK, GPI and PFK), glu- coneogenic enzymes, G-6-Pase, acid phosphatase (ACP), alkaline phosphatase (ALP) and glucose, were observed in treated groups, while LDH, glycogen phos- phorylase, AST and ALT enzymes activities showed significant (p < 0.01) reduction. The present results also, showed that significant reduction in glycogen, total protein, total cholesterol, uric acid, urea, and creatinine in treated mice as compared to the normal healthy control group. However, normal control mice treated with tamoxifen and vitamin C showed no side effects of most parameters compared to the normal healthy control group. It was concluded that vitamin C may prevent tamoxifen-induced testes toxicity in mice. Keywords: Adult Male Albino Mice; Tamoxifen Vitamin C; Liver Tissue Homogenates 1. INTRODUCTION Tamoxifen is one of the most effective synthetic non- steroidal ant estrogenic compounds, it is widely used in the treatment of advanced hormone-dependent breast cancer which is the most worldwide common form of cancer in women [1] by binding to estrogen receptors and suppressing epithelial proliferation [2,3] and as ad- juvant therapy following surgery in early stages of the disease. Tamoxifen is also, proposed for the prevention of cancer amongst high risk women [4]. Such an ap- proach requires objective and accurate evolution of the side effects which could result from the administration of this drug. Some studies showed that tamoxifen has adverse side effects on the cardiovascular system, bone metabolism and liver. Also, tamoxifen caused cytotoxicity on pri- mary cells from human multiple organs: Kidney, liver and lung [5]. Vitamin C is an antioxidant agent that limits the injury produced by drugs. Vitamin C is an essential nutrient that functions as a non-enzymatic antioxidant in the cy- tosol. The various experimental studies indicated that this vitamin is effective in preventing the oxidative renal damage and stress [6]. It is well known that the liver is one of the major tar- get organs affected by drug, where the most metabolic processes are usually located. The most important me- tabolic pathways are glycolysis, gluconeogenesis and glycogenolysis contributions of these pathways elucidate the metabolic relationship between glucose, glycogen and energy release. The characteristic pattern of change in some enzymes activity representing glycolytic path- ways as (hexokinase (HK), pyruvate kinase (PK), phos- phofructokinase (PFK), lactate dehydrogenase (LDH), and glucose phosphate isomerase (GPI); glycogenolytic pathways as glycogen phosphorlase, glucose-6-pho- sphatase (G-6-Pase); gluconeogenic pathways as fruc- tose-1-6 diphosphatase (F-D-P ase), phosphoenolpyru- vate carboxykinase (PEPCK). as well as glycogen con- tent in soft tissue and glucose in serum as bioenergetics parameters of critical importance in reflecting the phy- siological alteration of animals under the stress [7]. Carbohydrates and amino acids are one of the impor- tant parameters which could be used as another indicator  Z. Zuhair et al. / Advances in Biological Chemistry, 2011, 1, 15-23 Copyright © 2011 SciRes. ABC 16 of the stress by molluscicides [8]. The aim of the present work is to cast more light on the toxic effect of tamoxifen on some liver enzymes in mice representing metabolic pathways are glycolysis, gluconeogenesis and glycogenolysis and the role of vita- min C in minimizing the toxicity induced by tamoxifen. 2. MATERIALS AND METHODS 2.1. Experimental Animals Adult male albino mice in the present investigation were obtained from Schistosome Biological Supply Program (SBSP), Theodor Bilharz Research Institute, Cairo, Egypt. They were 3 months old and of an average weigh of 25 - 30 g. They were fed ad labium with a standard diet. They were kept in cages and acclimatized in the laboratory for 7 days prior to experimentation. 2.2. Applied Drugs Tamoxifen and vitamin C were purchased from Che- mical Industries Development (CID) Company, Al-Harm, Giza, Egypt as tablets for oral administration, each tab- let contains 40 mg and 500 mg of the active ingradi- ents, respectively. The therapeutic dose of each drug for mice was calculated according to the table given by Paget and Barnes [9].The therapeutic doses of ta- moxifen and vitamin C were calculated as 0.1 mg/kg and 1.25 mg/kg for mice, respectively. The doses were given orally and estimated according to the body weight of the mouse. 2.3. Experimental Design The mice were divided into three equal groups. The 1st group served as control and received distilled water. The 2nd group was daily administrated 0.1 mg tamoxifen. the 3th group were given daily 1.25 mg vitamin C simulta- neously with the dose of 0.1 mg tamoxifen. All the doses were given daily for 28 days and sacrificed after month. Animals’sacrifice and examination stared 4 weeks post treatment; it was done on successive days. In each day only two animals from each group were sacrificed. Each liver was then taken and divided into 0.25 g portions. The liver portion were taken and covered with aluminum foil and stored at –4˚C until used for homogenization and biochemical assays. All mouse were subjected to determine HK, PK, GPI, LDH, PFK, FD Pase, PEPCK, G-6-Pase, AST, ALT, ADP, ALP and glycogen phos- phorylase enzyme activities as well as, glycogen and protein content in liver tissue. Glucose, total cholesterol uric acid, urea, and creatinine in sera. 2.4. Preparation of Liver Tissue Homogenates On the day of each the following enzyme parameter assay, one portion weighing 0.25 g from each liver aluminium package was taken and homogenized in 2.5 ml of the spe- cific recorded solution to give 10% concentration and then used for assay. Similar periods elapsed between homog- enization and enzyme was assayed in two livers from each group on the same days. 2.5. Bleeding and Preparation of Serum Blood samples were collected, after 4 weeks post-treat- ment. Collected blood samples were centrifuged at 3000 rpm for 10 minutes and sera were stored immediately at –80°C until time of analysis. 2.6. Enzyme Assays All physiological parameters determined in this study were determined spectrophotometrically, using reagent kits purchased from BioMerieux Company, France. Hexokinase (HK) was assayed according to the me- thod of Uyeda and Raker [10]. Pyruvatekinase (PK) ac- cording to the method of McManus and James [11]. Glucose phosphate isomerase (GPI) according to the method of King [12], Phosphofructokinase (PFK) ac- cording to the method of Zammit et al. [13] and Lactate dehydrogenase (LDH) activity according to the method of Cabaud and Wroblewski [14]. Phosphoenolpyruvate carboxykinase (PEPCK) according to the method of Suarez et al. [15] and Glucose-6-phos-phatase according to the method of Swanson [16]. Fructose-1, 6-diphosphatase (FD Pase) according to the method of Sand et al. [17] and Glycogen phosphorylase according to Hedrick and Fischer [18]. Aspartate and alanine ami- notransferases according to the method of Reitman and Frankel [19]. Acid phosphatase and alkaline phosphatase activities according to Fishman and Ferner [20] and King and King [21] respectively. Total protein according to the method of Lowry et al. [22]. Glycogen according to the method of Nicholas et al. [23]. Glucose according to the method of Trinder [24]. Sera were used for measuring concentrations of glu- cose, total cholesterol, uric acid, urea and creatinine. 2.7 Statistical Analysis Data were analyzed by comparing values for different treatment groups with the values for individual controls. Results are expressed as mean ± S.D. The significant differences among values were analyzed using analysis by student’s t-test for comparing the means of experimental and control groups [25]. 3. RESULTS The present results in the Tab le 1 and Figure 1 showed that very highly significant (p < 0.01) reduction in Lac- tate (LDH) enzyme activity in mice treated with Ta-  Z. Zuhair et al. / Advances in Biological Chemistry, 2011, 1, 15-23 Copyright © 2011 SciRes. ABC 17 moxifen (32.5 2.3) as compared to the normal control (42.32 2.6), while significant (p < 0.001) increase was noticed in other glycolytic enzymes hexokinase (HK), pyruvatekinase (PK), phosphofructokinase (PFK) and glucose phosphate isomerase (GPI) as compared to the normal healthy control. The enzymes activities in treated mice were 0.141 0.13, 7.8 2.1, 26.2 2.2 and 1201 2.1) and in control mice were (0.085 0.05, 5.8 2.1, 18.21 1.4 and 88.6 8.2) µ mol/min/mg protein, re- spectively. Moreover, treatment of mice with the ta- moxifen and vitamin C recorded no significant differ- ence in all glycolytic enzymes as compared to control group. A noticeable remark on the effect of tamoxifen with vitamin C pointed out to that there is no side effects on all glycolytic enzymes (LDH, HK, PK & GPI) as compared to the normal healthy control group. The present results (Table 2 and Figure 2) showed that the effect of tamoxifen on some gluconeogenic en- zymes. Significant increase (p < 0.001) in the levels of FDPase and PEPCK was noticed in treated group as compared to the normal group. The percentage of in- creases were 39.66% and 48.57%, respectively Figure 1, Ta ble 2 showed a very highly significant increase (p < 0.001) in G-6-Pase, while a highly significant reduction (p < 0.001) in glycogen phosphorylase was noticed in treated group as compared to the normal healthy control group. The percentage of increases was 35.15% and 51.54% respectively. Moreover, treatment of mice with the tamoxifen and vitamin C recorded no significant difference in all gly- colytic, gluconeogenic and glycogenolytic enzymes as compared to control group. A noticeable remark on the effect of tamoxifen with vitamin C pointed out to that there is no side effects on all glycolytic, gluconeogenic and Glycogenolytic enzymes as compared to the normal healthy control group. Ta ble 3 and Figure 3 showed that highly significant reductions (p < 0.01) in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) enzymes in mice treated with tamoxifen while significant (p < 0.01) in- creases were observed in acid phosphatase (ACP) and alkaline phosphatase (ALP) levels as compared to the normal healthy control group. Moreover, treatment of mice with the tamoxifen and vitamin C indicated that no significant difference in the level of liver function enzymes as compared to control group. Treatment of the normal healthy mice with the tamoxifen and vitamin C showed no side effects on the level of liver function enzymes. Ta ble 4 and Figure 4 showed that highly significant reductions (p < 0.01) in glycogen and total protein levels in treated mice with tamoxifen as compared to the nor- mal healthy control group. The percentages of reductions were 31.01% and 37.56% respectively. The table shows a highly significant increase (p < 0.01) in glucose level (28.1%). The present results in the Tabl e 4 showed that very highly significant (p < 0.01) reduction in total cho- lesterol, uric acid, urea and creatinine in mice treated with tamoxifen as compared to the normal healthy con- trol. The percentages of reductions were 25.71%, 34.2%, 27.42% and 19.32%, respectively. Moreover, treatment of mice with the tamoxifen and vitamin C recorded no significant difference in the level of glucose, total cho- lesterol, uric acid, urea and creatinine as compared to control group. Treatment of the normal healthy mice with the tamoxifen and vitamin C showed no side effects on the level of glucose, total cholesterol, uric acid, urea and creatinine. 4. DISCUSSION In the present study, significant increase in glycolytic enzymes HK, PK, GPI and PFK were observed in treated group with tamoxifen, while LDH enzyme activ- ity showed significant reduction. The enhancement in the activities of glycolytic enzymes in treated mice could be attributed to increase metabolic activities of treated liver tissues to compensate the inhibition of host Krebs’ cycle of host caused by treatment with tamoxifen [26,27]. LDH inhibition revealed the aerobic–anaerobic switch induced by treatment with tamoxifen [27]. Kuser et al. [28] indicated that lactate is accumulated and glycogen depleted confirming inhibition of aerobic respiration and stimulation of anaerobic glycolysis through hexokinase, a rate limiting enzymes of glycolysis. Some authors re- ported that tissue damage followed the release of cellular enzymes such as LDH [29,30]. Besides in spite of the decrease in LDH activity, there was insignificant change in D-lactate and pyruvate level as compared to untreated snails, as reported by Reddy et al. [31]. Concerning gluconeogenic enzymes activities (Fruc- tose-1,6-diphosphatase and Phosphoenolpyruvate Car- boxykinase), the present results showed significant ele- vations in treated mice, where the significant increase in gluconeogenic enzymes fructose-1,6-diphosphatase is due to depletion of glucose in the treated mice, where the ratio of glycogen to glucose levels in liver is known to be regulated by the balance between glycogen synthesis and degradation capacities. The increase influx of glu- cose into glycolytic flux and enhanced glycogen stored lead to stimulation of the enzymes [32]. The nature of the end product formed is dependent on the competition for PEP by the two enzymes pyruvate kinase (PK) and phosphoenolpyruvate carboxykinase (PEPCK). Stimula- tion of PK in treated animals ascertained the stimulation of the gycolytic flux previously reported by Horemans et al. [33] and Ahmed and Gad [26]. With respect to G-6- 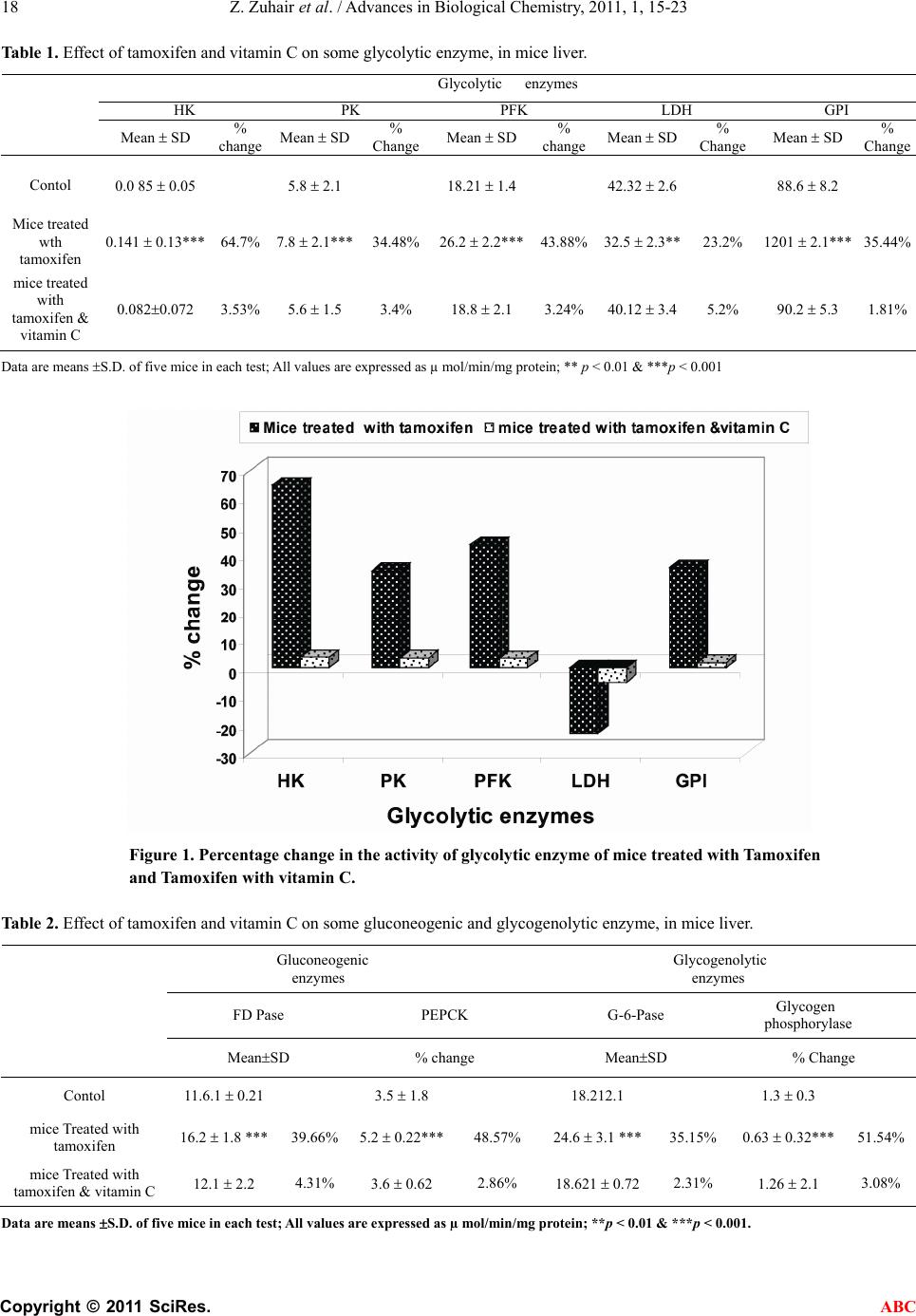 Z. Zuhair et al. / Advances in Biological Chemistry, 2011, 1, 15-23 Copyright © 2011 SciRes. ABC 18 Table 1. Effect of tamoxifen and vitamin C on some glycolytic enzyme, in mice liver. Glycolytic enzymes HK PK PFK LDH GPI Mean SD % change Mean SD % Change Mean SD% changeMean SD% Change Mean SD% Change Contol 0.0 85 0.05 5.8 2.1 18.21 1.4 42.32 2.6 88.6 8.2 Mice treated wth tamoxifen 0.141 0.13*** 64.7% 7.8 2.1*** 34.48% 26.2 2.2***43.88% 32.5 2.3**23.2% 1201 2.1***35.44% mice treated with tamoxifen & vitamin C 0.0820.072 3.53% 5.6 1.5 3.4% 18.8 2.1 3.24% 40.12 3.45.2% 90.2 5.3 1.81% Data are means S.D. of five mice in each test; All values are expressed as µ mol/min/mg protein; ** p < 0.01 & ***p < 0.001 Figure 1. Percentage change in the activity of glycolytic enzyme of mice treated with Tamoxifen and Tamoxifen with vitamin C. Table 2. Effect of tamoxifen and vitamin C on some gluconeogenic and glycogenolytic enzyme, in mice liver. Gluconeogenic enzymes Glycogenolytic enzymes FD Pase PEPCK G-6-Pase Glycogen phosphorylase MeanSD % change MeanSD % Change Contol 11.6.1 0.21 3.5 1.8 18.212.1 1.3 0.3 mice Treated with tamoxifen 16.2 1.8 *** 39.66% 5.2 0.22***48.57% 24.6 3.1 ***35.15% 0.63 0.32***51.54% mice Treated with tamoxifen & vitamin C 12.1 2.2 4.31% 3.6 0.62 2.86% 18.621 0.72 2.31% 1.26 2.1 3.08% Data are means S.D. of five mice in each test; All values are expressed as µ mol/min/mg protein; **p < 0.01 & ***p < 0.001.  Z. Zuhair et al. / Advances in Biological Chemistry, 2011, 1, 15-23 Copyright © 2011 SciRes. ABC 19 -60 -40 -20 0 20 40 60 %change FD PasePEPCKG-6-PaseGly.p h o sph o ryl ase G luc oneogenic and G lyc ogenoly t ic e nzyme s M ice T reate d with tam o xi fen Mice T reate d with tam o xi fen & vitam in C Figure 2. Percentage change in the activity gluconeogenic and glycogenolytic enzyme of mice treated with Tamoxifen and Tamoxifen with vitamin C. Table 3. Effect of tamoxifen and vitamin C on liver function enzymes in mice. Enzyme activity mol/min/mg protein Aspartate amino transferase (AST)) Alanine amino transferase (ALT) Acid phosphatase (ADP) Alkaline phosphatase (ALKP) Parameters Treatment Mean SD % change Mean SD % ChangeMean SD % change Mean SD % change Control 31.6 3.1 21.8 2.11 5.8 1.1 3.5 0.52 Mice treated with tamoxifen 20.5 1.03** –35.13 % 12.8 0.75***–41.28% 8.6 0.31***48.28% 5.6 ± 0.08*** 60% Mice treated with tamoxifen & vitamin C 30.7 0.04 –2.85% 22.1 + 0.86* –1.38% 6.2 0.38 6.9% 3.7 0.12 5.71% *p < 0.05, **p < 0.01 & ***p < 0.001 Figure 3. Percentage change in the activity of liver function enzymes of mice treated with Tamoxifen and Tamoxifen with vitamin C. 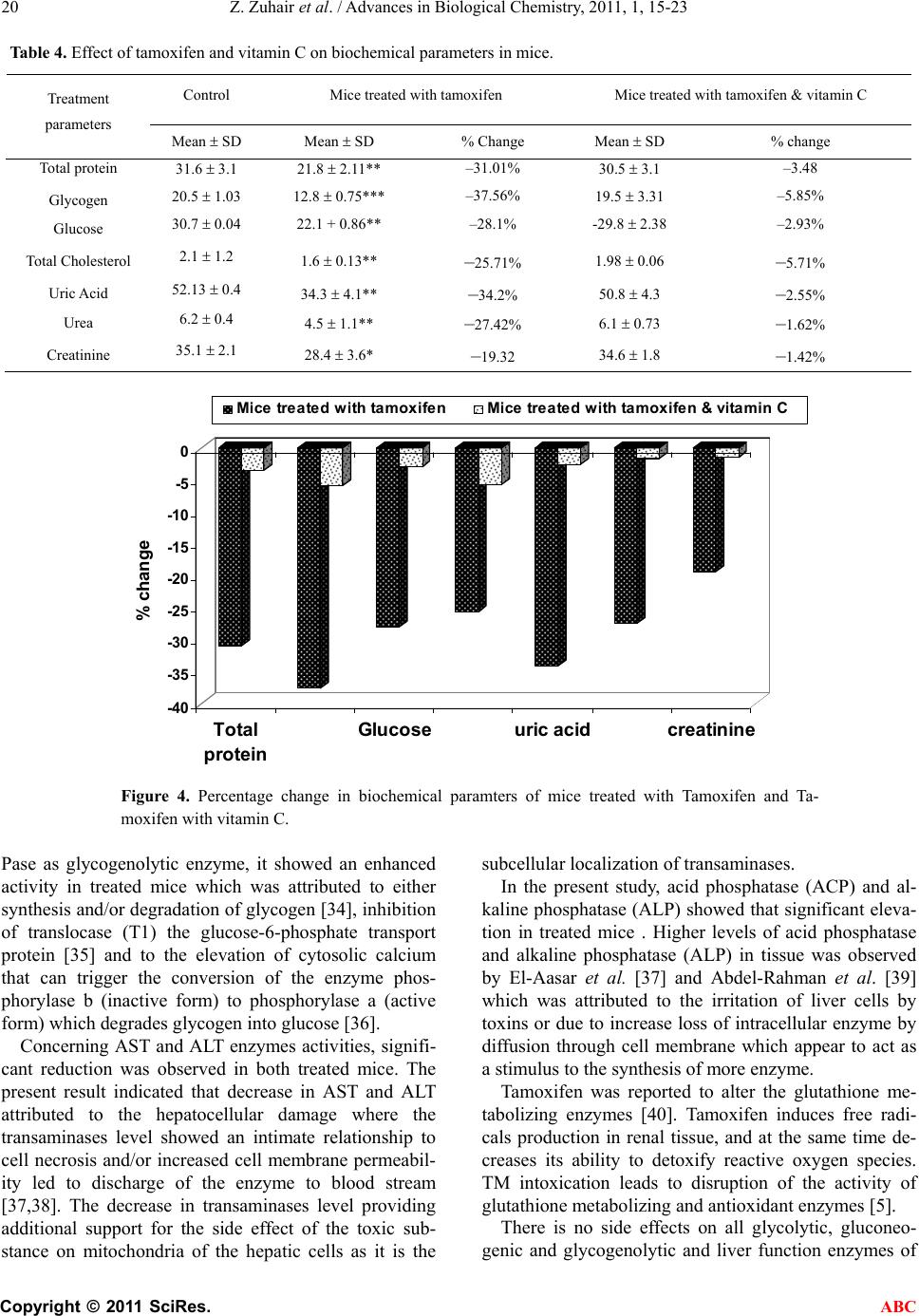 Z. Zuhair et al. / Advances in Biological Chemistry, 2011, 1, 15-23 Copyright © 2011 SciRes. ABC 20 Table 4. Effect of tamoxifen and vitamin C on biochemical parameters in mice. Control Mice treated with tamoxifen Mice treated with tamoxifen & vitamin C Treatment parameters Mean SD Mean SD % Change Mean SD % change Total protein 31.6 3.1 21.8 2.11** –31.01% 30.5 3.1 –3.48 Glycogen 20.5 1.03 12.8 0.75*** –37.56% 19.5 3.31 –5.85% Glucose 30.7 0.04 22.1 + 0.86** –28.1% -29.8 2.38 –2.93% Total Cholesterol 2.1 1.2 1.6 0.13** –25.71% 1.98 0.06 –5.71% Uric Acid 52.13 0.4 34.3 4.1** –34.2% 50.8 4.3 –2.55% Urea 6.2 0.4 4.5 1.1** –27.42% 6.1 0.73 –1.62% Creatinine 35.1 2.1 28.4 3.6* –19.32 34.6 1.8 –1.42% -40 -35 -30 -25 -20 -15 -10 -5 0 % change Total protein G lucoseu r ic acidcr eat i ni ne Mice treated with tamoxifen Mice treated with tamoxifen & vitamin C Figure 4. Percentage change in biochemical paramters of mice treated with Tamoxifen and Ta- moxifen with vitamin C. Pase as glycogenolytic enzyme, it showed an enhanced activity in treated mice which was attributed to either synthesis and/or degradation of glycogen [34], inhibition of translocase (T1) the glucose-6-phosphate transport protein [35] and to the elevation of cytosolic calcium that can trigger the conversion of the enzyme phos- phorylase b (inactive form) to phosphorylase a (active form) which degrades glycogen into glucose [36]. Concerning AST and ALT enzymes activities, signifi- cant reduction was observed in both treated mice. The present result indicated that decrease in AST and ALT attributed to the hepatocellular damage where the transaminases level showed an intimate relationship to cell necrosis and/or increased cell membrane permeabil- ity led to discharge of the enzyme to blood stream [37,38]. The decrease in transaminases level providing additional support for the side effect of the toxic sub- stance on mitochondria of the hepatic cells as it is the subcellular localization of transaminases. In the present study, acid phosphatase (ACP) and al- kaline phosphatase (ALP) showed that significant eleva- tion in treated mice . Higher levels of acid phosphatase and alkaline phosphatase (ALP) in tissue was observed by El-Aasar et al. [37] and Abdel-Rahman et al. [39] which was attributed to the irritation of liver cells by toxins or due to increase loss of intracellular enzyme by diffusion through cell membrane which appear to act as a stimulus to the synthesis of more enzyme. Tamoxifen was reported to alter the glutathione me- tabolizing enzymes [40]. Tamoxifen induces free radi- cals production in renal tissue, and at the same time de- creases its ability to detoxify reactive oxygen species. TM intoxication leads to disruption of the activity of glutathione metabolizing and antioxidant enzymes [5]. There is no side effects on all glycolytic, gluconeo- genic and glycogenolytic and liver function enzymes of  Z. Zuhair et al. / Advances in Biological Chemistry, 2011, 1, 15-23 Copyright © 2011 SciRes. ABC 21 mice treated with tamoxifen with vitamin C as compared to the normal healthy control group. The results of the present study indicated that the exogenously adminis- tered vitamin C may prevent tamoxifen-induced testes toxicity in mice. The protective effect of vitamin C is probably due to a counter action of free radicals by its antioxidant nature. Vitamin C may be recommended as an adjuvant therapy with certain anticancer. Protective effects of vitamin C against chemically-induced damage in various rodent organs have been demonstrated by many investigators [41,42]. Vitamin C was found to be effectively protecting chemically-induced oxidative re- nal damage in animals [42-44] reported that mega-dose of vitamins rendered significant protection of renal damage induced by anticancer and the effect of vitamin C was higher than that of vitamin E. The protective ef- fects may be partially mediated by preventing the renal antioxidant status. Increasing the glucose concentration stimulated gly- cogen synthesis and decreased the activity of glycogen phosphorylase. An inverse relationship was shown be- tween the actual glycogen content and the rate of glyco- genesis. So there is a substrate cycling that occurred between glucoses-6- phosphate and glycogen content, i.e. glucose was incorporated into glycogen during period of net glycogen breakdown, and vice versa; glycogen deg- radation occurred during periods of net glycogen synthe- sis which depends on glucose concentration [27]. Our data recorded enhancement levels of glucose-6-phos- phatase and glucose. The present results showed that significant reduction in total protein content in treated mice which could be attributed to cellular damage caused by toxins [30]. The main fraction of total protein content is albumin in turn may result from decrease anabolism or increase catabo- lism [45]. The significant decrease in total protein is mainly due to increase in messenger RNA degradation which is the possible cause for the hypoalbuminemia [46]. Also, the present results showed that very highly significant reduction in total cholesterol, uric acid, urea and creatinine in mice treated with tamoxifen as com- pared to the normal healthy control. 5. CONCLUSIONS Normal control mice treated with tamoxifen showed side effects of most parameters compared to the normal healthy control group. Moreover, normal control mice treated with tamoxifen and vitamin C showed no side effects of most parameters compared to the normal healthy control group. Hence, vitamin C may prevent tamoxifen-in- duced testes toxicity in mice. REFERENCES [1] Phillips, D.H. (2001) Underatanding the genotoxicity of tamoxiifen. Carcinogenesis, 22, 839-849. doi:10.1093/carcin/22.6.839 [2] Rang, H.P., Dale, M.M. and Ritter, J.M. (1995) The Re- productive system Pharmacology. Fourth edn. Churchill Livingstone, Edinburgh, London, New York, Philadel- phia, Sydney and Toronto. [3] Pirkko, H., Annukka, A. and Eero, M. (2002). Toxicity of antiestrogen. The Br east Journal (U.S.A), 8, 92-96. [4] Gamboa-da-costa, G., Marques, M.M., Beland, F.A., Freeman, J.P., Churchwell, M.L. and Doerge, D.R. (2003) Quantification of Tamoxifen DNA adducts using on lines sample preparation and HPLC-electrospray ionization tandem mass spectrometry. Chemical Research in To- xicology, 16, 357-366. doi:10.1021/tx020090g [5] Albert, P.L., Bode, C. and Sakai, Y. (2004). A novel in vitro system, the integrated discrete multiple organ cell culture (I dMOC)) system, for the evaluation of human toxicity: Comparative cytotoxicity tamoxifen towards normal human cells from five major organs and MCF-7 andenocarcinoma breast cancer. Chemico-Biological In- teractions, 150, 129-136. doi:10.1016/j.cbi.2004.09.010 [6] Kadkhodaee, M., Khastar, H., Faghih, M., Ghaznavi, R. and Zahmarkesh, M.,2005. Effect of co-suplementation and of vitamin E and C on gentamicin-induced nephro- toxicity in rat. Experimental Physiology, 90, 571-576. doi:10.1113/expphysiol.2004.029728 [7] Abd El-Monem, S.; Bakry, F.A. and Ismail, S.M. (2006) Influence of and niclosimide on some biological and physiological parameters of lymnaea natalensis snails as intermediate host of fasciola gigantica. Egyptian Jour- nal of Zoology, 46, 227-244. [8] Sakran A.M.A and Bakry F.A (2005) Biological and physiological studies on Biomphalaria alexandrina snails exposed to different plant molluscicides. Journal of Egypt German Society of Zoology, 48A, 237-256. [9] Paget, G. and Barners, J. (1964) Toxicity tests. In: Laur- ence, D.R. and Bacharach, A.L. Eds., Evalution of Drug Activities: Pharmacometrics, Academic Press, London and New York, 135-166. [10] Uyeda, K. and Racker, E. (1965): Regulatory mecha- nisms in carbohydrate metabolism. VII. Hexokinase and phosphofructokinase. The Journal of Biological Chemis- try, 240, 4682-4688. [11] McManus, D.P. and James, B.L. (1975) Anaerobic glu- cose metabolism in the digestive gland of Littorina saxa- tilis rudis (Maton) and the daughter sporocysts Microphal- lus Similis (jag). Comparative Biochemistry and Physiol- ogy, 51, 293-297. [12] King, J. (1965) Glucose phosphate isomerase. In: Practi- cal Clinical Enzymology. Van Nostr and Co. Ltd., Lon- don, 1113-1117. [13] Zammit, V.A., Beis, I. and Newsholme, E.A. (1978) Maximum activities and effects of fructose bisphosphate on pyruvate kinase from muscles of vertebrates and in- vertebrates in relation to the control of glycolysis. Bio- chemical Journal, 174, 989-998. [14] Cabaud, P. and Wroblewski, F. (1958) Colorimetric mea- surement of lactic acid dehydrogenase activity of body fluids. American Journal of Clinical Pathology, 30, 234- 236. [15] Suarez, R.K., Mallet, M.D., Doxboeck, C. and Hochacjka, P.W. (1986) Enzymes of energy metabolism and glu-  Z. Zuhair et al. / Advances in Biological Chemistry, 2011, 1, 15-23 Copyright © 2011 SciRes. ABC 22 coneogenesis in acid blue malin, Makaira nigricans. Canadian Journal of Zoology, 64, 694-697. doi:10.1139/z86-102 [16] Swanson, M.A. (1955) Glucose-6-phosphatase from liver. Methods in Enzymology, 2, 541-543. doi:10.1016/S0076-6879(55)02247-7 [17] Sand, O., Petersen, L.M. and Emmersen, B.K. (1980) Changes in some carbohydrate metabolizing enzymes and glycogen in liver, glucose and lipid in serum during vitellogenesis and after induction by estradiol-1-7-B in the flounder Platichtys flesus L. Comparative Biochem- istry and Physiology, 65, 327-332. doi:10.1016/0305-0491(80)90021-8 [18] Hedrick, J.L. and Fischer, E.H. (1965) On the role of pyridoxal-5-phosphate in phosphorylase, absence of classical vitamin B6 dependent enzymatic activities in- muscle glycogen phoshorylase. Biochemistry, 4, 1337- 1344. [19] Reitman, S. and Frankel, S. (1957) A colorimetric method for the determination of serum glutamic oxa- loacetic and glutamic pyruvic transaminases. American Journal of Clinical Pathology, 28, 56-63. [20] Fishman, W.H. and Ferner, F. (1953) A method for esti- mating serum acid phosphatase of prostatic origin. The Journal of Biological Chemistry, 200, 89-97. [21] King, P.R. and King, E.J. (1954) Estimation of plasma phosphatase by determination of hydrolysed phenol (with amino-antipyrine). Journal of Clinical Pathology, 7, 322-326. doi:10.1136/jcp.7.4.322 [22] Lowery, O.H., Rose Brough, N.J., Farr, A.L. and Randall, R.J. (1951) Protien measurement with the folin-phenol reagent. The Jour na l of Biological Chemistry, 2, 265-275 [23] Nicholas, V., Carroll, R., Longley, W. and Joseph, H.R. (1956) The determination of glycogen in liver and mus- cle by the use of anthrone reagent. The Journal of Bio- logical Chemistry, 220, 583-593. [24] Trinder, P. (1969): Determination of glucose in blood using glucose oxidase with an alternative oxygen ac- ceptor. Annals of Clinical Biochemistry, 6, 24-27. [25] Spiegel, R.M. (1981) Theory and problems of Statistics, Schaum’s outline series. McGraw-Hill, Singapore. [26] Ahmed, S.A. and Gad, M.Z. (1995) Effect of schisto- somal infection and its treatment on some key enzymes of glucose metabolism in mice livers Arznein. Forsch, 45, 1324-1330. [27] Tielens, A.G. (1997) Biochemistry of Trematode. In: Fried, B. and Graczyk, T.K. Eds., Advances in Trematode Biology, CRC Press, Boca Raton, 309-343. [28] Kuser, P.R. Krauchrenco, S., Antunes, O.A. and Polikar- pov, I. (2000) The high resolution crystal structure of yeast hexokinase PII with the correct primary sequence provides new insights into its mechanism of action. The Journal of Biological Chemistry, 275, 20814-20821. [29] Paul, J., Bekker, A.Y. and Duran, W.N. (1990) Calcium entry prevents leakage of macromolecules induced by ischemia-reperfusion in skeletal muscle. Circulation Re- search, 66, 1636-1642. [30] Parasad, M.R., Popeseu, L.M., Moraru, I.I, Liu, X., Maity, S., Engelman, R.M. and Das, D.K. (1991) Role of pho- spholipase A and C in myocardial ischemic reperfusion injury. American Journal of Physiology, 29, H877-H883. [31] Reddy, A.N., Venugopal, N.B.R.K. and Reddy, S.L.N. (1995) Effect of endosulphan 35 EC on some bioche- mical changes in the tissues and haemolymph of a fresh water field crab, Barytelphusa guerini. Bulletin of Envi- ronmental Contamination and Toxicology, 55, 116-121. doi:10.1007/BF00212397 [32] El-Ansary, A., El-Bardicy, S., Soliman, S.M. and Zayed, N. (2000) Sublethal concentration of Ambrosia maritima (Damsissa) affecting compatibility of Biomphalaria al- exandrina snails to infection with Schistosoma mansoni through disturbing the glycolytic pathway. Journal of the Egyptian Society of Parasitology, 30, 809-819. [33] Horemans, A.M.C., Tielens, A.G.M. and Van Den Bergh, S.G. (1992) The reversible effect of glucose on the en- ergy metabolism of Schistosoma mansoni cercariae and schistosomual. Molecular and Biochemical Parasitology, 51, 73-79. doi:10.1016/0166-6851(92)90202-U [34] Mchael, A.L., Wahab, R.A., Guriguis, N.M., El-Gazayerli, L.M. and Hamza, S. (1979) Effect of Schistosoma man- soni infection in mice on hydrolytic enzyme activity of the liver. Journal of the Egyptian Society of Parasitology, 6, 37-44. [35] Scott, H.M., Coughtrie, M.W. andBurchell, A. (1991) Steroid sulfates inhibit rat hepatic microsomal glucose-6- phosphatasesystem. Biochemical Pharmacology, 41, 1529-1532. doi:10.1016/0006-2952(91)90572-M [36] Exton, J.H. (1982) Regulation of carbohydrate Metabo- lism by Cyclic Nucleotides. In: Kehalian, J.W. and Na- thasan, J.A. Eds., Cyclic Nucleotides. II. Handbook of Experimental Pharmacology, 58, Springer-Verlag, Berlin, 3-88. [37] El-Aasar, A.A., El-Merzabani, M.M, Zakhary, N.I., Farag, H.I, Abdeen, A.M, Abd El-Salam, I. and Mokhtar, N.M. (1989) Biochemical and biophysical studies on schisto- somal liver of mice. Egypt. J. Bilh., 11, 19-33. [38] El-Shazly, A.M., Soliman, M., El-Kalla, M.R., Rezk, H., El-Nemr, H.E., Handousa, A.E. and Helmy, M.M. (2001) Studies on patients with Schistosoma mansoni; HCV and/or typhoid fever. Journal of the Egyptian Society of Parasitology, 31, 583-592. [39] Abdel-Rahman, H.M., El-Shanawani, F.M., Hassan, M.M., Salem, M. and El-Salhy, A.M. (1993) Alkaline phosphatase isoenzymes abnormalities in hepatic schis- tosomiasis. Egypt iournal of Bilharzia, 15, 41-48. [40] El-Beshbishy, H. (2005) Hepatoprotective effect of green tea (Camellia sinensis) extract against tamoxifen-induced liver injury in rats. Journal of Biochemistry and Molecu- lar Biology, 38, 563-570. doi:10.5483/BMBRep.2005.38.5.563 [41] Zaidi, S.M. and Banu, N. (2004) Antioxidant potential vitamin A, E and C in modulating oxidative stress in rat brain. Clinica Chimica Acta, 33, 229-340. doi:10.1016/j.cccn.2003.11.003 [42] Ajith, T.A., Usha, S. and Nivitha, V. (2007) Ascorbic acid and α-Tocopherol protect renal damage. Clinical and Experimental Nephrology, 9, 24-30 [43] Naziroglu, M., Kuraoglu, A. and Aksoy,A. 2004. Sele- nium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal and lens tis- sue in rat. Toxicology, 195, 221-230. doi:10.1016/j.tox.2003.10.012 [44] Abraham, P. (2005) Vitamin C may be beneficial in the prevention of paracetamol-induced renal damage. Clini-  Z. Zuhair et al. / Advances in Biological Chemistry, 2011, 1, 15-23 Copyright © 2011 SciRes. ABC 23 cal and Experimental Nephrology, 9, 24-30. doi:10.1007/s10157-004-0335-6 [45] El-Fakahany, A.F.M., Abdalla, K.F., El-Hady, H.M., El-Aziz, S.M.A. and Afifi, L.M. (1993) The effect of Pra- ziquantel treatment on the liver function, worm burden, and granuloma size using two drug regimens in marine Schistosoma mansoni infection. Journal of the Egyptian Society of Parasitology, 23, 877-882. [46] Metwally, A.A., Jaku, I., Komper, F., Khayyal, M.T., Fbeid, F.A. and Botros, S.S. (1990) Effect of schisto- somiasis infection on the clearance of phenazone in mice. Arzneimittel Forschung, 40, 206-209. |

