Journal of Materials Science and Chemical Engineering
Vol.04 No.07(2016), Article ID:68971,8 pages
10.4236/msce.2016.47006
Luminescence Properties of Phosphate Phosphor: Barium Tungstate Doped with Dy
Peiju Hu1, Wei Zhang1*, Zhengfa Hu1*, Zuyong Feng1, Lun Ma2, Xiuping Zhang3, Xia Sheng1, Jie Luo1
1School of Physics & Optoelectronic Engineering, Guangdong University of Technology, Guangzhou, China
2Department of Physics, The University of Texas at Arlington, Arlington, TX, USA
3Cancer Center of Guangzhou Medical University, Guangzhou, China



Received 17 March 2016; accepted 21 July 2016; published 25 July 2016

ABSTRACT
A series of barium-tungstate-based phosphors doped with different concentrations of Dy3+ were synthesized by solid-state reaction method. Photoluminescence properties and decay lifetime of Dy3+-doped BaWO4 samples were studied. The results indicated that luminescent properties of BaWO4:Dy3+ depended on the Dy3+ concentration, and the inner energy could transfer from 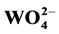 to Dy3+. The quality of the light was checked by estimating CIE parameters, and the results showed that BaWO4:Dy3+ was a potential candidate as blue-green luminescent materials in white LED because of its excellent emission spectrum excited by UV light.
to Dy3+. The quality of the light was checked by estimating CIE parameters, and the results showed that BaWO4:Dy3+ was a potential candidate as blue-green luminescent materials in white LED because of its excellent emission spectrum excited by UV light.
Keywords:
LED, BaWO4:Dy3+, Phosphors, Decay Lifetime, Luminescent Properties

1. Introduction
Recently, metal tungstates have received much attention due to its luminescence properties, structure performance and its wide application in various fields [1]-[3]. As matrix, tungstate is rather promising for optoelectronic application in which it can emit blue light. Among these metal tungstates, barium tungstate with a tetragonal scheelite-like structure possesses good chemical and thermal stability [4]-[6]. The BaWO4 crystallites with good crystal properties can be prepared by a simple and convenient electrochemical method [7] [8]. For example, BaWO4 luminescent materials are applied as luminescent matrix by doping lanthanide ions, which have been widely investigated and show excellent luminescence properties [9].
The BaWO4 luminescent materials doped with Tb3+ or Eu3+ have been reported that they can be synthesized by a simple hydrothermal route, and show potential applications in the field of phosphor-converted white LEDs [10] [11]. Alkaline-earth metal tungstate AWO4 (A = Ca2+, Sr2+, Ba2+) was used to study as phosphor self-ex- cited under UV excitation, exhibiting blue or green light [12]. Up to now, Rare-earth (Nd3+,Tm3+, Er3+, Ho3+ and Yb3+) doped AWO4 (A = Ca2+, Sr2+, Ba2+) phosphors have been successfully synthesized and studied the energy transfer mechanism in the matrix as well as the connection between luminescence properties and doped ion [13]-[15]. Otherwise, Dy3+ ions have two dominant emission bands and the yellow and blue emission intensities can be adjusted. Hence it is possible to obtain white light emission with Dy3+-activated luminescence materials [16] [17].
The existence of the inner energy transfer from the host to Dy3+ ions with BaWO4 as matrix, including the luminescence properties and its internal energy transfer mechanism, is attracting more research interests. In this paper, a series of BaWO4 phosphors with different Dy3+ doping concentrations are prepared by solid state method and their photoluminescence properties are investigated under UV excitation.
2. Experimental
A series of Dy3+-doped barium tungstate phosphors were prepared by the conventional solid-state-reaction method. The reagents including BaCO3 (A. R.), WO3 (A. R.) and Dy2O3 (99.9%) were used as the raw materials without any further purification. Raw materials with stoichiometric ratio (Ba1−xWO4: xDy3+ (x = 0, 0.01, 0.02, 0.03, 0.04, 0.05, 0.06)) were weighed, sufficiently mixed and ground together in an agate mortar. The mixture was annealed for 2 h at 500˚C and re-annealed at 1000˚C for 8 hours, and then was slowly cooled down to room temperature. As a result, white phosphor samples were produced.
The phase structure of the product was characterized by using XD-2 X-ray powder diffractometer (CuKα, 36 KV, 20 mA). A scan rate of 0.02˚/s was applied to record the patterns in 2θ range 10˚ - 70˚. The excitation and emission spectra of as-synthesized phosphors BaWO4:Dy3+ were recorded on Japan’s Hitachi F-7000 fluorescence photometer. Luminescence decay curves were measured with a PLS920P spectrometer, using nanosecond flash-lamps for excitation. All measurements were performed at room temperature.
3. Results and Discussion
The XRD patterns of the Ba1−xWO4:xDy3+ (x = 0 ~ 0.06) prepared by solid state reaction method were shown in Figure 1. It can be seen that the XRD diffraction peaks of the samples matched well with the standard card of BaWO4 (PDF 43-0646), and the diffraction peak intensity were high without any other additional peak of Dy2O3 [18]. Therefore, the as-prepared BaWO4 samples were crystallization with pure phase that is tetragonal phase structure, belonging to the I41/a (88) space group. The result shows that the Dy3+ ions in these samples should have substituted the Ba2+ ions sites, because small quantities of Dy3+ can enter the crystal lattice and do not change the crystal structure of the matrix BaWO4. A small right-shift of diffraction peak with the increasing Dy3+ concentration appeared in Figure 1. This may be due to the fact that the ionic radius of the Dy3+ (91.2 pm) is smaller than that of Ba2+ (135 pm) in the samples, causing smaller diffraction angle based on Bragg’s law (dsinθ = kλ).
Figure 1. XRD patterns of Ba1−xWO4:xDy3+ (x = 0 ~ 0.06).
The luminescence properties were investigated by measuring the PL spectra of Ba1−xWO4: xDy3+ (x = 0 ~ 0.06) powders at room temperature. Figure 2 shows the excitation and emission spectrum of the pure BaWO4 without doping Dy3+. When monitoring at 355 nm emission wavelength, the excitation spectrum of BaWO4 contains the wide band from 250 nm to 300 nm with its maximum at 259 nm which belongs to the W-O charge transfer belt (CTB). No other peak was observed in the excitation spectra of un-doped BaWO4. According to the principle of atomic orbital, the strong absorption band of BaWO4 in ultraviolet region comes from the high vibration energy transition that the electrons of group 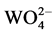 jump from the ground state 1A1 to 1B(1T2). The wide peak will be emitted at around 350 nm if the electrons jump from 1B(1T2), the minimum vibration energy level, back to 1A1 ground state [3].
jump from the ground state 1A1 to 1B(1T2). The wide peak will be emitted at around 350 nm if the electrons jump from 1B(1T2), the minimum vibration energy level, back to 1A1 ground state [3].
The excitation and emission spectrum of Ba0.99WO4:0.01 Dy3+ were recorded in Figure 3. Four main absorption peaks were presented in the 300 - 400 nm by monitoring the emission at 571 nm, which indicates that the near-UV LEDs based on the Dy3+ doped BaWO4 phosphor can be used as pumping sources to obtain efficient emission. The spectrum presents four bands centered at 323 nm, 350 nm, 363 nm, 386 nm, corresponding to the f ® f transitions within the Dy3+ ion: 6 H15/2 ® 4K15/2, 6H15/2 ® 4M15/2, 6H15/2 ® 4P3/2, 6H15/2 ® 4M21/2, and the main peak is located at 350 nm [19]. The characteristics of the emission peaks at 483 nm (blue) and 571 nm (green) can be detected excited by 350 nm. The two peaks correspond to the electron transition of 4F9/2 - 6H15/2 and 4F9/2-6H13/2, respectively. Generally speaking, the phosphor luminescence performance can be affected by the matrix structure. The emitting light is mainly blue when Dy3+ ions occupy the high symmetry of inversion center lattices in the matrix, while the emitting light is mainly green when Dy3+ ions occupy the low symmetry of none inversion center lattices in the matrix. It can be shown from Figure 3 that the green light part at 571 nm is much stronger than the blue one at 483 nm, which can be deduced that the Dy3+ ions occupy the low symmetry lattices in BaWO4 [20]. Comparing the emission spectrum with the excitation spectrum in Figure 2 and
Figure 2. Excitation and emission spectra of the un-doped BaWO4 samples.
Figure 3. Excited and emission spectrum of Ba0.99WO4:0.01Dy3+.
Figure 3, it can be found that the wide emission peak have much more overlap with the excitation peak of the Dy3+ ions at about 350 nm, when the electrons of 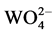 groups transfer from the minimum vibration energy level of 1B(1T2) state back to the 1A1 ground state [21].
groups transfer from the minimum vibration energy level of 1B(1T2) state back to the 1A1 ground state [21].
The emission spectrums of Ba0.99WO4:0.01Dy3+ excited at 350 nm and 259 nm as a comparison were shown in Figure 4. The emission peaks of the sample excited at 259 nm include not only the characteristics of Dy3+ ions located at 483 nm (blue) and 571 nm (green), but also the wide ones of 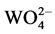 groups whose electrons transfer from the minimum vibration energy level of 1B (1T2) state back to the 1A1 ground state. The emission peak shapes of Dy3+ ions under the 259 nm excitation is the same as the ones under the 350 nm excitation. However, obviously, the emission peak intensity under 259 nm is stronger, which can be deduced that there exists energy transfer from
groups whose electrons transfer from the minimum vibration energy level of 1B (1T2) state back to the 1A1 ground state. The emission peak shapes of Dy3+ ions under the 259 nm excitation is the same as the ones under the 350 nm excitation. However, obviously, the emission peak intensity under 259 nm is stronger, which can be deduced that there exists energy transfer from 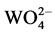 groups to Dy3+ ions and consequently strengthens the emission peaks of Dy3+ in Ba0.99WO4:0.01Dy3+.
groups to Dy3+ ions and consequently strengthens the emission peaks of Dy3+ in Ba0.99WO4:0.01Dy3+.
The emission spectra of Ba1-xWO4: xDy3+ (x = 0 ~ 0.06) phosphors excited at 350 nm were illustrated in Figure 5. The emission spectrum of all samples contains two characteristic peaks at 483 nm (blue) and 571 nm (green) which have no obvious changed with the increasing Dy3+ ions. The emission intensity of Dy3+ at 483 nm and 571 nm gradually increases with the increase of the Dy3+ content until the concentration of Dy3+ reaches to 0.05, and then the intensity decreases with the further increase of the Dy3+ content because of concentration quenching. Meanwhile, the emission peak intensity is relatively high, which shows that Ba1−xWO4:xDy3+ phosphors can obtain ideal emission light under 350 nm excitation.
Figure 4. Emission spectrum of Ba0.99WO4:0.01Dy3+ excited at 350 and 259 nm.
Figure 5. Emission spectrum of Ba1−xWO4:xDy3+ (x = 0 ~ 0.06) phosphors excited at 350 nm.
In order to investigate the difference in emission spectra resulted from various excitations, the PL emission spectra of Ba1−xWO4:xDy3+ (x = 0 ~ 0.06) powders excited at 259 nm (host excitation) were shown in Figure 6. The emission spectra of the Ba1−xWO4:xDy3+ phosphors consist of two main bands peaking at 483 and 571 nm which did not be changed as Dy3+ concentration changed. Within the emission transitions, the green band and the blue band are the predominant transitions. The emission intensity of Dy3+ at 483 nm and 571 nm gradually increases with the increasing of the Dy3+ content until the concentration of Dy3+ reaches to 0.05, and then it decreases with the further increase of the Dy3+ content because of concentration quenching. Comparing with the emission intensity difference at 483 and 571 nm, the emission peak of the characteristic peaks of Dy3+ is gradually strengthened with the increase of Dy3+ ions, indicating stronger energy transfer from the host to Dy3+ ions. The absorption rate of emission peak from the host is enhanced as the increase of Dy3+ ions, so that the emission peak intensity of the host is weakened while the characteristics emission peak of Dy3+ ion is relatively enhanced. Finally, the characteristics emission peak of Dy3+ ion is reduced as the increasing of Dy3+ ions, which is due to the shorter distance between different Dy3+ ions, and the stronger interact of ions. No radioactive transition is produced, leading to the concentration quenching effect.
The decay curves for the luminescence of BaWO4:xDy3+ (x = 0.01, 0.03, 0.05) phosphors as monitoring 575 nm under 259 nm excitation were shown in Figure 7. Based on the decay curves and loading time, the decay lifetimes of the samples were calculated to be τ = 13.693, 10.799 and 9.514 μs corresponding with the content (x = 0.01, 0.03, 0.05). The decreasing of the host emission lifetime value of BaWO4:xDy3+ supports the presence of the energy transfer from 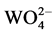 to Dy3+ in BaWO4 host.
to Dy3+ in BaWO4 host.
G. Blasse has pointed out that when a sensitizer or activator replaces the lattice in the matrix, the critical G. Blasse has pointed out that when a sensitizer or activator replaces the lattice in the matrix, the critical distance of the energy transfer meets the formula [22] [23]:
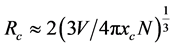
where V is the volume of the unit lattice, N is the atomic number of the unit lattice, xc is the critical concentration. For the BaWO4 host, V = 0.4002 nm3, and N = 4, the Dy3+ concentration xc is 0.05, hence the average distance (Rc = 0.576 nm) between Dy3+ can be calculated. The energy transfer mechanism is the very close to the distance between the ions. The electron cloud produces overlap, which leads to the exchanging electrons and the exchanging of energy. However, the critical distance of energy transfer is 0.5 nm, this value is less than the average distance Rc = 0.576 nm between Dy3+ ions. Hence the reason why concentration quenching of Dy3+ ions is not the energy transfer but the effect of the electric multipole moment-electric multipole moment effect.
According to the available CIE standard, we computed the CIE chromaticity coordinates (x, y) of the BaWO4: xDy3+ (x = 0 ~ 0.06) samples under the 259 nm excitation, which are marked as points 1 ~ 6 in Figure 8. The chromaticity coordinates are (0.3294, 0.3954), (0.3283, 0.3945), (0.3247, 0.3939), (0.3128, 0.3849), (0.3097, 0.3713) and (0.2946, 0.3657) corresponding with the increasing of Dy3+ concentration from x = 0 to 0.06. As
Figure 6. Emission peak of Ba1−xWO4:xDy3+ (x = 0.01 ~ 0.06) between 450 and 650 nm excited at 259 nm.
Figure 7. Photoluminescence decay curves of BaWO4:xDy3+ (x = 0.01, 0.03, 0.05) monitored by 575 nm excited at 259 nm.
Figure 8. The chromaticity coordination (x, y) of BaWO4:xDy3+ (x = 0 ~ 0.06) phosphors excited under 259 nm in the CIE 1931 chromaticity diagram.
shown in Figure 8, the color tones slowly shift from green (represented by point 1, 2) to blue (represented by point 5, 6) with increasing concentration of Dy3+ ion. In summary, a fine tuning emission color can be easily realized by adjusting the emission proportion between green and blue light in the emission spectrum of Dy3+, and the Ba1−xWO4:xDy3+ can be applied in white LED as blue-green phosphor.
4. Conclusion
In summary, BaWO4:xDy3+ phosphors are synthesized by conventional solid state method. XRD studies confirm the formation of single phase in these samples, and there is no change in the structure by doping a small amount of Dy3+. The efficient energy transfer takes place within the 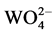 groups to the Dy3+ ions, which is demonstrated by the photoluminescence excitation and photoluminescence decay times. The CIE coordinates show that the emission light color of BaWO4:xDy3+ samples changes from green to blue with the increasing of Dy3+ concentration. The PL emission intensity increases with Dy3+ content to its maximum at the optimum concentration of about 5 mol% Dy3+ in BaWO4:xDy3+ phosphors, and then decreases due to the concentration quenching. Therefore, these phosphors can be efficiently excited by near-UV light, and be potential candidates for application in white LED.
groups to the Dy3+ ions, which is demonstrated by the photoluminescence excitation and photoluminescence decay times. The CIE coordinates show that the emission light color of BaWO4:xDy3+ samples changes from green to blue with the increasing of Dy3+ concentration. The PL emission intensity increases with Dy3+ content to its maximum at the optimum concentration of about 5 mol% Dy3+ in BaWO4:xDy3+ phosphors, and then decreases due to the concentration quenching. Therefore, these phosphors can be efficiently excited by near-UV light, and be potential candidates for application in white LED.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 11304045), Guangdong Excellent Talents Project (Grant No. 400140095), and Guangdong provincial science and technology projects (Grant No. 2013B090700006).
Cite this paper
Peiju Hu,Wei Zhang,Zhengfa Hu,Zuyong Feng,Lun Ma,Xiuping Zhang,Xia Sheng,Jie Luo, (2016) Luminescence Properties of Phosphate Phosphor: Barium Tungstate Doped with Dy. Journal of Materials Science and Chemical Engineering,04,37-44. doi: 10.4236/msce.2016.47006
References
- 1. Ignatyev, B.V., Isaev, V.A., Lebedev, A.V. and Plautskiy, P.G. (2014) Simultaneous Dual-Wavelength Stimulated Raman Scattering in Ba(MoO4)x(WO4)1-x Solid Solution Single Crystals. Optics Letters, 39, 5479-5482. http://dx.doi.org/10.1364/OL.39.005479
- 2. Bi, C.H. and Meng, Q.Y. (2013) Luminescent Properties and Energy Transfer Mecha-nism of CaWO4:Sm3+ Phosphors. Acta Physica Sinica, 62, 750-754.
- 3. Spassky, D., Mikhailin, V., Nazarov, M., Ahmad-Fauzi, M.N. and Zhbanov, A. (2012) Luminescence and Energy Transfer Mechanisms in CaWO4 Single Crystals. Journal of Luminescence, 132, 2753-2762. http://dx.doi.org/10.1016/j.jlumin.2012.05.028
- 4. Sahi, S., Chen, W. and Jiang, K. (2015) Luminescence Enhancement of PPO/PVT Scintillators by CeF3 Nanoparticles. Journal of Luminescence, 159, 105-109. http://dx.doi.org/10.1016/j.jlumin.2014.11.004
- 5. Chen, X.P., Xiao, F., Ye, S., Huang, X.Y., Dong, G.P. and Zhang, Q.Y. (2011) ZnWO4:Eu3+ Nanorods: A Potential Tunable White Light-Emitting Phosphors. Journal of Alloys and Compounds, 509, 1355-1359. http://dx.doi.org/10.1016/j.jallcom.2010.10.061
- 6. Mao, Y.B. and Wong, S.S. (2004) General, Room-Temperature Method for the Synthesis of Isolated as Well as Arrays of Single-Crystalline ABO4-Type Nanorods. Journal of the American Chemical Society, 126, 15245-15252. http://dx.doi.org/10.1021/ja046331j
- 7. Song, Z.W., Ma, J.F., Sun, H.Y., Sun, Y., Wang, W., Fang, J.R., et al. (2009) Electro-chemical Synthesis of Barium Tungstate Crystallites with Different Morphologies: Effect of Electrolyte Components. Journal of the American Ceramic Society, 92, 2447-2450. http://dx.doi.org/10.1111/j.1551-2916.2009.03234.x
- 8. Mazur, M.M., Velikovskiy, D.Yu., Mazur, L.I., Pavluk, A.A., Pozhar, V.E. and Pustovoit, V.I. (2014) Elastic and Photo-Elastic Characteristics of Laser Crystals Potassium Rare-Earth Tungstates KRE(WO4)2, Where RE = Y, Yb, Gd and Lu. Ultrasonics, 54, 1311-1317. http://dx.doi.org/10.1016/j.ultras.2014.01.009
- 9. Hou, S.Y., Xing, Y., Ding, H., Liu, X.C., Liu, B. and Sun, X.J. (2010) Facile Synthesis and Photoluminescence Properties of Dumbbell-Like Ln-Doped BaWO4 (Ln=Nd, Er, Yb) Microstructures. Materials Letters, 64, 1503-1505. http://dx.doi.org/10.1016/j.matlet.2010.04.004
- 10. Liao, J.S., Qiu, B., Wen, H.R., You, W.X. and Xiao, Y.J. (2010) Synthesis and Optimum Luminescence of Monodispersed Spheres for BaWO4-Based Green Phosphors with Doping of Tb3+. Journal of Lumines-cence, 130, 762-766. http://dx.doi.org/10.1016/j.jlumin.2009.11.028
- 11. Jia, G., Dong, D.B., Song, C.Y., Li, L.F., Huang, C.M. and Zhang, C.M. (2014) Hydrothermal Synthesis and Luminescence Properties of Monodisperse BaWO4:Eu3+ Submicrospheres. Materials Letters, 120, 251-254. http://dx.doi.org/10.1016/j.matlet.2014.01.090
- 12. Thongtem, T., Phuruangrat, A. and Thongtem, S. (2008) Characterization of MeWO4 (Me = Ba, Sr and Ca) Nanocrystallines Prepared by Sonochemical Method. Applied Surface Science, 254, 7581-7585. http://dx.doi.org/10.1016/j.apsusc.2008.01.092
- 13. Kang, F.W., Hu, Y.H., Chen, L., Wang, X.J., Wu, H.Y. and Mu, Z.F. (2013) Luminescent Properties of Eu3+ in MWO4 (M=Ca, Sr, Ba) Matrix. Journal of Luminescence, 135, 113-119. http://dx.doi.org/10.1016/j.jlumin.2012.10.041
- 14. Dabre, K.V., Dhoble, S.J. and Lochab, J. (2014) Synthesis and Luminescence Properties of Ce3+ Doped MWO4 (M=Ca, Sr and Ba) Microcrystalline Phosphors. Journal of Luminescence, 149, 348-352. http://dx.doi.org/10.1016/j.jlumin.2014.01.048
- 15. Liao, J.S., Liu, L.B., You, H.Y., Huang, H.P. and You, W.X. (2012) Hydro-thermal Preparation and Luminescence Property of MWO4:Sm3+ (M = Ca, Sr, Ba) Red Phosphors. Optik, 123, 901-905. http://dx.doi.org/10.1016/j.ijleo.2011.07.002
- 16. Liu, X.H., Xiang, W.D., Chen, F.M., Hu, Z.F. and Zhang, W. (2013) Synthesis and Photoluminescence Characteristics of Dy3+ Doped NaY(WO4)2 Phosphors. Materials Research Bulletin, 48, 281-285. http://dx.doi.org/10.1016/j.materresbull.2012.10.050
- 17. Hu, Z.F., Meng, T., Zhang, W., Ye, D.H., Cui, Y.P., Luo, L. and Wang, Y.H. (2014) Synthesis and Luminescence of Dy3+-Activated NaSrPO4 Phosphors for Novel White Light Generation. Journal of Ma-terials Science: Materials in Electronics, 25, 1933-1937. http://dx.doi.org/10.1007/s10854-014-1823-4
- 18. Janka, S., Ivanova, M.E., Meulenberg, W.A., Doris, S. Detlev, S., Scherb, T., et al. (2013) Synthesis and Characterization of Nonsubstituted and Substituted Proton-Conducting La(6−x)WO(12−y). Inorganic Chemistry, 52, 10375-10386. http://dx.doi.org/10.1021/ic401104m
- 19. Ambast, A.K., Kunti, A.K., Som, S. and Sharma, S.K. (2013) Near-White-Emitting Phosphors Based on Tungstate for Phosphor-Converted Light-Emitting Diodes. Applied Optics, 52, 8424-8431. http://dx.doi.org/10.1364/AO.52.008424
- 20. Liu, S.X., Zhang, W., Hu, Z.F., Feng, Z.Y., Sheng, X. and Liang, Y.L. (2013) Synthe-sis and Luminescent Properties of Eu3+ and Dy3+ Doped BiPO4 Phosphors for Near UV-Based White LEDs. Journal of Materials Science: Materials in Electronics, 24, 4253-4257. http://dx.doi.org/10.1007/s10854-013-1393-x
- 21. Feng, W.L., Zhao, M.F., Xue, J.Y. and Tian, X.J. (2012) Photoluminescence Properties of (Ba1-xEux)WO4 Red Synthesized by the Coprecipitation/Calcination Method. Journal of Alloys and Compounds, 521, 146-149. http://dx.doi.org/10.1016/j.jallcom.2012.01.098
- 22. Sun, J.Y., Zhang, X.Y., Xia, Z.G. and Du, H.Y. (2011) Synthesis and Lumi-nescence Properties of Novel LiSrPO4:Dy3+ Phosphor. Materials Research Bulletin, 46, 2179-2182. http://dx.doi.org/10.1016/j.materresbull.2011.07.033
- 23. Dhobale, A.R., Mohapatra, M., Natarajan, V. and Godbole S.V. (2012) Synthesis and Photoluminescence Investigations of the White Light Emitting Phosphor, Vanadate Garnet,Ca2NaMg2V3O12 Co-Doped with Dy and Sm. Journal of Luminescence, 132, 293-298. http://dx.doi.org/10.1016/j.jlumin.2011.09.004
NOTES
*Corresponding author.










