Open Access Library Journal
Vol.02 No.08(2015), Article ID:68584,8 pages
10.4236/oalib.1101717
The Significance of Procalcitonin and C-Reactive Protein in Diagnostic Tests for Respiratory Adenovirus Infections in Children
Wolfgang Kunze1*, Thorsten Klemm2, Jan-Peter Streidl1
1Department of Children and Adolescent Medicine, Community Hospital Wurzen, Wurzen, Germany
2MVZ Laboratory Dr. Reising-Ackermann and Colleagues, Leipzig, Germany
Email: *kunze@krankenhaus-muldental.de, t.klemm@labor-leipzig.de, jstreidl@web.de
Copyright © 2015 by authors and OALib.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 6 August 2015; accepted 24 August 2015; published 27 August 2015

ABSTRACT
Background: This study aims to answer the question if procalcitonin tests allow distinguishing bacterial from AdV infections in cases in which acute phase values are significantly elevated. Methods: 40 patients of infant and toddler age were divided into two patient groups (n = 23) and (n = 17) for comparison based on PCT 0.5 µg/L. AdV was determined by polymerase-chain-reaction. Results: The PCT value in Group 1 is 0.21 ± 0.12 µg/L, median 0.2, and 1.56 ± 1.07 µg/L, median 1.3 (p = 0.0001) in Group 2. At a cut-off value of <0.5 µg/L, C-reactive protein, leucocytes, age, fever temperature, and the duration of fever did not differ significantly. This conclusion also applies to a PCT value to <0.9 µg/L. Conclusions: In AdV infections, CRP values of >40 mg/L are observed in more than 80% of patients. A clinically relevant conclusion of our findings is that independent of CRP levels antibiotic therapy is not indicated up to PCT levels of 0.5 µg/l.
Keywords:
Adenovirus, Respiratory Infection, Procalcitonin, C-Reactive Protein, Antibiotics, Children
Subject Areas: Virology

1. Introduction
Adenovirus (AdV) infections differ in type and severity. The most prevalent are infections of the airways. The percentage of febrile infections is approximately 10%, according to the literature [1] - [4] .
In a fraction of patients with respiratory AdV infections, an increase in acute phase values is observed. The physician is confronted with the question as to whether this is a distinctive feature of the AdV infection or due to bacterial superinfection. Antibiotic therapy would be indicated in bacterial infection, but not in the former case.
This study aims to answer the question as to whether Procalcitonin (PCT) tests allow to distinguish bacterial from AdV infections in cases in which acute phase values are significantly elevated [3] [4] .
2. Patients and Methods
The investigations were carried out in the pediatric department of a local hospital as part of routine medical care.
This involves the retrospective analysis of data from 40 children with Polymerase-chain-reaction (PCR) verified, clinically significant respiratory AdV infections who have the following diagnosed medical conditions: Tonsillopharyngitis (n = 18), upper airway infection (n = 16), pneumonia (n = 4), obstructive bronchitis (n = 2). To answer the research question of this study, we divided the patients into 2 groups: Group 1: PCT < 0.5 µg/L (n = 23), group 2 ≥ 0.5 µg/L (n = 17).
The collected patient information included the patient’s age and gender, temperature, length of hospital stay, length and type of therapy as well as the month of disease onset.
Diagnostic testing comprised the following: C-reactive protein (CRP), leukocytes and percentage of neutrophils, chest X-rays if pneumonia is suspected, PCT (KRYPTOR, ThermoFisher, Henningsdorf, Germany), Interleucin (IL)-6 (Elescys IL-6 test, Roche Diagnostics GmbH Mannheim, Germany), blood cultures (BACTEC FX, Heidelberg, Germany), throat swabs for group A streptococci (StrepA test) (möLab GmbH, Langenfeld, Germany); influenza virus RNA detection by PCR.
AdV testing was performed by collecting a nasopharyngeal swab specimen for human AdV DNA testing by real-time polymerase chain reaction (PCR) [5] on the first and second day of fever (median 1.5).
Statistics
Statistical analysis was performed using the SPSS program (SPSS Inc., an IBM Company Headquarters, 233 S. Wacker Drive, 11th floor Chicago, Illinois 60606).
The analysis involved the calculation of the median, minimum and maximum values due to the non-normal distribution of the data. Comparison of the rates was performed using the Wilcoxon rank test for paired samples and the Mann-Whitney U test for independent samples.
Rates were calculated by chi2 test or Fisher’s exact test. The correlation was determined by calculation of correlation coefficient “r” and Spearman’s rank correlation coefficient “rs”. The results are statistically significant with a p-value of ≤0.05.
3. Results
The data from patient group 1 (n = 23) was compared to the data from patient group 2 (n = 17) based on PCT < 0.5 µg/L and ≥0.5 µg/L. All additional results follow this method (Table 1 and Table 2).
The PCT value in Group 1 is 0.21 ± 0.12 µg/L (mean ± SD), median 0.21, and 1.56 ± 1.07 µg/L, median 1.3 (p = 0.0001) in Group 2.
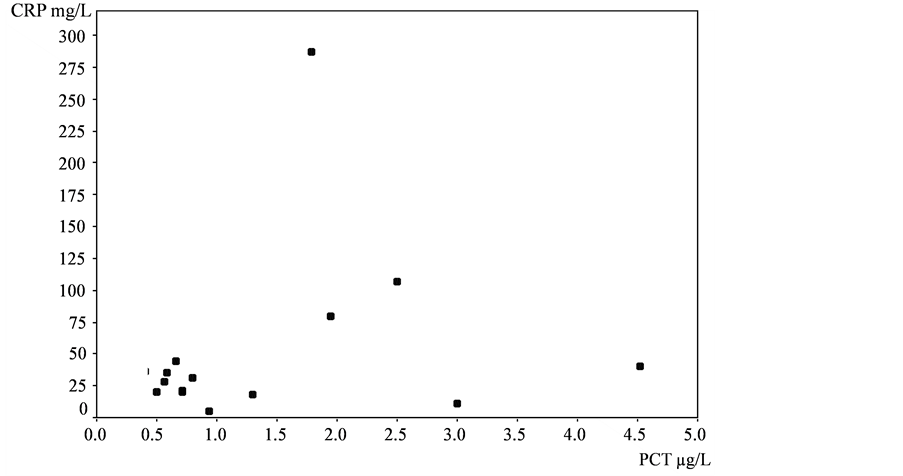
High PCT values of 1.79; 2.3 and 2.5 µg/L correlated with CRP values of 287.3; 115.5 and 106.4 µg/L. In contrast, CRP values of 18.5; 11.0 and 40.0 mg/L does net correlated with increased PCT values of 1.3; 3.0 and 4.52 µg/L.
No antibiotic therapy was given or therapy was discontinued prematurely in patients with PCT values of 1.3; 2.3; 3.0 and 4.52 µg/L (upper airway infection) who experienced spontaneous defervescence.
The CRP and leucocyte values (total, relative and absolute) also do not reveal significant differences. The IL-6 values are 41.6 ± 41.3 ng/L (mean ± SD), median 24.7 and 92.4 ± 68.8 ng/L, median 76.3 (p = 0.022), respectively.
Information regarding the age, temperature (˚C), duration of fever (days), length of hospital stay and therapy is shown in Table 2. None of the parameters revealed significant differences between the groups.
In group 2, 7 out of 17 patients had a documented otitis media while group 1 had no similar case.
The PCT groups do not differ significantly with respect to therapy versus no therapy (9:14) and (8:9).
Table 1. Clinical parameters in both patient groups for age, temperature, fever, duration of hospitalization and therapy.
Table 2. Laboratory parameters in both patient groups for PCT, CRP, white blood cell count, IL-6.
The gender ration of male (n = 23) to female (n = 17) does not achieve statistical significance.
Blood cultures were obtained 11 times (27.9%, Group 1 n = 5, Group 2 n = 6) and all tested pathogen-nega- tive. In particular, all cultures in Group 2 with CRP values above 40 (−287.3) mg/L were sterile.
StrepA throat swabs tested 14 times (35%) negative, particularly in tonsillopharyngitis cases, without evidence of a significant difference in the PCT groups.
Chest X-rays were performed in 5 patients, of which 4 (80%) had infiltrations consistent with pneumonia. The distribution of the infiltrations between the two groups was 75 and 25%, respectively.
34 patients (85%) were tested for influenza virus A and B infection by PCR with negative results.
In group 1 and 2, the distribution of upper airway infections, tonsillopharyngitis, pneumonia and obstructive bronchitis was 56.3 vs. 43.7, 55.6 vs. 44.4, 75 vs. 25 and 50 vs. 50%, 57.5 vs. 42.5% related to all diagnoses, respectively (Table 3).
Antibiotic therapy was prescribed 17 times (42.5%), 9 of them (52.9%) were discontinued prematurely. Twenty three patients (57.5%) did not receive medication. The prescription rate was evenly distributed. No significant difference in the two groups with respect to the duration of therapy and treatment was observed. No correlations between the individual findings were found overall (rs = 0.141) (Figure 1).
4. Discussion
There is wide agreement in the medical literature that acute phase values, CRP in particular, may be elevated in respiratory AdV infections. The data vary depending on study design.
Data on the relationship between CRP in AdV infections to PCT are not available.
The following data on average CRP values in AdV infections has been documented: 50.4 mg/L [6] , 68.3 mg/L (8 - 196.7) [7] . An in-house investigation of 265 PCR-verified respiratory AdV infections revealed the following CRP distribution: <40 mg/L 58.9%, ≥40 - <80 mg/L 26.8%, ≥80 - <120 mg/L 9.8%, >120 mg/L 4.5% (unpublished results). Other investigators established CRP values of >60 mg/L in 35.8% [8] , >70 mg/L in 22.5% [9] , >40 mg/L in 56%, >100 mg/L in 20% [10] . Lin et al. found CRP values of >40 mg/L in 80.4% and >100
Figure 1. Correlation PCT-CRP (rs = 0.141).
Table 3. Data on the percentage in both patient groups for gender, diagnostic and therapeutic parameters.
Mann-Whithey U-test or Fisher’s Exact test were used for statistical analysis. Differences between results are significant at the p-value of <0.05.
mg/L in 37%. Only 5.4% of these patients had a value <10 mg/L [11] . In-house tests (n = 106) revealed CRP values of <20, ≥20 - <50, ≥50 - <100, ≥100 - <120 and ≥120 mg/L in 34, 23.6, 30.2, 8.5 and 3.7%, respectively. Values of >200 mg/L were observed in 4 patients [12] . Farng et al. determined a CRP of 67.1 ± 49.9 in AdV infections compared to a CRP of 27.8 ± 35.9 mg/L in non-AdV infections (p < 0.001) [13] . Values of 32 ± 23 mg/L in AdV infections were significantly elevated compared to influenza (7.1 ± 6.2) and RS-virus infections (9.7 ± 8.1) [14] . Comparable results have been established by Oda et al. and Appenzeller et al. The CRP of 57 ± 41 mg/L in AdV infections relative to 12 ± 25 mg/L in RSV infections [15] and the CRP of 49 relative to 9 mg/L in Influenca infections [16] was significantly elevated.
The values for leukocytes and absolute neutrophils follow the acute phase reaction principle and are increased in AdV infections [7] - [9] [11] . These values are significantly increased compared to influenza and RS-virus infections as well [12] - [16] .
With respect to the diagnostic value of IL-6 in differentiating AdV from bacterial infections it must be noted that this parameter is not suitable to answer the research question, since it follows the acute phase reaction principle identical to CRP and leukocytes [14] . Pathogen detection by blood culture is regarded as proof of bacterial infection. In the literature available to us, we did not find any publication indicative of a PCR-verified AdV infection followed by a blood culture-verified bacterial infection. The detection of pathogen is most likely due to mixed bacterial infection or coinfection, which are reported at an incidence rate of 3% - 5% [6] [8] [12] [17] . Superinfection was ruled out in several studies by blood culture, liquor, urine and throat swab (for A streptococci) tests that tested negative [7] [13] [14] [16] - [18] .
CRP was considered the main criterion for differentiating viral from bacterial infections in the 1980s [19] . According to a study by Korppi et al., bacterial coinfection was present in 45% of AdV infections. This interpretation resulted in the prescription of antibiotics [20] .
The literature reports that AdV infections may clinically (including laboratory values) and radiologically mimic bacterial infections [7] [9] . As a result, antibiotics were administered in a CRP-value-dependent manner in 60% [16] , 75.7% [6] , 76% [10] and 91.2% [8] of cases, and administration was discontinued only after the test result was AdV positive. Other studies demonstrate that antibiotics were not administered initially in 64% [11] and 71% [18] of cases. In a study in which 38% of patients received therapy, therapy was discontinued prematurely in 62% of cases without negative consequences [12] . Rocholl et al. treated 46% of patients in which a bacterial cause was found in only 2.1% of cases [17] .
Diverse antibiotics were prescribed, which turned out to be not indicated in each case [8] [12] [21] . CRP remains without effect [16] .
The determination of PCT, which contrary to acute phase values is an immunomodulator with its own diagnostic profile, makes it possible to differentiate bacterial from viral infections.
Based on the combination PCT <0.5 µg/L and CRP <40 mg/L, Galetto-Lacour et al. demonstrated a sensitivity of 97% and specificity of 61%, ruling out severe bacterial infection with a probability of <1% and <3% [22] . PCT values of 0.26 (± 0.17) and 0.8 (0 - 4.4) have been established in viral infections [23] [24] .
PCT values of < 0.71 µg/L were observed in children who had viral infections [25] .
Toikka et al. specify a PCT value of ≥2.0 µg/L associated with probability of bacterial pneumonia [26] .
PCT values of ≤0.5 and <0.1 - 0.42 µg/L, respectively, have been established in Epstein-Barr and RS virus infections [27] [28] . In H1N1 influenza virus infections, values of 0.4 (0.1 - 6.1) µg/L have been found [29] . A PCT value of <0.8 µg/L in agreement with the clinical progression makes bacterial infection unlikely. Values of >0.8 µg/L indicate bacterial coinfection with a high degree of sensitivity and specificity [30] [31] .
An upper PCT limit of 0.25 and 0.5 µg/L was shown to be useful in clinical practice also with respect to the prescription of antibiotics [32] - [35] .
Unlike the mentioned viral infections, a special constellation applies to respiratory AdV infections.
It offers the possibility to trigger an immediate inflammatory response associated with an increase in acute phase values, CRP in particular, which is similar to bacterial infection [16] .
No plausible explanation could be found for the cause of this phenomenon, neither by using the currently available diagnostic methods nor in the literature.
To differentiate an AdV infection associated with increased CRP from bacterial superinfection using the established diagnostic methods was therefore not possible in this medical condition.
Only one report by Elenius et al. involving 16 children between 0.9 - 8.8 years of age is available on this topic. Fourteen of sixteen patients had a CRP of >40 mg/L and a PCT of <0.5 µg/L. Children with moderately elevated PCT (0.13 - 0.8 µg/L) had pneumonia or otitis media consistent with bacterial coinfection [21] .
These observations were confirmed in our patients with median PCT of 1.3 µg/L.
A PCT value of <0.5 µg/L is useful to distinguish medical conditions associated with increased CRP (Inflammation) and is therefore suitable for differentiating AdV infections from bacterial infections that may require antibiotic therapy. Based on our results, the same is true for PCT values between ≥0.5 and ≤1.3 µg/L.
The clinical constellations in patients with PCT values 2 - 5 µg/L with normal CRP, clinically unremarkable course, no detectable pathogens in blood cultures or Strep A tests, spontaneous decrease of fever, and no antibiotic therapy is pathogenetically not entirely clear but also most likely associated with bacterial coinfection.
This finding confirms the experience shared by many investigators in that no conclusive evidence of systemic bacterial infection can be established despite elevated acute phase values.
Values above 0.5 µg/L may be caused by local or minor bacterial infection or coinfection, provided the infection exceeds a certain threshold of severity.
PCT reactions are induced by both systemic inflammation and bacterial infection. The highest values occur in septic shock. Substantial PCT induction does not occur if the systemic inflammatory reaction is absent during infection. The PCT usually increases to approximately 0.5 µg/L [4] .
Severe cell damage that may progress to cell necrosis is being discussed as a possible induction pathway for elevated PCT values. The invasion of adherent monocytes into the damaged tissue triggers chemotactic reactions that break down apoptotic or dead cells and therefore eventually induces PCT production. This inflammatory mechanism can be initiated, for example, by severe tissue trauma, burns, extensive surgery, persistent perfusion defect, cardiogenic shock and also by severe AdV infections via the release of cytokines (interleukin-6, tumor necrosis factor) without bacterial infection being the cause [14] . Monocytes can induce the significant increase of circulating procalcitonin by recruiting parenchymal cells in the infected tissue [36] [37] . This process may result in PCT values between 0.5 and 2.0 µg/L, but these values must be assessed with caution and in consideration of the patient’s medical condition, since various non-bacterial (nonspecific) disorders may induce PCT [4] . It is conceivable that this induction pathway may also be involved in respiratory AdV infections associated with severe cell damage (tissue trauma). Th1 activation with subsequent increase in acute phase values occurring in AdV but not in RSV infections is an additional possible mechanism described in the literature [38] .
A clinically relevant conclusion of our findings is that independent of CRP levels antibiotic therapy is not indicated up to PCT levels of 0.5 (−2.0) µg/L. In higher PCT values, antibiotic therapy with close monitoring of PCT values is indicated due to the suspicion of a bacterial coinfection. Once PCT levels fall to below 0.5 µg/L antibiotic therapy should be terminated.
The limitations of the study are that it is based on the retrospective investigation of a small number of patients. Additional studies will be essential.
Acknowledgements
The authors wish to thank Michael Meisner, MD, PhD, Hospital of Dresden-Neustadt for comments and fruitful discussion.
Conflict of Interest
The authors declare that there is no actual or potential conflict of interest in relation to this article.
Cite this paper
Wolfgang Kunze,Thorsten Klemm,Jan-Peter Streidl, (2015) The Significance of Procalcitonin and C-Reactive Protein in Diagnostic Tests for Respiratory Adenovirus Infections in Children. Open Access Library Journal,02,1-8. doi: 10.4236/oalib.1101717
References
- 1. Pädiatrische Infektiologie (Hrsg.) (2013) DGPI Handbuch Infektionen bei Kindern und Jugendlichen. 6. vollst. überarb. Aufl., G. Thieme Verlag, Stuttgart, New York, 142-146.
- 2. Lynch, J.P., Fishbein, M. and Echavarria, M. (2011) Adenovirus. Seminars in Respiratory and Critical Care Medicine, 32, 494-511.
http://dx.doi.org/10.1055/s-0031-1283287 - 3. Irwin, A.D. and Carrol, E.D. (2011) Procalcitonin. Archives of Disease in Childhood—Education & Practice Edition, 96, 228-233.
http://dx.doi.org/10.1136/archdischild-2011-300178 - 4. Meisner, M. (2010) Procalcitonin—Biochemie und klinische Diagnostik. 1. Auflage-Bremen: UNI-MED.
- 5. Heim, A., Ebnet, C., Harste, G., et al. (2003) Rapid and Quantitative Detection of Human Adenovirus DNA by Real-Time PCR. Journal of Medical Virology, 70, 228-239.
http://dx.doi.org/10.1002/jmv.10382 - 6. Cheng, C.C., Huang, L.M., Kao, C.L., et al. (2008) Molecular and Clinical Characteristics of Adenoviral Infections in Taiwanese Children in 2004-2005. European Journal of Pediatrics, 167, 633-640.
http://dx.doi.org/10.1007/s00431-007-0562-4 - 7. Chuang, Y.Y., Chiu, C.H., Wong, K.S., et al. (2003) Severe Adenovirus Infection in Children. Journal of Microbiology, Immunology and Infection, 36, 37-40.
- 8. Chen, H.L., Chiou, S.S., Hsioao, H.P., et al. (2004) Respiratory Adenoviral Infections in Children: A Study of Hospitalized Cases in Southern Taiwan in 2001-2002. Journal of Tropical Pediatrics, 50, 279-284.
http://dx.doi.org/10.1093/tropej/50.5.279 - 9. Dominguez, O., Rojo, P., de las Heras, S., et al. (2005) Clinical Presentation and Characteristics of Pharyngeal Adenovirus Infections. The Pediatric Infectious Disease Journal, 24, 733-734.
http://dx.doi.org/10.1097/01.inf.0000172942.96436.2d - 10. Tabain, I., Ljubin-Sternak, S., Cepin-Bogović, L., et al. (2012) Adenovirus Respiratory Infections in Hospitalized Children: Clinical Findings in Relation to Species and Serotypes. The Pediatric Infectious Disease Journal, 31, 680-684.
http://dx.doi.org/10.1097/INF.0b013e318256605e - 11. Lin, C.H., Huang, Y.C., Chiu, C.H., Huang, C.G., Tsao, K.C. and Lin, T.Y. (2007) A Cluster of Adenovirus Serotype 3 Infections in Children in Northern Taiwan: Clinical Features and Laboratory Findings. Journal of Microbiology, Immunology and Infection, 40, 302-309.
- 12. Kunze, W., Beier, D. and Gröger, K. (2010) Adenovirus Respiratory Infections in Children. Do They Mimic Bacterial Infections? WebMedCentral PAEDIATRICS, 1, Article ID: WMC001098.
http://www.webmedcentral.com/article_view/1098 - 13. Farng, K.T., Wu, K.G., Lee, Y.S., Lin, Y.H. and Hwang, B.T. (2002) Comparison of Clinical Characteristics of Adenovirus and Non-Adenovirus Pneumonia in Children. Journal of Microbiology, Immunology and Infection, 35, 37-41.
- 14. Kawasaki, Y., Hosoya, M., Katayose, M. and Suzuki, H. (2002) Correlation between Serum Interleukin 6 and C-Reactive Protein Concentrations in Patients with Adenoviral Respiratory Infection. The Pediatric Infectious Disease Journal, 21, 370-374.
http://dx.doi.org/10.1097/00006454-200205000-00004 - 15. Oda, K. and Yamamoto, Y. (2008) Serum Interferon-γ, Interleukin-4, and Interleukin-6 in Infants with Adenovirus and Respiratory Syncytial Virus Infection. Pediatrics International, 50, 92-94.
http://dx.doi.org/10.1111/j.1442-200X.2007.02522.x - 16. Appenzeller, C., Amman, R.A., Duppenthaler, A., Gorgievski-Hrisoho, M. and Aebi, C. (2002) Serum C-Reactive Protein in Children with Adenovirus Infection. Swiss Medical Weekly, 132, 345-350.
- 17. Rocholl, C., Gerber, K., Daly, J., Pavia, A.T. and Byington, C.L. (2004) Adenoviral Infections in Children: The Impact of Rapid Diagnosis. Pediatrics, 113, e51-e56.
http://dx.doi.org/10.1542/peds.113.1.e51 - 18. Van Lierde, S., Corbeel, L. and Eggermont, E. (1989) Clinical and Laboratory Findings in Children with Adenovirus Infections. European Journal of Pediatrics, 148, 423-425.
http://dx.doi.org/10.1007/BF00595902 - 19. Korppi, M. and Kroger, L. (1993) C-Reactive Protein in Viral and Bacterial Respiratory Infection in Children. Scandinavian Journal of Infectious Diseases, 25, 207-213.
http://dx.doi.org/10.3109/00365549309008486 - 20. Korppi, M., Leinonen, M., Mäkelä, P.H. and Launiala, K. (1991) Mixed Infection Is Common in Children with Respiratory Adenovirus Infection. Acta Paediatrica, 80, 413-417.
http://dx.doi.org/10.1111/j.1651-2227.1991.tb11875.x - 21. Elenius, V., Peltola, V., Ruuskanen, O., Yliharsila, M. and Waris, M. (2011) Plasma Procalcitonin Levels in Children with Adenovirus Infection. Archives of Disease in Childhood, 97, 582-583.
http://dx.doi.org/10.1136/archdischild-2011-301308 - 22. Galetto-Lacour, A., Zamora, S.A. and Gervaix, S.A. (2003) Bedside Procalcitonin and C-Reactive Protein Tests in Children with Fever without Localizing Signs of Infection Seen in a Referral Center. Pediatrics, 112, 1054-1060.
http://dx.doi.org/10.1542/peds.112.5.1054 - 23. Hatherill, M., Tibby, S.M., Sykes, K., Turner, C. and Murdoch, I.A. (1999) Diagnostic Markers of Infection: Comparison of Procalcitonin with C-Reactive Protein and Leucocyte Count. Archives of Disease in Childhood, 81, 417-421.
http://dx.doi.org/10.1136/adc.81.5.417 - 24. Fernández Lopez, A., Luaces Cubells, C., García García, J.J. and Fernández Pou, J., Spanish Society of Pediatric Emergencies (2003) Procalcitonin in Pediatric Emergency Departments for the Early Diagnosis of Invasive Bacterial Infections in Febrile Infants: Results of a Multicenter Study and Utility of a Rapid Qualitative Test for This Marker. The Pediatric Infectious Disease Journal, 22, 895-904.
http://dx.doi.org/10.1097/01.inf.0000091360.11784.21 - 25. Limper, M., Smit, P.M., Bongers, K.M., van Zanten, A.P., Smits, P.H.M., Brandjes, D.P.M., et al. (2010) Procalcitonin in Children with Suspected Novel Influenza A (H1N1) Infection. Journal of Infection, 61, 351-353.
http://dx.doi.org/10.1016/j.jinf.2010.07.005 - 26. Toikka, P., Irjala, K., Juvén, T., Virkki, R., Mertsola, J., Leinonen, M. and Ruuskanen, O. (2000) Serum Procalcitonin, C-Reactive Protein and Interleukin-6 for Distinguishing Bacterial and Viral Pneumonia in Children. The Pediatric Infectious Disease Journal, 19, 598-602.
http://dx.doi.org/10.1097/00006454-200007000-00003 - 27. Matesanz, J.L., Fernández, E., Fernández, J.M. and Viejo, G. (2003) Plasma Procalcitonin and C-Reactive Protein Concentrations in Pediatric Patients with Epstein-Barr Virus Infection. Clinical Chemistry, 49, 2103-2104.
http://dx.doi.org/10.1373/clinchem.2003.023499 - 28. Resch, B., Gusenleitner, W. and Müller, W. (2003) Procalcitonin, Interleukin-6, C-Reactive Protein and Leukocyte Counts in Infants with Bronchiolitis. The Pediatric Infectious Disease Journal, 22, 475-476.
http://dx.doi.org/10.1097/01.inf.0000066196.23839.b0 - 29. Piacentini, E., Sánchez, B., Arauzo, V., Calbo, E., Cuchi, E. and Nava, J.M. (2011) Procalcitonin Levels Are Lower in Intensive Care Unit with H1N1 Influenza A Virus Pneumonia than in Those with Community-Acquired Bacterial Pneumonia: A Pilot Study. Journal of Critical Care, 26, 201-205.
http://dx.doi.org/10.1016/j.jcrc.2010.07.009 - 30. Ingram, P.R., Inglis, T., Moxon, D. and Speers, D. (2010) Procalcitonin and C-Reactive Protein in Severe 2009 H1N1 Influenza Infection. Intensive Care Medicine, 36, 528-532.
http://dx.doi.org/10.1007/s00134-009-1746-3 - 31. Cuquemelle, E., Soulis, F., Villers, D., Roche-Campo, F., Ara Somohano, C., Fartoukh, M., et al., A/H1N1 REVA-SRLF Study Group (2011) Can Procalcitonin Help Identify Associated Bacterial Infection in Patients with Severe Influenza Pneumonia? A Multicenter Study. Intensive Care Medicine, 37, 796-800.
http://dx.doi.org/10.1007/s00134-011-2189-1 - 32. Albrich, W.C., Dusemund, F., Bocher, B., Meyer, S., Thomann, R., Kühn, F., et al. (2012) Effectiveness and Safety of Procalcitonin-Guided Antibiotic Therapy in Lower Respiratory Tract Infections in “Real Life”: An International Multicenter Poststudy Survey (ProREAL). Archives of Internal Medicine, 172, 715-722.
http://dx.doi.org/10.1001/archinternmed.2012.770 - 33. Burkhardt, O., Ewing, S., Haagen, U., Giersdorf, S., Hartmann, O., Wegscheider, K., et al. (2010) Procalcitonin-Guidance and Reduction of Antibiotic Use in Acute Respiratory Tract Infection. European Respiratory Journal, 36, 601-607.
http://dx.doi.org/10.1183/09031936.00163309 - 34. Schützle, H., Forster, J., Superti-Furga, A. and Berner, R. (2009) Is Serum Procalcitonin a Reliable Diagnostic Marker in Children with Acute Respiratory Tract Infections? A Retrospective Analysis. European Journal of Pediatrics, 168, 1117-1124.
http://dx.doi.org/10.1007/s00431-008-0899-3 - 35. Baer, G., Baumann, P., Buettcher, M., Heininger, U., Berthet, G., Schäfer, J., et al. (2013) Procalcitonin Guidance to Reduce Antibiotic Treatment of Lower Respiratory Tract Infection in Children and Adolescents (ProPAED): A Randomized Controlled Trial. PLoS ONE, 8, e68419.
http://dx.doi.org/10.1371/journal.pone.0068419 - 36. Linscheid, P., Seboek, D., Schaer, D.J., Zulewski, H., Keller, U. and Müller, B. (2004) Expression and Secretion of Procalcitonin and Calcitonin Gene-Related Peptide by Adherent Monocytes and by Macrophage-Activated Adipocytes. Critical Care Medicine, 32, 1715-1721.
http://dx.doi.org/10.1097/01.CCM.0000134404.63292.71 - 37. Higginbotham, J.N., Seth, P., Blaese, R.M. and Ramsey, W.J. (2002) The Release of Inflammatory Cytokines from Human Peripheral Blood Mononuclear Cells in Vitro Following Exposure to Adenovirus Variants and Capsid. Human Gene Therapy, 13, 129-141.
http://dx.doi.org/10.1089/10430340152712683 - 38. Diaz, P.V., Calhoun, W.J., Hinton, K.L., Avendaño, L.F., Gaggero, A., Simon, V., et al. (1999) Differential Effects of Respiratory Syncytial Virus and Adenovirus on Mononuclear Cell Cytokine Responses. American Journal of Respiratory and Critical Care Medicine, 160, 1157-1164.
http://dx.doi.org/10.1164/ajrccm.160.4.9804075
NOTES

*Corresponding author.