Open Access Library Journal
Vol.02 No.04(2015), Article ID:68350,21 pages
10.4236/oalib.1101297
Gaskets with Expandable Graphite Treated with Nitric, Sulphuric, Phosphoric Acids and Ferric Chloride
Heinrich Horacek
Free Consultancy, Linz, Austria
Email: heinrich.horacek@aon.at
Copyright © 2015 by author and OALib.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 1 April 2015; accepted 25 April 2015; published 30 April 2015
ABSTRACT
The task was to find out the most appropriate expandable graphite intercalation compound GIC for intumescent seals. The application in fire seals required GICs, which exerted high expansion at low burning rates. Therefore graphite was treated with nitric, sulphuric, phosphoric acids and ferric chloride. Phosphoric acid with its flame retardant properties was the favourite, but graphite treated with it, did not exfoliate, therefore expandable graphite was post treated with phosphoric acid. Elemental analysis and thermogravimetric measurements led to chemical formulas of GICs. Their heats of exfoliation were determined by differential scanning calorimetry, by the work done given by the product of increased specific volume and atmospheric pressure, by the Arrhenius plot of exfoliated specific volume dependent on temperature and were calculated in a complete balance of weights and heats of formation. It turned out that the sum of heats of intercalation and exfoliation corresponded with the heats of decomposition and gasification of the intercalated compounds. When the heat of intercalation was added to the lattice energy of graphite, the lattice energy of GIC was obtained. Electron microscopy indicated that expansion did not happen in monolayers but in nanoplatelets consisting of about 40 atomic layers. The expandable GICs were incorporated into polyvinyl acetate strips and applied as fire gaskets in gaps. In a fire test, the gap protected by the strip comprising the GIC treated with sulphuric and phosphoric acid showed the best performance, which corresponded with the highest observed expansion and with the second highest temperature of maximum speed of combustion.
Keywords:
Nanomaterials, Thermal Properties, Industrial Application, Exfoliated Graphite
Subject Areas: High Polymer Chemistry, Nanometer Materials, Physical Chemistry, Thermochemistry

1. Introduction
Intercalated graphite compounds GICs were intensively discussed in literature [1] - [3] . Less information was available concerning expandable intercalated graphite compounds [4] [5] . In order to receive detailed data about maximum expanded specific volumes, temperatures of maximum rate of combustion, heats of formation, heats of intercalation and exfoliation as well as lattice energies between the layers of graphite, expandable GICs were prepared by incorporating nitric, sulphuric, phosphoric acids as well as ferric chloride [6] .
In 1840, Schafhäutl [7] discovered expandable graphite intercalation compounds.
Until now only one type of expandable GIC was available commercially, such one treated by sulphuric acid (H2SO4).
In literature also other types of expandable GICs were described such as graphite treated with nitric acid (HNO3), bromine (Br2), ferric chloride (FeCl3), chromo oxide (CrO3) potassium tetrahydrofuran (K(THF)2) and potassium permanganate (KMnO4) [8] - [12] .
Also the influence of synergistic additives was investigated: alcohols, aldehydes, sugars, hydrogen peroxide (H2O2) [13] and phosphoric acid (H3PO4) [10] .
These efforts can be understood under the background of growing applications as foils, seals, electrically conductive or shielding material and flame retardant. Graphenes and related materials received much attention, when their thicknesses approached atomic levels. Microelectronics was focused on monolayer graphenes. Fuel cells, batteries, solar cells, sensors and polymer composition extended their interest to nanoplatelets consisting of up to 10 atomic layers which deviate from bulk graphite. Pure single layer graphene was manufactured by self assembling approach [14] and from GIC by microexplosion method [15] . According to the nomenclature and terminology [16] - [20] graphite intercalation compounds were described as donor acceptor complexes or as graphite salts:
 (1)
(1)
In Equation (1), C stands for graphite, HX for acid, n for the stage index and m for the stoichiometric factor.
The thickness of the constitutional repeating unit Ic, which was the distance between two successive intercalated layers, were calculated according to Equation (2).
Ic was given by the thickness of the intercalated layer ds plus the product of the stage index n minus 1 multiplied by the original distance between two adjacent graphenes co =3.35 Angstrom.
 (2)
(2)
Stage index 2 Stage index 3
_____________ _____________ graphene
0000000000000 0000000000000 intercalated compound
_____________ _____________
_____________ _____________
0000000000000 _____________
00000000000000
Chung [5] described intercalation and exfoliation of bromine GIC C8Br CAS [12079-58-2] and wondered, that the heat of exfoliation amounted to 71 kJ/mole, which did not correspond with 31 kJ/mole the heat of vaporization of liquid bromine. This discrepancy was eliminated, when the heat of vaporization 31 kJ/mole was not put in relation with 71 kJ/mole, the heat of exfoliation, but with the added heats of intercalation and exfoliation: −40 + 71 = 31. Anderson [21] cited several heats of intercalation for bromine and graphite −41.9 kJ/mole second stage heat of intercalation, −45.2 kJ/mole third stage and −46.1 kJ/mole fourth stage. In the following the mass balances of the molecular weights MW (g/mole) and the heat balances of the heats of formation H (kJ/mole) were set up for each formula F, which led to heats of intercalation Hinter of −40 kJ/mole or hinter of 0.21 kJ/gC:
Intercalation: hinter = −40/192 = −0.21 kJ/gC; −40/352 = −0.11 kJ/gGIC
F1: 2*8 C + Br2 = 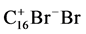
MW (g/mole): 192 + 160 = 352
H (kJ/mole): 0 − 40 = −40
Exfoliation: hexf = 71/192 = 0.37 kJ/gC; 71/352 = 0.20 kJ/gGIC, maximum expansion (l-lo)/lo = 32.5 lo initial thickness, l thickness after expansion [5]
F2: 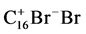 = 16 Cexf + Br2gas
= 16 Cexf + Br2gas
MW: 352 = 192 + 160
H: −40 + 71 = 31
F1 + F2: 2*8 C+ Br2 = 16 Cexf + Br2gas
H: 0 + −40 + −40 + 71 = −40 + 31
Vaporization of bromine at 59˚C:
F3: Br2 = Br2gas
H: 0 + 31 = 31
From the above formula it became obvious that the heat of intercalation Hinter plus the heat of exfoliation Hexf was equal to the heat of vaporization Hvap:
 (3)
(3)
−40 +71 = 31 kJ/mole
For a second stage intercalation of bromine graphite the identity distance Ic was 10.4 Angstrom.
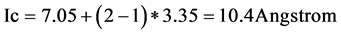 (4)
(4)
The lattice energy was defined as the energy accompanying the process of bringing layers, when separated from each other by an infinite distance, to the distance they occupy in a stable lattice. By definition the lattice energy of GIC LGIC should be equal to the sum of the lattice energy of graphite LC plus the heat of intercalation hinter related to 1gC:
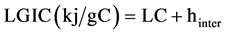 (5)
(5)
Dappe [23] published a graphite lattice energy LC of −65 meV, which correlated with −520 J/g or −0.52 kJ/g at a layer distance of Ic = 3.1 - 3.2 Angstrom. (1 meV = 1.6 × 10−22 J) × (NA = 6.023 × 1023 mole−1) = 96.4 J/mole divided by 12 g/mole the molecular weight of graphite gave the conversion factor 7.88. Novoselov [24] measured −0.07 eV complying with −0.56 kJ/g at an equilibrium distance of 3.35 Angstrom. In Gmelins Handbook [25] −334 erg/cm2 were stated, which were converted to −0.445 kJ/g by extending the volume of 1 cm2 × 3.35 Angstrom to 1 cm3 by the factor 3 × 107 divided by the density of graphite 2.25 g/cm3.
Henning [22] determined −1.3 eV lattice energy for the bromine graphite with the molecular weight of 352 g/mole GIC and 192 g/mole C. (1eV = 1.6 × 10−19 J) × (NA = 6.023 × 1023 mole−1) = 96.4 kJ/mole, therefore the bromine GIC had a lattice energy of −1.3 × 96.4/352 = −0.356 kJ/g GIC or −0.653 kJ/gC at a layer distance of 10.4 Angstrom. When the heat of intercalation hinter = −0.21 kJ/gC was subtracted from the lattice energy of the GIC C16Br2 −0.653 kJ/gC according to Equation (5), then a graphite lattice energy of −0.443 kJ/gC was obtained in Equation (6). Comparing the data in literature Gmelins Handbook [25] with −0.445 kJ/g made the best fit, nevertheless the recent published lattice energy of −0.56 kJ/g [24] was taken for further calculations.
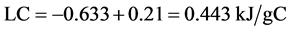 (6)
(6)
C16Br2 was not considered for the application of fire gaskets, because of the low MAK value of 0.7 mg/m3 of bromine. The wanted GICs should have low temperatures of expansion, a high volume increase by expansion and a low burning rate. Indications for the suitability of GICs were expected from the properties of the intercalated compounds, such as their temperatures of decomposition Tdec, of vaporization Tvap and of sublimation Tsubl as well as from their relevant heats H:
2H3PO4 = P2O5 + 3H2O; H dec = 205 kJ/mole H3PO4; Tdec: 250˚C, 350˚C, 500˚C
H2SO4 = H2O + SO3; H dec = 246 kJ/mole H2SO4; Tdec: 338˚C/450˚C
2HNO3 = H2O + 2NO2 + 0.5O2; H dec = 86 kJ/mole HNO3; Tdec: 84˚C
2FeCl3 = Fe2Cl6gas; H subl = 138 kJ/mole Fe2Cl6; Tsub: 315˚C
2CrO3 = Cr2O3 + 1.5O2; H dec = 11 kJ/moles CrO3; Tdec: 250˚C
K(THF)2 = Kgas + 2THFgas; H vap = 142 kJ/mole K(THF)2; Tdec: 500˚C
Cancer causing chromo oxide (CrO3) was considered as harmful and was rejected. Nitrogen dioxide NO2 with a MAK value of 9 mg/m3 and sulphur trioxide (SO3) with such one of 1 mg/cm3 were pardoned. In comparison tetrahydrofuran (THF) showed a high MAK value of 50 ppm, but was highly burnable and therefore unfit.
The research purpose was to find out the most appropriate expandable GIC for the application in intumescent fire seals. Starting from this state of knowledge GICs were prepared from nitric, sulphuric, phosphoric acids and ferric chloride. They were characterized, applied in intumescent strips and screened in a simplified fire door test. The fire performance was correlated with the properties of GICs such as maximum expansion and temperature of maximum burning rate.
2. Experimental
For the preparation of the expandable GICs as well as for intumescent strips the chemicals of Table 1 were used.
2.1. Preparation of GICs
2.1.1. No. 1 Nitric Acid GIC
100 g nitric acid was added to 100 g of graphite in 15 minutes at such a velocity that the temperature did not surpass 45˚C. Intercalation took place under heat development. In a calorimeter −0.11 kJ/gC (kJ related to 1g graphite) heat development was measured. A pressure of 2 bar nitrogen was put upon the mixture which was stirred for further 30 minutes. The graphite was separated and dried in a nitrogen stream. Elemental analysis confirmed a nitrogen content of 2.1%.
Then 70 ml of water were added under stirring so that the temperature did not exceed 45˚C.
The water was sucked off. This operation was repeated, until the water was weakly acidic. The graphite was dried by flowing nitrogen for 5 minutes.
60 ml of 4% ammonia water were added for further neutralization. The mixture was stirred for 5 minutes, afterwards the ammonia water was sucked off and the graphite was dried under streaming nitrogen for 45 minutes at RT and for 4 h at 60˚C.
The graphite so prepared showed a nitrogen content of 2.0% and a weight loss during exfoliation of 10%. These data led to the formula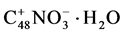 .
.
2.1.2. No. 2 Phosphoric and Nitric Acids GIC
Searching in literature for phosphoric acid intercalated compounds graphite phosphate with the common formula CxH3PO4 CAS [79081-31-6] was detected. A stage 2 GIC with the identity distance 2 × 11.38 Angstrom and the formula C++H2P2O7−− was described, when graphite was treated by 100% phosphoric acid in the presence of chromo oxide at 80˚C - 100˚C [10] . When graphite was stored in 100% phosphoric acid at RT as well as at 90˚C for 5 hours the treated graphite exerted no expansion. Chromo oxide was not added, because of its danger of cancer. Therefore the expandable product  was applied for further treatment.
was applied for further treatment.
100 g of 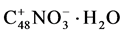 were dispersed in 100 g 80 w% phosphoric acid.
were dispersed in 100 g 80 w% phosphoric acid.
After 5 h at 65˚C H3PO4 was sucked off and the residue was blown dry by a stream of nitrogen. Then twice 100 ml water was added under stirring and filtered off. After filtration the graphite was dried under flowing nitrogen for 45 minutes at RT and dried for 4 h at 80˚C.
The graphite treated by phosphoric acid comprised 1.9% N and 4.2% P, which corresponded with the formula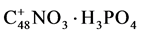 .
.
Table 1. Chemicals used.
2.1.3. No. 3 Sulphuric Acid GIC
To 100 g graphite 10 ml 30 w% H2O2 and afterwards 150 ml 96 w% sulphuric acid with a density of 1.84 g/cm3 were added under stirring. When the dosage was complete, the mixture was stirred further for 15 minutes at RT. The procedure of washing and drying was repeated twice until the pH-value of the waste water amounted to more than 4. Then the product was dried for 45 minutes at RT and for 4 hours at 60˚C.
The so prepared graphite had a sulphur content of 2.9% and a weight loss of 18% during exfoliation. These data were in agreement with a formula of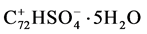 .
.
2.1.4. No. 4 Phosphoric and Sulphuric Acids GIC
100 g 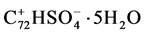 were dispersed in 100 g 80% phosphoric acid and stirred 5 h at 65˚C.
were dispersed in 100 g 80% phosphoric acid and stirred 5 h at 65˚C.
After filtration the residue was dried under streaming nitrogen. After washing and drying for two times, as already described, the product accomplished an elementary analysis of 2.8% P and 2.9% S, which corresponded with the formula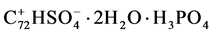 .
.
2.1.5. No. 5 Ferric Chloride GIC
100 g graphite was mixed with 300 ml nitro methane and 250 g FeCl3.
The mixture was stirred at RT for 18 hours, then washed two times with 150 ml nitro methane and blown dry under nitrogen. The ultimate drying was performed at 60˚C for 4 hours.
The graphite obtained was specified by an iron content of 13.5% and a weight loss of 39% after exfoliation. The expected formula was C21FeCl3.
2.2. Characterization by TMA, TGA, DSC and Muffle Furnace Test
A Mettler Toledo TMASDTA 840 was used for thermogravimetric analysis TGA, for thermomechanic analysis TMA and for differential scanning calorimetry DSC. In the case of TGA and DSC the samples were placed in aluminium oxide crucibles of 900 µl (ME 51119, 960) with 12 mm diameter covered by punctured lids. TGA were performed in air at 20 K/min heating rate and DSC in nitrogen at 20 K/min. The TMA investigations were exerted in aluminium crucibles with 7 mm diameter and 4.6 mm height covered by 6 mm lids in air at a heating rate of 20 K/min and a load of 1 N which is equal to a pressure of 0.36 bar.
In the muffle furnace test a 1 g sample was placed in a calibrated quartz tube and put into a muffle furnace of defined temperature for 5 minutes. The specific volume (ml/g) was determined immediately after the withdrawal from the furnace.
2.2.1. No. 1
In order to achieve agreement with elemental analysis and heat development during intercalation the formula F4 had to be assumed, followed by the reaction of washing in formula F5.
Intercalation: hinterex/cal = −0.11/−0.13 kJ/gC (ex = experimental/cal = calculated)
F4: 24*2C + 4 HNO3 + 0.5O = C48 + 
MW(g/mole) 576 + 4*63 + 8 = 827 + 9
H(kJ/mole) 4*174 ? 74 = −648.5 − 121.5
Washing: hwashing ex/cal = −/+0.23 kJ/gGIC
F5: 

MW: 827 + 18 = 656 + 3*63
H: −648.5 − 287 + 189.5 = −224 − 3*174
The reaction with nitric acid led to a stage 2 GIC with stoichiometric factor 4 under heat development of −0.11 kJ/gC with a calculated constitutional repeating unit Ic of 11.4 Angstrom.

The theoretical intercalation was described by F6.
Intercalation: hinterex/cal = −/+0.16 kJ/gC = 0.14 kJ/gGIC
F6: 48C + HNO3 + 0.5H2O + 0.25O2 =
MW: 576 + 63 + 9 + 8 = 656
H: −174 + −143 + 93 = −224
Exfoliation: hexf ex/cal = 0.02/0.02 kJ/gGIC; 0.026 kJ/gC; Rex/cal = 90%/88%
F7: 
MW: 656 = 576 + 46 + 18 + 16
H: −224 + 15 = 0 + 34 − 243
F6 + F7: 48C + HNO3 + 0.5H2O + 0.5O = 48C ex + NO2 + H2Ogas +0.5 O2
MW: 576 + 63 + 9 + 8 = 576 + 46 + 18 + 16
H: −174 + −143 + 93 + 15 = 34 − 243
Decomposition and vaporization at 84˚C:
F8: HNO3 = NO2 + 0.5H2O + 0.5O
MW: 63 = 46 + 9 + 8
H 173.5 + 86 = 34 + −121.5
F9: 0.5*(H2O = H2Ogas)
H: 0.5*(−286 + 43 = −243)
F8 + F9 HNO3 + 0.5H2O = NO2 + H2O + 0.5O
MW: 63 + 9 = 46 + 18 + 8
H: −173.5 ? 143 + 107.5 = 34 − 243
The sum of the heats of intercalation and of exfoliation corresponded to the sum of the heats of decomposition and vaporization:

93 + 15 ^ 86 + 21.5
In Figure 2 a sample of the GIC was subdued the thermal mechanical analysis.
Expansion started at 150˚C and reached a maximum of 2250% at 250˚C. Above 600˚C combustion was observed and reached its maximum rate at 900˚C (Figure 1).
A 1 g sample was placed in a calibrated quartz tube and heated for 5 minutes at defined temperatures in a muffle furnace.
In Figure 2 the specific volume V (ml/g) increased proportionally with the temperature T (˚C) according to Equation (9).
Figure 1. TMA: expansion% versus temperature of No. 1
Figure 2. Furnace test: Specific volume V (ml/g) versus temperature T of No. 1 

In Figure 2 the sample extended to a specific volume of 40 ml/g at 200˚C and such one of 275 ml/g at 800˚C. At low temperatures up to 300˚C the thermal history of the samples subdued to TMA with a heating rate of 20 K/min and furnace test with a heating time of 5 min was were similar and the results were some how comparable, for instance in TMA an extension of 2000% measured at 200˚C. As the initial specific volume was 1.66 cm3/g at 200˚C, a volume of 1.66*2000%/100 = 33.2 ml/g resulted.
The specific volume at 800˚C was put into the ideal gas equation and the moles of gases evolved during exfoliation were calculated.

According to Equation (10) 1.86 moles of gases were evolved.
When the GIC expanded fully, the only counter force was the atmospheric pressure of 1 bar. The work done to expand the bulk volume from 1.66 to 275 cm3/g was wexf, which was given by the product of the maximum specific volume in Figure 2 and the pressure of 1 bar.

When the data of Figure 2 were treated according Clausius Clapeyron or Arrhenius in Table 2, 13.8 kJ/mole heat of exfoliation Hexf or herf 0.021 kJ/gGIC was determined.
In a plot lgV versus 103/T the inclination dlgV/d(103/T) was −0.72 and Hexf was calculated by Equation (12):

In differential scanning calorimetry DSC exfoliation occurred under heat uptake of 0.020 kJ/g GIC or 13.1 kJ/mole at 200˚C in Figure 3. During exfoliation a weight loss of 10% was measured by TGA investigation in Figure 4.
Equation (13) allowed the calculation of the lattice energy LGIC.
Table 2. Clausius Clapeyron or Arrhenius: heat of exfoliation.
Figure 3. DSC: Heat mW versus temperature of No. 1

During exfoliation 2.5 moles of gases, namely NO2, H2O and 0.5O2, were evolved according F6 under 15 kJ/mole heat consumption. When the maximum expanded specific volume was introduced into the ideal gas law only 1.86 moles of gases were determined. DSC measurements delivered 13.1 kJ/mole or 0.020 kJ/g heat of exfoliation and the Clausius Clapeyron plot of Figure 2 led to 13.8 kJ/mole or 0.019 kJ/gGIC heat of exfoliation.
The work of maximum exfoliation wexf with 0.027 kJ/gGIC was higher than those derived from DSC with 0.020 kJ/gGIC and from the specific volume in dependence of temperature with 0.019 kJ/gGIC.
2.2.2. No. 2
The expandable nitric acid GIC was post treated with phosphoric acid and characterized by elemental analysis. In Figure 5 the maximum expansion was reduced to 1150% and the temperature of maximum heat of combustion was shifted from 900˚C to 1100˚C.
Phosphoric acid did not contribute to expansion but acted as a flame retardant.
13.3% phosphoric acid shifted the temperature of maximum combustion Tmax comb to higher temperatures, namely from 900˚C to 1030˚C. With increasing content of phosphoric acid the temperature of combustion was
Figure 4. TGA: weight residue % versus temperature of No. 1
Figure 5. TMA: expansion% EF versus temperature T of No. 2
shifted to higher values, which was documented in Figure 6. content for
Tmax comb was proportional with the phosphoric acid content.

2.2.3. No. 3
According to literature [26] the intercalation process proceeded in first stage intercalation reaction under formation of 
Intercalation hinter ex/cal = −/− 0.006 kJ/gC
F9: 24*6C + 7H2SO4 + 0.5O2 = 
MW 1728 + 686 + 16 = 2*1206 + 18
H 7* − 889 ? 11 = 2* − 3117
Washing hwash ex/cal = −/0.36 kJ/gGIC.
F10: 

MW 1206 + 5*18 = 1051 + 2.5*98
H: −3117 + 5* − 287 + 435.5 = −1894 + 2.5* − 889
14.71 Angstrom as repeating unit was calculated for a third stage intercalation.

In Figure 7 the specific volume of the GIC was a linear correlation with the temperature in the muffle furnace and followed Equation (16):

The sample was also investigated by TMA in Figure 8. Expansion started at 200˚C and reached a maximum expansion of 2250% at 300˚C.
A sample taken out from the muffle furnace at 800˚C was investigated by secondary electron microscopy in Figure 9. From the picture an expanded layer distance of about 1 - 2 mm was estimated.
Figure 6. Temperature of maximum rate of combustion T max comb in relation with the H3PO4 content for
Figure 7. Furnace test: Specific volume ml/g versus temperature of No. 3
Figure 8. TMA: expansion% versus temperature of No. 3
From the observed averaged distance of about 1.5 mm resulted a 10 µm expanded interlayer distance Ic exp, when the enlargement of 150 was taken in account.
A rough calculation was established under the assumption, that one platelet had the area A and the thickness x1*Ic, when Ic was the interlayer distance and x1 the numbers of interlayer distances in one platelet.
The specific volume at room temperature V (RT) in the unexpanded state resulted from N1 such platelets in 1
Figure 9. SEM of No. 3 
g. After expansion at 800˚C the specific volume increased from V (RT) to V(800˚C, because the platelets expanded due to the growing thickness of x2*Ic exp by exfoliation. Therefore Equation (17) was valid.

Under the assumption that neither the number of platelets N1 nor their area A had changed during expansion Equation (17) simplified to Equation (18).

When 10 µm, the observed Ic exp, was introduced into Equation (18), then resulted 40 for (x2/x1), which meant, that platelets of about 40 layers had developed, or in the case of monolayers an expanded interlayer distance of 0.25 µm*150 = 37 µm = 0.037 mm was expected, which was not observed in Figure 9.
In Figure 10 the thermogravimetric analysis showed that exfoliation happened under a weight loss of 18%.
In a graph of lgV versus 103/T a heat of exfoliation Hexf = 15 kJ/mole or 0.014 kJ/gGIC was obtained which was half the heat of exfoliation of 0.029 kJ/gGIC at 300˚C measured by DSC investigation in Figure 11.
When the ideal gas law was applied and 240 ml/g at 800˚C from Figure 8 were introduced, 3 moles of gases were obtained according to Equation (19).

As atmospheric pressure was the only counter force against expansion, the work done by exfoliation wexf was calculated to be 0.03 kJ/g in Equation (20), when 300 ml/g the maximum specific volume of Figure 7 was taken.
Figure 10. TGA: weight residue temperature of No. 3
Figure 11. DSC: Heat mW versus temperature of No. 3

The chemical equations were set up for the theoretical intercalation and for exfoliation:
Intercalation: hinterex/cal = −/0.51kJ/gC = −/0.39kJ/gGIC
F11: 72C + H2SO4 + 4.5H2O + 0.5O =
MW: 864 + 98 + 81 + 8 = 1.051
H: −886 ? 1.291.5 + 414 = −1.763.5
Exfoliation: hexf ex/cal = 0.029/0.029 kJ/gGIC; Rex/cal = 82/82%
Exfoliation under expansion hex/cal = −/0.01kJ/gGIC
F12a: 

MW: 1.051 = 997 + 54
H: −1.763.5 + 10 = −1.024.5 − 729
Exfoliation under decomposition hex/cal = −/0.019kJ/gGIC
F12b: 
MW: 997 = 864 + 80 + 45 + 8
H: −1.024.5 + 20 = −397 − 607.5
F12ab: 
MW: 1.051 = 864 + 80 + 99 + 8
H: −1.763.5 + 30 = −397 ? 1.336.5
F11 + F12ab: 72C + H2SO4 + 4.5H2O = 72Cex + SO3 + 5.5H2O
MW: 864 + 98 + 81 = 864 + 80 + 99
H: −886 ? 1.291.5 + 444 = −397 ? 1.336.5
Decomposition and vaporization at 338˚C:
F13: H2SO4 = SO3 + H2O
MW: 98 = 80 + 18
H 86 + 246 = −397 − 243
F14: 4.5*(H2O = H2Ogas)
H 4.5*(−286 + 43 = −243)
F13 + F14: H2SO4 + 4.5H2O = SO3 + 5.5H2O
MW: 98 + 81 = 80 + 99
H: −886 ? 1287 + 439.5 = −397 ? 1336.5
Then the heats of intercalation and exfoliation were added and put in relation to the heats of decomposition and vaporization:



The formula F12 postulated 6.75 moles of evolved gases under heat evolution of 0.029 kJ/gGIC, which correlated with a weight loss of 18% measured by TGA accompanied with a heat evolution by exfoliation of about 0.029 kJ/gGIC measured by DSC and a work of expansion of 0.03 kJ/gGIC. Whereas 3 moles of gases were determined, when the ideal gas equation was applied to the expanded specific volume accompanied with 0.014 kJ/gGIC heat of exfoliation measured by the expanded specific volume in dependence of temperature. These results led to the conclusion, that exfoliation happened in two steps: one accompanied with expansion F12a and one without F12b.
In Equation (22) the lattice energy LGIC of −0.566 kJ/gC was calculated.

2.2.4. No. 4
In the TMA measurement given in Figure 12 two maxima of expansion were observed at 350˚C and 800˚C. 8.9% incorporated phosphoric acid shifted the maximum rate of combustion from 900˚C to 990˚C in Figure 6.
2.2.5. No. 5 C21FeCl3
C21FeCl3 was interpreted as a mixture of 50% C24FeCl3 (stage 3) and 50% C18FeCl3 (stage 4).
16.1 Angstrom for stage 3 and 19.45 Angstrom for stage 4 were calculated for the constitutional repeating units Ic:

Figure 12. TMA: expansion versus temperature of No. 4
The intercalation process proceeded in the absence of water and no change was observed by washing with nitro methane. For the first stage intercalation-121 kJ/mol FeCl3 building heat was cited in literature [19] .
Intercalation and exfoliation were described:
Intercalation: hinter ex/cal = −/0.515 kJ/gC = −/0.31 kJ/gGIC
hinter ex/cal = −/−0.58 kJ/gC; −0.33 kJ/gGIC
F15a: 3*6C + FeCl3 = C18FeCl3
MW: 216 + 162.5 = 378.5
H: −402 − 125 = −527
hinter ex/cal= −/−0.45 kJ/gC; −0.29 kJ/gGIC
F15b: 4*6C + FeCl3 = C24FeCl3
MW: 288 + 162.5 = 450.5
H: −402 − 130 = −532
Exfoliation: hexf ex/cal = −/0.03 kJ/gGIC expansion; 0.45 kJ/gGIC decomposition; Rex/cal = 61/61%
Exfoliation and expansion at 300˚C hex/cal = 0.03 kJ/gGIC
F16a: C18FeCl3 = C18FeCl + Cl2
MW: 378.5 = 307.5 + 71
H: −527 + 14 = −513
Exfoliation under decomposition hex/cal = −/0.48
F16b: C18FeCl = 18Cexf + FeClgas
MW: 307.5 = 216 + 91.5
H: −513 + 183 = −330
Exfoliation and expansion at 400˚C hex/cal = −/0.03 kJ/gGIC
F17a: C24FeCl3 = C24FeCl + Cl2
MW: 450.5 = 379.5 + 71
H: −532 + 14 = −518
Exfoliation and decomposition hex/cal= −/0.42 kJ/gGIC
F17b: C24FeCl = 24Cexf + FeClgas
MW: 379.5 = 288 + 91.5
H: −518 + 188 = −330
Sublimation of the dimer of ferric chloride at 315˚C:
F18: Fe2Cl6 = Fe2Cl6gas
H: −804 + 138 = −666
The molar heats of intercalation in F15 Hinter = 0.5*(−125 − 130) and exfoliation in F16 and F17 Hexf = 0.5*(14 + 183 + 14 + 188) were more or less equivalent with the heat of sublimation Hsubl in F18.

The TMA investigation in Figure 13 indicated that the GIC expanded in two steps at 210˚C (3.stage) and 400˚C (4.stage). Above 450˚C the last 5% of FeCl3 were evaporated and a maximum expansion of 2400% was reached at 500˚C. The GIC exerted its maximum speed of combustion at 900˚C.
The sample in the muffle furnace obeyed Equation (25)

When the data of Equation (25) were plotted according to Arrhenius 0.033 kJ/gGIC was determined as the heat of exfoliation.
Figure 13. TMA: expansion% versus temperature of No. 5 C21FeCl3, 20 K/min, air.
The specific volume at 800˚C was applied to the ideal gas law and 0.94 mole of gas was the result of the calculation in Equation (26).

0.0275 kJ/gGIC was the calculated work of exfoliation wexf in Equation (27).

The lattice energies calculated in Equation (28) were unexpected high due to the high heats of intercalation.

2.3. Manufacture and Application of Intumescent Strips as Fire Gaskets
Mixtures of 55 parts of polyethylene vinylacetate, Evatane 24-03, and 45 parts of the before investigated GICs were fed to a single screw extruder Alpha 45 from Cincinnati Company. The temperature of extrusion was 130˚C and the output 5 kg/h. The extrudates passed a rectangular die, so that strips of the perimeter 10 × 2 mm2 were obtained.
The strips were used as gaskets for gaps in a fire test simulating fire doors. All tests were performed in a furnace heated according the standard ISO temperature curve. The front side of the furnace was closed by a heat resistant 40 mm thick Calcium silicate board of Promatect. The board showed six 1000 × 8.5 × 40 mm3 gaps. Five gaps were equipped with 1000 mm long strips glued to the 40 mm wide wall in such a way that they were placed in the middle. Iron Constantan thermocouples were situated above the empty and above the five protected joints. Then a 40 mm thick Rockwool was placed above the whole front side for thermal insulation. In the fire test the gaps closed after 2 minutes at 300˚C. After closure the temperature dropped to 50˚C. When the gaps opened, a jump in temperature was detected. In Figure 14 a difference in temperature between furnace and open gap of about 500˚C and between closed and open gap of about 300˚C was observed.
3. Results and Discussion
In Table 3 all data of the five GICs were collected. Some discrepancies of the data could be explained by the
Figure 14. Fire test in gaps of 8.5 mm width, 40 mm depth and 1000 mm length: temperature of furnace, of open gap and of gaps protected by10 × 2 mm2 intumescent strips No. 1-5.
Table 3. Characteristic data of GICs.
circumstance that not all evolved gases contributed to expansion. In respect to the suitability as fire gaskets the GICs with the numbers 2 and 4, which exerted high expansion and high temperature of maximum combustion, were expected to be the most suitable. In the course of this investigation it turned out that also heavy metals were considered to be environmental harmful. Therefore No. 5 was not treated with phosphoric acid nevertheless the untreated sample was incorporated in a strip and tested as fire gasket with good results.
In Table 4 the data of the gaskets were summarized. The task was to find out the correlation between the properties of GICs and their performance as gaskets.
At a first view the gaskets based on GICs with phosphoric acid No. 2 and No. 4 showed better results, than those without. The time of closure tc increased with increasing amount of phosphoric acid.
GIC intercalated with nitric acid: 
GIC intercalated with sulphuric acid: 
The same type of dependence had been already derived for the maximum temperature of combustion, namely
GIC intercalated with nitric acid: 
When the gaskets based on GICs without phosphoric acid were further investigated, then a correlation
Table 4. Fire Gaskets: tc time until the joints remained closed and dT difference in temperature between open and protected gap.
between tc and EFmax was detected: tc increased with increasing maximum expansion EFmax independent of the chemical composition.
For GICs intercalated with nitric, sulphuric acids and ferric chloride Equation (31) was valid:

The furnace was characterized by the heat flux per area Q/F. The heat flux per area Q/F could be estimated from the difference in temperature dT between furnace and open gap using a heat transmission number a of 3 W/m2∙K for free convection and 0.04 W/m∙K for the heat conductivity 

For the protected joints the difference in temperature dT between open and protected gap was calculated according to Equation (33) for the case of 10 mm wide protective strip with a heat conductivity 

304˚C was in good agreement with the experimental values of dT in Table 4.
4. Conclusions
Intumescent strips manufactured from GICs and polyethylene vinylacetate were applied as gaskets in gaps and subdued fire tests. The strips containing GICs treated with phosphoric acid showed better fire performance than those without phosphoric acid. The strip comprising the GIC treated with phosphoric and sulphuric acids closed the gap for the longest time, which corresponded with the second highest temperature of maximum speed of combustion but the highest specific volume at 800˚C. The GIC treated with FeCl3 showed also good fire performance, but was not further considered in respect to environmental incrimination by heavy metal. Phosphoric acid was not intercalated and did not contribute to expansion, but shifted the temperature of maximum speed of combustion from 900˚C to about 1000˚C, which was important for the application as expandable GICs in intumescent gaskets. Gaskets based on GICs without phosphoric acid performed better with GICs with high expansions irrespective of chemical composition. The influence on the time of closure was significant larger for phosphoric acid than for the maximum expansion illustrated by the factors of correlation 9.5 for phosphoric acid and 0.2 for expansion factor.
Intercalation and exfoliation were measured and described by formula as well as by balances of weights and heats. The original heats of intercalation were exotherm determined in a calorimeter. After washing stable compounds with calculated heats of intercalation were obtained. Data could be given for the heat of intercalation and exfoliation, which were derived from DSC measurements, from specific volumes determined in muffle furnace and treated according to Clausius Clapeyron and from the work of exfoliation given by the product of atmospheric pressure and the volume of maximum expansion. Not all intercalated compounds contributed to expansion. High expansion was observed, when high weight loss of the intercalated compound contributing to expansion occurred. Exfoliation happened in nanoplatelets consisting of about 40 atomic layers. The heats of exfoliation were endotherm in every case. The theoretical heats of intercalation together with the heats of exfoliation corresponded with the heat of evaporation, sublimation and or decomposition of the intercalated compound. The lattice energy of the GIC was the sum of the graphite lattice energy plus the work or heat of intercalation.
Cite this paper
Heinrich Horacek, (2015) Gaskets with Expandable Graphite Treated with Nitric, Sulphuric, Phosphoric Acids and Ferric Chloride. Open Access Library Journal,02,1-21. doi: 10.4236/oalib.1101297
References
- 1. Dresselhaus, M.S. and Dresselhaus, G. (2002) Intercalation Compounds of Graphite. Advances in Physics, 51, 1-186.
http://dx.doi.org/10.1080/00018730110113644 - 2. Ebert, L.B. (1967) Intercalation Compounds of Graphite. Annual Review of Material Science, 6, 191-211.
- 3. Enoki, T., Suzuki, M. and Endo, M. (2012) Application of Graphite Intercalation Compounds. Oxford University Press, Oxford.
- 4. Kang, F., Zhang, Y.P. and Wang, H.N. (2002) Effect of Preparation Conditions on the Characteristics of Exfoliated Graphite. Carbon, 40, 1575-1581.
http://dx.doi.org/10.1016/S0008-6223(02)00023-4 - 5. Chung, D.D.L. (1987) Review-Exfoliation of Graphite. Journal of Materials Science, 22, 4190-4198.
http://dx.doi.org/10.1007/BF01132008 - 6. Rüdorff, W., Stumpp, E., Spriessler, W. and Sieke, F.W. (1963) Reaktion des Graphits mit Metallchloriden. Angewandte Chemie, 75, 130-136.
http://dx.doi.org/10.1002/ange.19630750204 - 7. Schafhäutl, C. (1840) über die Verbindungen des Kohlenstoffes mit Silicium, Eisen und anderen Metallen, welche die verschiedenen Arten von Gusseisen, Stahl und Schmiedeeisen bilden. Journal für praktische Chemie, 21, 159-174.
http://dx.doi.org/10.1002/prac.18400190125 - 8. Skowronsky, J.M. (1988) Exfoliation of Graphite CrO3 Intercalation Compound in Hydrogen Peroxide Solution. Journal of Materials Science, 23, 2243-2246.
http://dx.doi.org/10.1007/BF01115794 - 9. Skowronsky, J.M. (1997) Graphite Intercalation Compounds. In: Nalwa, H.S., Ed., Handbook of Organic Conductive Molecules and Polymers, John Wiley and Sons, New York, 621-687.
- 10. Rüdorff, W. and Hofmann, U. (1938) über Graphitsalze. Zeitschrift für Anorganische Allgemeine Chemie, 238, 1-50.
http://dx.doi.org/10.1002/zaac.19382380102 - 11. Inagaki, M., Muramatsu, K., Maeda, Y. and Maekawa, K. (1983) Production of Exfoliated Graphite from Potassium-Graphite-Tetrahydrofuran Ternary Compounds and Its Application. Synthetic Methods, 8, 335-342.
http://dx.doi.org/10.1016/0379-6779(83)90117-0 - 12. Messaoudi, A., Inagaki, M. and Beguin, F. (1991) Chemical Reduction of FeCl3-Graphite Intercalation Compounds with Potassium-Naphthalene Complexes in Tetrahydrofuran. Journal of Materials and Chemistry, 1, 735-738.
http://dx.doi.org/10.1039/jm9910100735 - 13. Kang, F., Leng, Y. and Zhang, T.Y. (1996) Influence of H2O2 on Synthesis of H2SO4-GICs. Physical Chemistry of Solids, 57, 889-892.
http://dx.doi.org/10.1016/0022-3697(95)00368-1 - 14. Zhang, W., Cui, J., Toa, C., Wu, Y., Li, Z., Wen, Y. and Li, G. (2009) A Strategy for Producing Pure Single-Layer Graphene Sheets Based on a Confined Self Assembling Approach. Angewandte Chemie, 121, 5978-5982.
http://dx.doi.org/10.1002/ange.200902365 - 15. Zeng, F., Kuang, Y., Wong, Y., Huang, Z., Fu, C. and Zhou, H. (2011) Facile Preparation of High Quality Graphene Scrolls from Graphite Oxide by Microexplosion Method. Advanced Materials, 23, 4929-4932.
http://dx.doi.org/10.1002/adma.201102798 - 16. Kagan, H.B. (1957) Graphite Insertion Compounds. Chemtech, 510-515.
- 17. Schlögl, R. (1994) Graphite—A Unique Host Lattice. In: Mueller-Warmuth, W. and Schoeller, R., Eds., Progress in Intercalation Research, Volume 14, Kluwer Academic Publishers, Dordrecht, 83-176.
http://dx.doi.org/10.1007/978-94-011-0890-4_2 - 18. Hwan, D.M. (1992) Structure and Dynamic. In: Zabel, H. and Solin, S.A., Eds., Graphite Intercalation Compounds I, Material Science, Springer-Verlag, Heidelberg.
- 19. Gmelins (1968) Handbuch der anorganischen Chemie Kohlenstoff 14C TlB Lfg3. Graphitsalze, 19, 865-912.
- 20. Boehm, H.P., Setton, R. and Stumpp, E. (1994) Nomenclature and Technology of Graphite Intercalation Compounds. Pure and Applied Chemistry, 66, 1893-1901.
http://dx.doi.org/10.1351/pac199466091893 - 21. Anderson Axdal, S.H. and Chung, D.D.L. (1987) Effect of Surface Acidity of Activated Carbon on Hydrogen Storage. Carbon, 25, 161-319.
- 22. Hennig, G.R. (1959) Interstitial Compounds of Graphite. In: Cotton, F.A., Ed., Progress in Inorganic Chemistry, Volume 1, John Wiley & Sons, Inc., Hoboken, 125-205.
http://dx.doi.org/10.1002/9780470166024.ch2 - 23. Dappe, Y.J., Basant, M.A., Flores, F. and Ortega, J. (2006) Weak Chemical Interaction and van der Waals Forces between Graphene Layers: A Combined Density Functional and Intermolecular Perturbation Theory Approach. Physical Review B, 74, 205434-205443.
http://dx.doi.org/10.1103/PhysRevB.74.205434 - 24. Novoselov, K.S., Geim, A.K., Morozov, S.V., Jiang, D., Dubonos, S.V., Grigorieva, I.V. and Fisov, A.A. (2004) Electric Field Effect in Atomically Thin Carbon Films. Science, 306, 666-669.
http://dx.doi.org/10.1126/science.1102896 - 25. Gmelins (1968) Handbuch der anorganischen Chemie Kohlenstoff 14. Graphite, 8, 432-438.
- 26. Aronson, S., Frishberg, C. and Frankl, G. (1971) Thermodynamic Properties of Graphite Bisulfate Lamellar Compounds. Carbon, 9, 715-723.
http://dx.doi.org/10.1016/0008-6223(71)90004-2 - 27. Horacek, H. and Pieh, S. (2000) The Importance of Intumescent Systems for Fire Protection of Plastic Materials. Polymer International, 49, 1106-1114.
http://dx.doi.org/10.1002/1097-0126(200010)49:10<1106::AID-PI539>3.3.CO;2-9





























