Open Access Library Journal
Vol.02 No.02(2015), Article ID:68043,8 pages
10.4236/oalib.1101102
In Vitro anthelmintic Activity of Mucuna pruriens (DC) and Canarium schweinfurthii (Engl) on Ascaris suum
B. J. Okoli1,2*, R. G. Ayo1, J. D. Habila1, S. L. Japhet3, G. I. Ndukwe1
1Department of Chemistry, Ahmadu Bello University, Zaria, Nigeria
2Department of Chemical Sciences, Bingham University, Abuja, Nigeria
3National Agency for Drug Administration and Control, Abuja, Nigeria
Email: *okolibj@binghamuni.edu.ng
Copyright © 2015 by authors and OALib.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 13 January 2015; accepted 29 January 2015; published 2 February 2015
ABSTRACT
The in vitro anthelmintic evaluation of the crude extracts and solvent partitions on Pheretima posthuma and Ascarissuum (eggs and L2 stage) respectively showed significant results at the same concentration (10, 20, 40 and 80 mg/ml). This study provides a clear evidence for usage of Mucuna pruriens and Canarium schweinfurthii as an anthelmintic. The most potent ovicidal partition of C. schweinfurthii (Engl) leaves and bark and leaves of M. pruriens (DC) is hexane (100.00 ± 0.33), chloroform (98.20 ± 0.12) and chloroform (98.70 ± 0.88) partitioned crude respectively at 80 mg/ml while the most potent larvicidal partition is hexane (0.06%), chloroform (0.1%) and chloroform (0.2%). The active compounds are predominantly in the non-polar solvent which supports the tegumental diffusing activity of the compounds.
Keywords:
Anthelmintic Activity, Mucuna pruriens, Canarium schweinfurthii, Pheretima posthuma, A. suum, Eclodibility
Subject Areas: Organic Chemistry, Pharmacology

1. Introduction
Helminth infections are among the most widespread infections in humans, distressing a huge population of the world. The majority of infections due to helminthes are generally restricted to tropical regions, cause enormous hazards to health and contribute to the prevalence of malnourishment, anemia, eosinophilia and pneumonia [1] . Ever since the advent of broad-spectrum anthelmintics in the 1960s, anthelmintics of the macrocyclic lactone family have a significantly longer residual effect in comparison with the other anthelmintics.
Over time, questions have been raised concerning the long-term impact of the massive application of these highly efficacious, broad-spectrum anthelmintic compounds on the environment. Due to the bioavailability of some of these drugs, high percentages of these substances are being excreted unchanged after oral or systemic administration [2] [3] . To this day however, we remain oblivious to what the possible long-term ecological effects of these drugs and their residues are on pasture fauna and flora [4] . The gastro-intestinal helminthes are becoming resistant to both the benzimidazole and macrocyclic lactone which are the available anthelminthic, therefore there is a foremost problem in treatment of helminthes diseases [5] . This concern, among others, is stimulating the need for alternative, more biological measures of parasitecontrol. Hence, there is an increasing demand towards new anthelminthics, which may take time before they become less effective.
In biological farming, the use of traditional synthetic drugs is not allowed, and therefore organic farmers prefer a phytopharmaceutical approach for the control of parasitic infections on their farms [6] . Nevertheless, continued efforts can be made to standardize the plant extracts with good anthelmintic activity and formulate best alternative herbal preparations to replace or complement the synthetic drugs, which are currently in use.
M. pruriens is a tropical legume known as velvet bean and devils bean. The genus Mucuna belongs to the family Leguminosae and consists of 100 species of climbing vines and shrubs. The chemical compounds responsible for the itch are a protein, mucunain [7] and serotonin. C. schweinfurthii (Burseraceae) is a wild tree found mostly in Africa, which produces fruits similar to olives. It is commonly known as African elemi; incense tree, bush candle tree or purple canary tree [8] . In Nigeria, the trees are found mostly in the north-central Nigeria which includes Pankshin, Mangu, BarkinLadi and Bokkos LGAs of Plateau State as well as Niger State [9] . These plants have a wide reputation among natives of being curative for helminthiases like elephantiasis [10] , intestinal worms, genito-urinary diseases, black tongue, round worm, gonorrhea and stomach disorders [11] . The aim of this study is to establish the anthelmintic potential of M. pruriens and C. schweinfurthii of the crude extract on Pheritima posthuma and partioned crude on two stages of A. suum.
2. Materials and Methods
2.1. Plants
The leaves of M. pruriens and C. schweinfurthii were procured from University of Ibadan Botanical garden and the Pankshin area of Jos, Plateau staterespectively. The plant materials were identified and authenticated by the curator; Mallam Musa Mohammed in the Department of Biological Sciences, Ahmadu Bello University Zaria, Kaduna State. Voucher numbers of 392 and 7232 were deposited for M. pruriens and C. schweinfurthii respectively.
2.2. Extraction of Mucuna pruriens and Canarium schweinfurthii
The powdered plant materials were extracted with methanol using both the soxhlet and maceration method. Crude extract of M. pruriens leaves and C. schweinfurthii leaves and bark were subjected to column fractionation with solvent of different polarity starting with n-hexane, chloroform, ethylacetate and methanol, in order of increasing polarity. The different solvent fraction obtained were evaporated to dryness and subjected to bioassays.
3. Biological assay
3.1. Helminthes
The helminthes were collected according to the method described by Jabbar et al. [12] . Briefly, the P. posthuma and A. suum were identified by Mallam Yusuf Magagi, Department of Veterinary medicine, Parasitology and Entomology, Ahmadu Bello University Zaria.
3.2. Anthelmintic studies on Pheretima posthuma
This was carried out according to the method described by Marie-Magdeleine et al. [13] , to establish the potency of the plant as claimed by the ethno-medicinal usage. The choice of P. posthuma is as a result of the anatomical similarity to that of the parasite.
3.3. Egg Hatch Assay
The egg hatch assay was conducted as published by McGaw et al. (2007) [14] and Bizimenyera et al. (2006) [15] . The counted number of eggs in a 0.5 mL of egg suspension was pipetted into a 96-well microtitre plate. Wells 1 to 3 were used for Mucuna pruriens leaves experiments, well 5 to 7 for Canarium schweinfurthii leaves experiments, wells 9 to 11 Canarium schweinfurthii bark and wells 12 to 14 were used for both negative and positive controls. In addition, 0.5 mL of plant extract at different concentrations of 10, 20, 40 and 80 mg/mL were added. A commercial anthelmintic drug Albendazole ® (Levamisole, Afrivet, South Africa) was used as the positive control at the same concentrations and DMSO water used as a negative control. All tests were repeated 3 times.
The plate was incubated under humidified conditions at 25˚C temperature for 48 hours thereafter a drop of Lugol’s iodine solution was added to each well to stop further hatching. All unhatched eggs and L1 larvae were then counted.
Inhibition percentages were calculated using a formula by Cala et al. (2012) [16] .
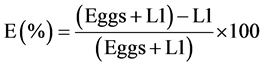
3.4. Larval Development Assay
The larval development assay was conducted as described by Bizimenyera et al. (2006) [15] . The counted number of eggs in a 0.5 mL of the egg suspension was put into each well in a 96-microtitre plate with a 100 μl of lyophilized penicillin-streptomycin to combat fungal growth. The contents of the wells were then mixed, and the plates placed in an incubator under humidified conditions at ambient temperature for 48 hours for incubation of the eggs. After 48 hours, 0.5 mL of the extracts of Mucuna pruriens and Canarium schweinfurthii as well as Albendazole ® (Levamisole, Afrivet, South Africa) as a positive control at 10, 20, 40 and 80 mg/mL were added to respective plates. The negative control plates received 0.5 mL of DMSO. All experiments were replicated three times. Incubation of the plates was continued for 21 days, after which all the plates were examined to determine the survival of larvae at different concentrations. All the L3 stage larvae in each well were counted and a percentage inhibition of larval development was calculated using the formula (Cala et al., 2012) [16] :
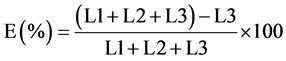
4. Result and Discussion
The hexane partitioned crude of C. schweinfurthii (Engl) and the chloroform partitioned crude of C. schweinfurthii (Engl) are yellowish oil and white crystals containing tannins and, flavonoids and tannins respectively while ethylaceate and methanol indicates the presence of alkaloid, cardiac glycoside, tannins, saponins and flavonoids which are potential antinematocidal compounds [16] .
4.1. In Vitro Anthelmintic Activity of the crude Extract on Pheretima posthuma
The Soxhletleave extract of M. pruriens (DC), C. schweinfurthii (Engl) and barks of C. schweinfurthii (Engl); time for paralysis at 10 mg/ml were 14.75 ± 0.71, 22.00 ± 0.41 and 27.00 ± 0.32 minutes, while death occurred at 22.00 ± 0.41, 30.50 ± 0.12 and 145.00 ± 0.07, minutes respectively. Macerated leave extract of M. pruriens (DC), C. schweinfurthii (Engl) and C. schweinfurthii (Engl) barks, paralysis time at 10 mg/ml were 7.50 ± 0.11, 14.00 ± 0.41 and 17.00 ± 0.30 minutes, whereas death took place at 22.00 ± 0.41, 66.00 ± 0.31, 36.5 ± 0.40 minutes respectively. The time for paralysis and death for albendazole at 10 mg/ml were 15.00 ± 0.50 and 35.00 ± 0.40 minutes respectively.
The crude extracts of the plants showed a significant anthelmintic activity (Table 1). The macerated extract contains thermolabile compounds that are potential anti-nematodical agent which its biological activity have been lost or affected by heat.
Crude macerated and soxhlet extract of M. pruriens (DC) showed significant difference in the time for paralysis but no significant difference in the time for death; this indicates that both extracts contains different tegumental diffusing agent (Table 1).
Table 1. In-vitro anthelmintic activity of various extracts on Pheretima posthuma.
MP: M. pruriens (DC) leaves; CSL: C. schweinfurthii (Engl) leaves; CSB: C. schweinfurthii (Engl) bark.
Alkaloids were absent in the soxhlet extract and research on HL60 tissue-culture cells have shown that some alkaloids block the narrow region of the channels, which subsequently cause muscle contractions in nematodes.
This leads to worm paralysis in a contractile state and, once rendered immobile, the worms are expelled (Martin and Robertson, 2007).The crude macerated and soxhlet extracts caused death in 22.5 ± 0.41 minutes, this is an indication that both extracts contains similar death-inducing compounds.
The macerated and soxhlet extracts of M. pruriens (DC) leaves contain more potent antinematodal agent than C. schweinfurthii (Engl) leave and bark extracts; and the positive control (Table 1). Generally the extracts obtained through the cold extraction procedure (maceration) are more potent compared to the extracts obtained by hot-continuous process. These indicate that the type of extraction and temperature plays an important role on the anthelmintic activity of various crude plant extracts (Table 1).
4.2. Effect of the extracts on the Unembryonated Eggs of Ascaris suum
The effect of the leaves and bark of C. schweinfurthii (Engl) and leaves of M. pruriens (DC) on the unembryonated A. suum egg are presented in Figures 1-3 and Table 2 as percentage inhibition. The partitioned crude extract of C. schweinfurthii (Engl) leaves and bark and Mucuna puriens (DC) yielded four, three and three partitioned crude respectively.
The most potent partition of C. schweinfurthii (Engl) leaves and bark, and leaves of M. pruriens (DC) are hexane, chloroform and chloroform partitioned crude respectively. There is no significant difference in the percentage inhibition of unembryonated eggs of A. suum for the C. schweinfurthii (Engl) leaves, C. schweinfurthii- (Engl) bark and M. pruriens (DC) leaves at all concentrations for all the partition extract. The methanol partition crude of C. schweinfurthii (Engl) bark, and chloroform and ethylacetate partition crude of M. pruriens (DC) leaves showed a significant difference at all concentration.
The methanol partitioned crude of C. schweinfurthii (Engl) bark showed a very low activity (Figure 2), which is an indication of the absence of ovicidal phytochemical agents (Table 2). The in vitro test of all the partition crude ovicidal activity at all tested concentrations and caused complete lyses of the eggs. Statistical differences (P < 0.05) were also observed among plant extracts.
The high activities of the partitioned crudeare due to the presence of potential antinematocidal as reported in the work of Athanasiadou et al. [17] , which target the unique molecular and physiological pathways of parasites.
4.3. Effect of the extracts on the Second Stage (L2) of A. suum
The bioactivity accounts for 20.06% of the total variance at P value are <0.0001. If bioactivity has no effect overall, there is a less than 0.01% chance of randomly observing an effect on the eclodibility. As the concentration of the partitioned crude increases, it reduces the mean hatching rate. The increase concentration accounts for 77.65% of the total variance at P value is <0.0001.
Table 2. Percentage inhibition of unembryonated eggs of A. suum.
Figure 1. Effect of partitioned crude of CSL on unembryonated eggs of A. suum.
Figure 2. Effect of partitioned crude of CSB on unembryonated eggs of A. suum.
Figure 3. Effect of partitioned crude of MP on unembryonated eggs of A. suum.
The effect is considered extremely significant. At all dose the most potent partitioned crude of the leaves of C. schweinfurthii (Engl), bark of C. schweinfurthii (Engl) and leaves of M. pruriens (DC) are the hexane, chloroform and methanol crude (Figures 4-6). The partitioned crude showed a concentration dependent activity (Figures 4-6).
For all the extracts, percentage eclodibility was lower than 10% at all concentrations except for methanol column fraction of C. schweinfurthii (Engl) bark with (Figure 5). An increase in concentration was characterized by a decrease in eclodibility rate, and there was a significant difference (P > 0.05) for the different concentrations tested. The most potent partitioned crudes showed a comparable potency with the reference drug, albendazole at all dose (Figure 7).
Figure 4. Effect of CSL on the eclodibility of L2 stage of Acaris suum.
Figure 5. Effect of CSB on the eclodibility of L2 stage of Acaris suum.
Figure 6. Effect of MP on the eclodibility of L2 stage of Acaris suum.
Figure 7. Effect of Albendazole on L2 stage of Acaris suum.
5. Conclusion
Non standardized procedures of extraction may lead to the degradation of the phytochemicals present in the plants and may lead to variations, thus leading to the lack of reproducibility (Table 1). This information can better equip entho-medicinal practitioners with practice and reduce the huge medical bills incurred in the treatment of this tropical infection, and hence alleviate poverty. The unembryonated egg of A. suum is the non- infectious stage of the parasite; the objective is to prevent the unembryonated eggs from developing into infectious embryo of A. suum (L1 stage). Overall, the column fractions of the C. schweinfurthii (Engl) and Mucuna puriens (DC) which were in vitro evaluated in the present study had ovicidal and larvicidal activities on A. suum, which were the stages of dissemination into the environment. The partitioned crudes were more ovicidal than the reference drug, but showed similar larvicidal potential with the reference drug. It is thought that these extracts penetrate the eggshell and stop the development of unembryonated eggs in the same way as albendazole (Dupouy-Camet, 2000). The present in vitro results suggest that the extracts can affect the biology of the parasitic eggs and larvae. Nonetheless, further experiments are still to isolate and characterize these anthelmintic compounds.
Cite this paper
B. J. Okoli,R. G. Ayo,J. D. Habila,S. L. Japhet,G. I. Ndukwe, (2015) In Vitro Anthelmintic Activity of Mucuna pruriens (DC) and Canarium schweinfurthii (Engl) on Ascaris suum. Open Access Library Journal,02,1-8. doi: 10.4236/oalib.1101102
References
- 1. Bundy, D.A. (1994) Immunoepidemiology of Intestinal Helminthic Infection I: The Global Burden of Intestinal Nematode Disease. The Royal Society of Tropical Medicine and Hygiene, 8, 259-261.
http://dx.doi.org/10.1016/0035-9203(94)90069-8 - 2. Farkas, R., Gyurcso, A. and Borzsonyi, L. (2003) Fly Larvicidal Activity in the Faeces of Cattle and Pigs Treated with Endectocide Products. Medical and Veterinary Entomology, 17, 301-306.
http://dx.doi.org/10.1046/j.1365-2915.2003.00443.x - 3. Plumb, D.C. (2008) Plumb’s Veterinary Drug Handbook. 6th Edition, Blackwell Publishing, Vancouver.
- 4. Spratt, D.M. (1997) Endoparasite Control Strategies: Implications for Biodiversity of Native Fauna. International Journal for Parasitology, 27, 173-180.
http://dx.doi.org/10.1016/S0020-7519(96)00147-6 - 5. Sondhi, S.M., Shahu, R. and Archana, M. (1994) Anti-Amoebic and Anthelmintic Evaluation of Heterocyclic Compounds Containing Nitrogen and/or Sulphur. Indian Drugs, 31, 317-320.
- 6. Van Krimpen, M.M., Binnendijk, G.P., Borgsteede, F.H. and Gaasenbeek, C.P. (2010) Anthelmintic Effects of Phytogenic Feed Additives in A. suum Inoculated Pigs. Veterinary Parasitology, 168, 269-277.
http://dx.doi.org/10.1016/j.vetpar.2009.11.004 - 7. Agharkar, S.P. (1991) Medicinal Plants of Bombay Presidency. Scientific Publication, Jodhpur, 1-2.
- 8. Weeks, A., Daly, D.C. and Simpson, B.B. (2005) The Phylogenetic History and Biogeography of the Frankincense and Myrrh Family (Burseraceae) Based on Nuclear and Chloroplast Sequence Data. Molecular Phylogenetics and Evolution, 35, 85-101.
http://dx.doi.org/10.1016/j.ympev.2004.12.021 - 9. Keay, R.W.J. (1989) Trees of Nigeria. 2nd Edition, C. Krendon Press, Oxford, 335-336.
- 10. Oudhia, P. (2002) Kapikachu or Cowhage (M. pruriens) Crop Fact Sheet. Version of 5-9.
- 11. Warrier, P.K., Nambiar, V.P.K. and Ramankutty, C. (1996) Indian Medicinal Plants. Vol. 4, Orient Longman, Chennai, 68-72.
- 12. Jabbar, A., Zaman, M.A., Iqbal, Z., Yassen, M. and Shamim, A. (2007) Anthelmintic Activity of Chemopodium album (L.) and Caesalpinia crista (L.) against Trichostrongylid Nematodes of Sheep. Journal of Ethnopharmacology, 114, 86-91.
http://dx.doi.org/10.1016/j.jep.2007.07.027 - 13. Marie-Magdeleine, C., Hoste, H., Mahieu, M., Varo, H. and Archimede, H. (2009) In Vitro Effects of Cucurbita moschata Seed Extracts on Haemonchus contortus. Veterinary Parasitology, 161, 99-105.
http://dx.doi.org/10.1016/j.vetpar.2008.12.008 - 14. McGaw, L.J., Rabe, T., Sparg, S.G., Jager, A.K., Eloff, J.N. and van Staden, J. (2000) An Investigation on the Biological Activity of Combretum Species. Journal of Ethnopharmacology, 75, 45-50.
- 15. Bizimenyera, E.S., Githiori, J.B., Eloff, J.N. and Swan, G.E. (2006) In Vitro Activity of Peltophorum africanum Sond. (Fabaceae) Extracts on the Egg Hatching and Larval Development of the Parasitic Nematode Trichostrongylus Colubriformis. Veterinary Parasitology, 142, 336-343.
- 16. Cala, A.C., Chagas, A.C.S., Oliveira, M.C.S., Matos, A.P., Borges, L.M.F., Sousa, L.A.D., Souza, F.A. and Oliveira, G.P. (2012) In Vitro Anthelmintic Effect of Melia azedarach L. and Trichilia classenii C. against Sheep Gastrointestinal Nematodes. Experimental Parasitology, 130, 98-102.
- 17. Hoste, H., Jackson, F., Athanasiadou, S., Thamsborg, S.M. and Hoskin, S.O. (2006) The Effects of Tannin-Rich Plants on Parasitic Nematodes in Ruminants. Review. Trends in Parasitology, 22, 253-261.
- 18. Athanasiadou, S. and Kyriazakis, I. (2006) Medicinal Plants for Helminth Parasite Control: Facts and Fiction. Animal, 1, 1392-1400.
- 19. Dupouy-Camet (2000) Pyrexie4. 117-121.
NOTES

*Corresponding author.









