Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2009, 2, 318-322 doi: 10.4236/jbise.2009.25047 Published Online September 2009 (http://www.SciRP.org/journal/jbise/ JBiSE ). Published Online September 2009 in SciRes.http://www.scirp.org/journal/jbise Dendritic compound of triphenylene-2,6,10-trione ketal-tri-{2,2-di-[(N-methyl-N-(4-pyridinyl) amino) methyl]-1,3-propanediol}: an easily recyclable catalyst for Morita-Baylis-Hillman reactions Rong-Bao Wei, Hong-Lin Li*, Ya Liang, Yan-Ru Zang School of Chemistry and chemical Engineering, Tianjin Institute of Technology, Tianjin, China. Email: hlli515@163.com Received 11 April 2009; revised 10 May 2009; accepted 13 May 2009. ABSTRACT A novel Dendritic Compound (2) of triphenylene- 2,6,10-trione ketal-tri-{2,2-di-[(N-methyl-N-(4-py- ridinyl) amino)methyl]-1,3-propanediol} was conveniently synthesized by aromatization of cyclohexanedione mono-ketal, ketal-exchange reaction with 2,2-dibromomethyl-1,3-propane- diol and nuclophilic substitution with N-methy- laminopyridine as nuclophilic reagent. The Mo- rita-Baylis-Hillman reaction of various aryl al- dehydes with methyl vinyl ketone and acryloni- trile in (DMF/cyclohexane, 1/1, v/v) has been investigated by using Dendritic Compound (2) as catalyst. The corresponding Morita-Baylis- Hillman adducts was obtained in good yields by using the recycled and reactivated dendritic catalyst. Keywords: Morita-Baylis-Hillman Reaction; Methyl Vinyl Ketone; Aryl Aldehydes; Dendritic Compound; DMAP 1. INTRODUCTION The Morita-Baylis-Hillman reaction possesses cheap and easy availability of starting materials, easing perform- ance, atom economy, forming chemo-specific functional groups in the product, providing an avenue for introduc- ing asymmetry, and fitting for simulation on the solid phase as a prelude for combinatorial synthesis represent some of the reasons, which have led to an exponential increase in the synthetic utility of this reaction [1,2,3,4,5, 6,7,8,9,10]. The Morita-Baylis-Hillman reaction can be promoted by using organic bases. However, almost all the Morita-Baylis-Hillman reactions reported so far use small molecular homogeneous catalyst, and it impedes the reusing of the catalyst. In addition, this reaction could be accomplished in a perfect atom-economic way if a recyclable Lewis base was employed as the promoter. Recently, Corma et al. [11] developed a heterogeneous catalyst system by using an insoluble Merrifield type resin-supported 4-(N-benzyl- N-methyl amino)pyridine as reusable catalyst for the Morita-Baylis-Hillman cou- pling of aromatic aldehydes and unsaturated ketones. Shi [12] reported the use of soluble polymer-supported Lewis bases, such as PEG4600-(PPh2)2 and linear poly (DMAP) in the Morita-Baylis-Hillman reactions of N- tosylimines with unsaturated ketones. Yang [13] employed the dendritic Lewis base as the catalyst together with a binary solvent system (DMF- cyclohexane, 1:1, v/v) ,which could become homogene- ous when heated up to 60◦C and then could be readily separated by cooling the system to room temperature. Several other examples of immobilized praline are re- ported in literature: poly-(ethylene glycol)-supported praline [14], proline immobilized on polyethyleneglycol grafted on cross-linked polystyrene [15], proline immo- bilized on mesoporous silica [16], polystyrene-supported praline [17] or polymer-supported proline-decorated dendrons [18,19]. Dendritic compound of triphenylene-2,6,10-trione ketal-tri-{2,2-di-[(N-methyl-N-(4-pyridinyl) amino) me- thyl]-1,3-propanediol} provided the following key ad- vantages: a) reaction system provides complete miscibil- ity under the reaction temperature; b) the structure of dendrier catalyst is well-defined; c) the dosage of cata- lyst is less than other catalysts. 2. RESULTS AND DISCUSSION We chose a dendritic compound of triphenylene-2,6, 10-trione ketal-tri-{2,2-di-[(N-methyl-N-(4-pyridinyl) amino)methyl-1,3-propanediol} 2 as catalyst for Mo- rita-Baylis-Hillman reaction, which was conveniently prepared according to following synthesis procedure (Scheme 1). 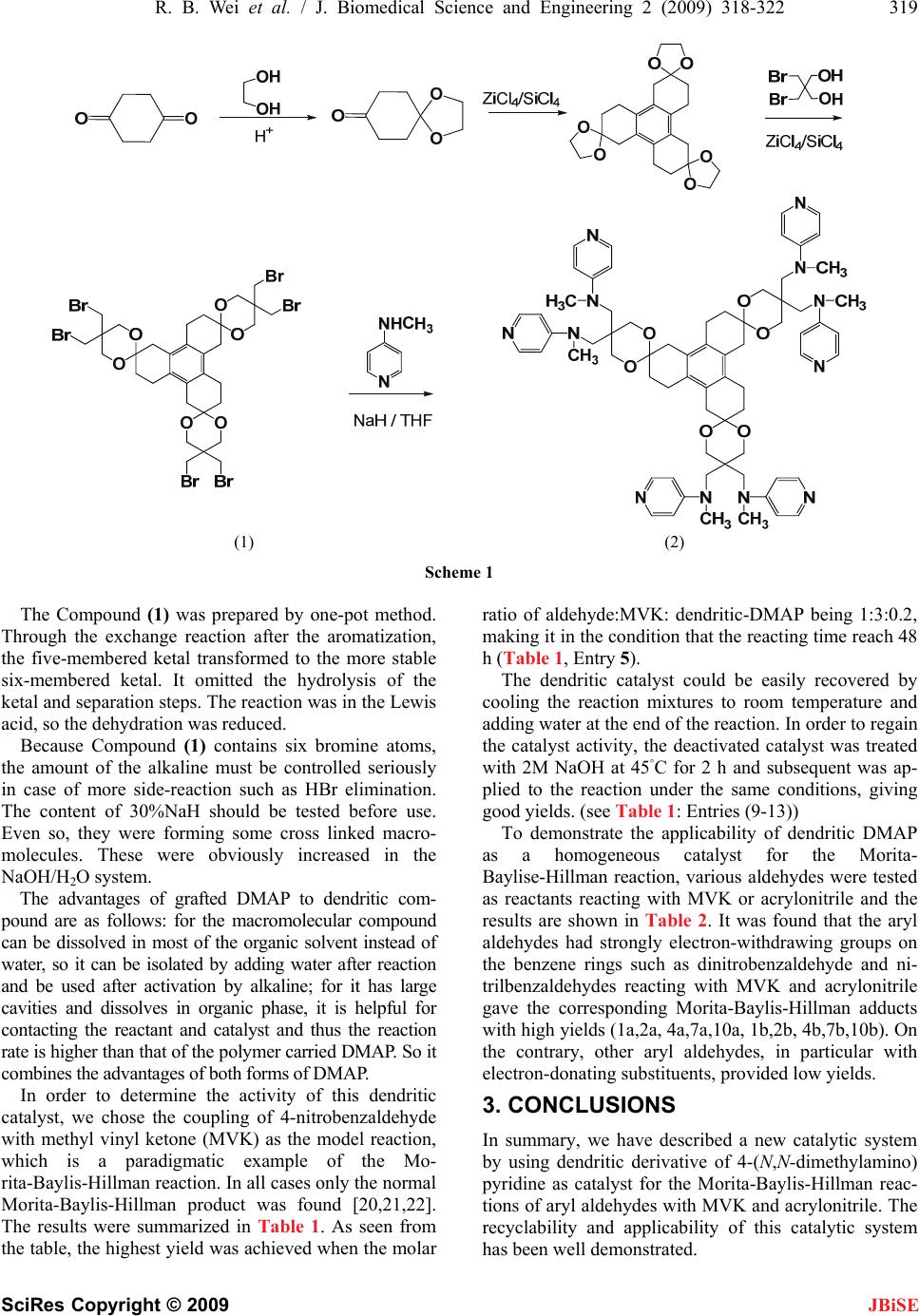 R. B. Wei et al. / J. Biomedical Science and Engineering 2 (2009) 318-322 319 SciRes Copyright © 2009 JBiSE (1) (2) Scheme 1 The Compound (1) was prepared by one-pot method. Through the exchange reaction after the aromatization, the five-membered ketal transformed to the more stable six-membered ketal. It omitted the hydrolysis of the ketal and separation steps. The reaction was in the Lewis acid, so the dehydration was reduced. Because Compound (1) contains six bromine atoms, the amount of the alkaline must be controlled seriously in case of more side-reaction such as HBr elimination. The content of 30%NaH should be tested before use. Even so, they were forming some cross linked macro- molecules. These were obviously increased in the NaOH/H2O system. The advantages of grafted DMAP to dendritic com- pound are as follows: for the macromolecular compound can be dissolved in most of the organic solvent instead of water, so it can be isolated by adding water after reaction and be used after activation by alkaline; for it has large cavities and dissolves in organic phase, it is helpful for contacting the reactant and catalyst and thus the reaction rate is higher than that of the polymer carried DMAP. So it combines the advantages of both forms of DMAP. In order to determine the activity of this dendritic catalyst, we chose the coupling of 4-nitrobenzaldehyde with methyl vinyl ketone (MVK) as the model reaction, which is a paradigmatic example of the Mo- rita-Baylis-Hillman reaction. In all cases only the normal Morita-Baylis-Hillman product was found [20,21,22]. The results were summarized in Table 1. As seen from the table, the highest yield was achieved when the molar ratio of aldehyde:MVK: dendritic-DMAP being 1:3:0.2, making it in the condition that the reacting time reach 48 h (Table 1, Entry 5). The dendritic catalyst could be easily recovered by cooling the reaction mixtures to room temperature and adding water at the end of the reaction. In order to regain the catalyst activity, the deactivated catalyst was treated with 2M NaOH at 45◦C for 2 h and subsequent was ap- plied to the reaction under the same conditions, giving good yields. (see Table 1: Entries (9-13)) To demonstrate the applicability of dendritic DMAP as a homogeneous catalyst for the Morita- Baylise-Hillman reaction, various aldehydes were tested as reactants reacting with MVK or acrylonitrile and the results are shown in Table 2. It was found that the aryl aldehydes had strongly electron-withdrawing groups on the benzene rings such as dinitrobenzaldehyde and ni- trilbenzaldehydes reacting with MVK and acrylonitrile gave the corresponding Morita-Baylis-Hillman adducts with high yields (1a,2a, 4a,7a,10a, 1b,2b, 4b,7b,10b). On the contrary, other aryl aldehydes, in particular with electron-donating substituents, provided low yields. 3. CONCLUSIONS In summary, we have described a new catalytic system by using dendritic derivative of 4-(N,N-dimethylamino) pyridine as catalyst for the Morita-Baylis-Hillman reac- tions of aryl aldehydes with MVK and acrylonitrile. The recyclability and applicability of this catalytic system has been well demonstrated.  320 R. B. Wei et al. / J. Biomedical Science and Engineering 2 (2009) 318-322 SciRes Copyright © 2009 Table 1. Morita-Baylis-Hillman Reactions of 4-nitrobenzaldehyde (1.0 equiv.) with MVK in the pre- sence of Cat 1. O 2 NCHO + O Cat 1 DMF-cyclohexane O OH O 2 N Entry Aldehyde: MVK: Cat 1(mol) Time (d) Ylide(%) 1 1:1:0.2 0.5 30.5 2 1:2:0.2 0.5 45.3 3 1:3:0.2 0.5 49.7 4 1:4:0.2 0.5 50.1 5 1:3:0.2 1 88.6 6 1:3:0.2 2 90.5 7 1:3:0.2 3 90.0 8 1:3:0.2 4 89.4 9 1:3:0.2 2 90.5 10 1:3:0.2 2 90.5 11 1:3:0.2 2 90.5 12 1:3:0.2 2 90.5 13 1:3:0.2 2 90.5 Cat 1 is the Compound (2) Table 2. The Baylis-Hillman reactions of aryl aldehydes with active alkene in the presence of Cat 1. ArCHO + EWG Cat 1 DMF-cycloh exane EWG OH Ar Enty Ar EWG Time(d) Yield(%) 1 4-NO2-C6H4CHO 2 1a 92.2 2 2-NO2-C6H4CHO 2 2a 92.2 3 3-NO2-C6H4CHO 2 3a 82.2 4 4-CN-C6H4CHO 2 4a 90.2 5 4-Cl-C6H4CHO 2 5a 82.2 6 4-Br-C6H4CHO 2 6a 72.2 7 4-NO2-C5H4NCHO 2 7a 90.2 8 4-CH3O-C6H4CHO 2 8a 32.2 9 3-CH3O-C6H4CHO 2 9a 52.2 10 3,4-dinitro-C6H3CHO COCH3 2 98.5 11 3,4-dinitro-C6H3CHO CN 2 97.9 12 4-NO2-C6H4CHO CN 2 95.2 13 2-NO2-C6H4CHO CN 2 94.7 14 3-NO2-C6H4CHO CN 2 87.9 15 4-CN-C6H4CHO CN 2 89.2 16 4-Cl-C6H4CHO CN 2 84.2 17 4-Br-C6H4CHO CN 2 79.5 18 4-NO2-C5H4NCHO 2 7b 95.3 19 4-CH3O-C6H4CHO 2 8b 40.3 20 3-CH3O-C6H4CHO 2 9b 56.2 21 C6H5CHO COCH3 2 52.6 22 C6H5CHO CN 2 47.2 Cat 1 is the Compound (2) JBiSE  R. B. Wei et al. / J. Biomedical Science and Engineering 2 (2009) 318-322 321 SciRes Copyright © 2009 JBiSE 4. EXPERIMENTAL 4.1. General Remarks Aryl aldehydes and acrylonitrile were stored under a nitrogen atmosphere before using. Other commercially supplied reagents were used as 4supplied without further purification. Organic solvents were dried by standard methods when necessary. 1H NMR spectra were recorded on an INOVA400 spectrometer for solution in CDCl3 with TMS as internal standard. Varian MAT 44S MS; Cavlo Eba 1106 ele- mental analysis instrument; WRR melting point appara- tus (Shanghai Precision Instrument Co. Ltd.). 4.2. Synthesis of Dendrimer Compound (2) ZrCl4/SiCl4 (0.5g, 1:1 in weight) was added to dissolving 1,4-cyclohexane-dione mono-ketal ethylene diol (1.56g, 10 mmol) in anhydrous ethanol (100mL). It was refluxed for 10~12 h and controlled by TCL until the 1, 4-cyclo- hexane-dione mono-ketal ethylene diol almost disap- peared. After cooling, 2,2-dibromomethyl-1,3-pro- panediol (3.93g, 15 mmol) was added and refluxed for 9~12 h. Then cooling to room temperature, Ethanol was removed to 1/3 by evaporation. The raw product was crystallized by isopropyl alcohol. Compound (1) (2.95g) was obtained. Yield: 88.2%. M.p.:247~248℃ (decom- posed). M+ :1013, C%: 39.14(39.08), H%:4.20(4.17), Br%: 47.27(47.28). IR(KBr,cm-1):2897,2860, 1465,1357; 1H- NMR (CDCl3,δppm): 3.55 (2H,s,O-CH2) 2.62 (2H, s,Br -CH2), 2.02 (2H,t, J = 7.4 Hz CH2), CH2), 1.85(2H,t, J = 7.4 Hz CH2), 1.52(2H,s,CH2). In the protection of N2, 30 % NaH (0.48 g, 0.006 mol) was added to dissolving N-methyl-4-aminopyridine (0.65 g,6 mmol) in anhydrous THF (100 mL). It was refluxed for 1~2 h. After cooling, Compound (1) (1.01g, 1mmol) was added and refluxed for 10~15 h until N-methyl-4-amino pyridine could not be examined by TCL. THF was removed to 2/3 by evaporation. After cooling and filtering, the product was crystallized by CHCl3:C2H5OH. Compound (2) (0.98g) was obtained. Yield: 83.5%. M.p.>300(decomposed).℃ M+:1176.5, C%: 70.44(70.38), H%: 7.23(7.19), N%: 14.30 (14.27 .IR(KBr,cm-1):3112, 3008,2875, 2861, 1605,1465,1375; 1H-NMR (CDCl3,δppm): 8.41(2H, d, J = 8.0 Hz, Ar), 7.85(2H, d, J = 8.0 Hz, Ar), 3.54 (2H, s,O-CH2) 2.64(2H,s,N-CH2), 2.02(2H,t, J = 7.4 Hz CH2), 1.98(2H,s, N-CH2),1.85(2H,t, J = 7.4 Hz CH2), 1.52 (2H,s,CH2). 4.3. General Procedure for the Mo- rita-Baylis-Hillman Reaction of Aryl Aldehydes with MVK and Acrylonitrile To a 50ml bottom flask charged with aryl aldehydes (5.0 mmol) and dendritic DMAP (0.2 mmol) in 20ml DMF was added methyl vinyl ketone (MVK) (15.0 mmol) or acrylonitrile (15.0 mmol) under nitrogen atmosphere and the reacted mixture was stirred 48 h at 60◦C. At the end of the reaction, the reacted mixture was cooled back to 25◦C and then adding 20ml water to the reacted mixture. The catalyst was recovered by filtration. The product was remained in the DMF/water layer, the solvent was removed under reduced pressure, and the residue was further purified by recrystalization 4.4. Characterization of the Morita- Baylis-Hillman Reaction Adducts Compounds 1a, 2a , 4a, 5a , 6a, 8a,11a, and 1b, 2b, 4b, 5b, 6b, 8b,11b have been successfully characterized in the literature [23]. Compound 3a 1H NMR (CDCl3, 400 MHZ, TMS): δ 2.35(3H, s, Me), 3.00 (1H, br., s, OH) 5.63 (1H, s, CH), 6.01 (1H,s, olefinic), 6.24 (1H, s, olefinic), 7.40 (1H, m, Ar), 8.12 (1H, m, Ar), 8.15 (1H, m, Ar), 8.25 (1H, m, Ar). Compound 3b 1H NMR (CDCl3, 400 MHZ, TMS): 3.00 (1H, br., s, OH) 5.63 (1H, s, CH), 6.11 (1H,s, ole- finic), 6.27 (1H, s, olefinic), 7.35 (1H, m, Ar), 8.00(1H, m, Ar), 8.10 (1H, m, Ar), 8.20 (1H, m, Ar). Compound 7a 1H NMR (CDCl3, 400 MHZ, TMS): δ 2.35 (3H, s, Me), 3.00 (1H, br., s, OH) 5.63 (1H, s, CH), 6.01 (1H,s, olefinic), 6.24 (1H, s, olefinic), 7.59 (2H, d, J = 8.4 Hz, Ar),8.62 (2H, d, J = 8.0 Hz, Ar). Compound 7b 1H NMR (CDCl3, 400 MHZ, TMS): 3.00 (1H, br., s, OH) 5.63 (1H, s, CH), 6.01 (1H,s, ole- finic), 6.24 (1H, s, olefinic), 7.50 (2H, d, J = 8.4 Hz, Ar),8.60 (2H, d, J = 8.0 Hz, Ar). Compound 9a 1H NMR (CDCl3, 400 MHZ, TMS): δ 2.00 (1H, br., s, OH), 2.13 (3H, s, Me), 3.87 (3H, s, OCH3), 5.80 (1H, s, CH), 5.83 (1H, s, olefinic), 6.02 (1H, s, olefinic), 6.87-7.47(4H, m, Ar). Compound 9b 1H NMR (CDCl3, 400 MHZ, TMS): δ 2.00 (1H, br., s, OH), 3.87 (3H, s, OCH3), 5.80 (1H, s, CH), 5.83 (1H, s, olefinic), 6.02 (1H, s, olefinic), 6.87-7.47(4H, m, Ar). Compound 10a 1H NMR (CDCl3, 400 MHZ, TMS): δ 2.35(3H, s, Me), 3.00 (1H, br., s, OH) 5.63 (1H, s, CH), 6.01 (1H,s, olefinic), 6.24 (1H, s, olefinic), 7.49 (1H, d, J = 8.4 Hz, Ar),8.12 (1H, d, J = 8.0 Hz, Ar) , 9.15 (1H, d, J = 3.0 Hz, Ar). Compound 10b 1H NMR (CDCl3, 400 MHZ, TMS): 3.20 (1H, br., s, OH) 5.70 (1H, s, CH), 6.21 (1H,s, olefinic), 6.27 (1H, s, olefinic), 7.47 (1H, d, J = 8.4 Hz, Ar),8.10 (1H, d, J = 8.0 Hz, Ar) , 8.95 (1H, d, J = 3.0 Hz, Ar). 5. ACKNOWLEDGEMENTS We appreciate the National Natural Science Foundation of China (G20472064) and the Tianjin Natural Science Foundation of China  322 R. B. Wei et al. / J. Biomedical Science and Engineering 2 (2009) 318-322 SciRes Copyright © 2009 [13] Yang, F. N., Gong, H., Tang, W. J., and Fan, Q. H. J., (2005) Molecular Cat. A: Chem., 233, 55. (040884311). [14] Benaglia, M., Cinquini, M., Cozzi, F., Puglisi, A., and Celentano, G., (2002) Adv. Synth. Catal., 344, 533. REFERENCES [1] Singh, V. and Batra, (2008) S., Tetrahedron, 64, 4511. [15] Akagawa, K., Sakamoto, S., and Kudo, K., (2005) Tet- rahedron Lett., 46, 8185. [2] Burke, M. D., Berger, E. M., and Schreiber, S. T., (2003) Science, 302, 613. [16] Calderon, F., Ferna′ndez, R., and Sa′nchez, F., (2005) Adv. Synth. Catal., 347, 1395. [3] Basavaiah, D., Rao, K. V., and Reddy, R. J., (2007) Chem. Soc. Rev., 36, 1581. [17] Font, D., Bastero, A., Sayalero, S., Jimeno, C., Pericas, M. A., (2007) Org. Lett., 9, 1943. [4] Masson, G., Housseman, C., and Zhu, J., (2007) Angew. Chem., Int. Ed., 46, 4614. [18] Kehat, T. and Portnoy, M., (2007) Chem. Commun., 2823. [5] Roy, D. and Sunoj, R. B., (2007) Org. Lett., 9, 4873. [6] Robiette, R., Aggarwal, V. K., and Harvey, J. N., (2007) J. Am. Chem. Soc., 129, 15513. [19] Giacalone, F., Gruttadauria, M., Marculescu, A. M., Anna, F., and Noto, R., (2008) Catalysis Commun., 9, 1477. [7] Leadbeater N. E. and Marco M., (2002) Chem. Rev., 102, 3217. [20] Shi, M., Li, C. Q., and Jing, J. K., (2001) Chem. Com- mun., 833. [8] McNamara, C. A., Dixon, M. J., Bradley, M., (2002) Chem. Rev., 102, 3275. [21] Krishna, P. R., Manjuvani, A., and Kannan, V., (2004) Tetrahedron Lett., 45,1183. [9] Basavaiah, D., Rao, A. J., and Satyanarayana, T., (2003) Chem. Rev., 103, 811. [22] Shi, M., Li, C. Q., and Jing, J. K., (2003) Tetrahedron, 59, 1181. [10] Singh V. and Batra S., (2003) Tetrahedron, 64, 4511. [11] Corma, A., Garcia, H., Leyva, A., (2003) Chem. Com- mun., 2806. [23] Yang, N. Y., Gong, H., Tang, W. J., Fan, Q. H., Cai, C. Q., and Yang, L. W., (2005) J Molecular Catal. A: Chem., 233, 55. [12] Huang, J. W. and Shi, M., (2003) Adv. Synth. Catal., 345, 953. JBiSE |

