 Open Journal of Urology, 2011, 1, 37-47 doi:10.4236/oju.2011.13009 Published Online August 2011 (http://www.SciRP.org/journal/oju) Copyright © 2011 SciRes. OJU Nomograms as P redictive Tools for Pros t a t e C a ncer Patients Who Had Radical Prostatectomy Saadettin Eskicorapci1, Cenk Acar1, Zafer Sinik1, Z afe r Aybek1, Michael Kattanb2 1Department of Ur ol o gy, Pamukkale University School of Medicine, Denizli, Turkey 2Department of Quantitative Health Sciences, Cleveland Clinic, Cleveland, USA E-mail: drsye@yahoo.com Received March 22, 2011; revised May 9, 2011; accepted May 18, 2011 Abstract Prostate cancer is the most common solid cancer for men in the developed countries. Radical prostatectomy is the most preferred treatment modality for localized prostate cancer. Individual decision making is neces- sary for each patient because of the diversities in the biological characteristics of the prostate cancer. The prediction of pathologic stage, prognosis and cancer specific mortality after curative therapy and quality of life issues are essential for counseling and tailoring treatment in possible candidates of radical prostatectomy. Several studies demonstrated that nomograms are the best predictive tools regarding the other prediction models. For better understanding the nomograms in radical prostatectomy patients, they should be classified according to categories for their use. PSA, Gleason grade and clinical stage are seemed to be the most im- portant prognostic factors in patients who are candidates for radical prostatectomy. Additionally, the patho- logical parameters are remarkable prognostic criteria. The Partin tables for predicting the radical prostatec- tomy pathology and Kattan nomograms for predicting the biochemical recurrences free survival rates are the most frequently used nomograms. Today, these nomograms should not replace the clinical decisions but they give significant information for the patients’ prognosis, treatment selection and follow up. Keywords: Nomogram, Prediction, Prostate Cancer, Prognosis, Radical Prostatectomy 1. Introduction Prostate cancer is the most common solid cancer for men in the developed countries [1]. Radical prostatectomy is the most preferred treatment modality for localized pros- tate cancer. Individual decision making is necessary for each patient because of the diversities of the biological characteristics of the prostate cancer. [2]. Selection of proper treatment for individual patient is crucial to im- proving the propensity of cure and survival. The predic- tion of pathologic stage, prognosis and cancer specific mortality after curative therapy and quality of life issues are essential for counseling and tailoring treatment in possible candidates of radical prostatectomy. Research- ers have developed predictive and prognostic tools that are based on statistical models for making more accurate risk estimation. Contemporarily, these tools are nomo- grams, risk groupings, artificial neural networks (ANN), probability tables such as “Partin staging tables” and CART (classification and regression tree) analyses [3-8]. Several studies demonstrated that nomograms are the best predictive tools regarding the other prediction mod- els [9,10]. 2. What Is a Nomogram? Statistically, a nomogram is defined as graphical calcu- lating scale for related mathematical formula. In medical science, nomograms are the methods for predicting spe- cific outcome (biochemical recurrences for prostate can- cer, breast cancer mortality etc.) and prognosis by using the significantly prognostic parameters of the disease. For prostate cancer, it is aimed to make an assumption by using the prostate cancer data (prostate specific antigen (PSA), digital rectal examination (DRE), Gleason score, age, race, etc.) (3). Despite the fact that nomograms are developed for each stage of the prostate cancer, they have intensively been studied for localized prostate can- cer in recent years. Commonly used nomograms are Partin nomogram (tables) for predicting the radical prostatectomy (RP) pathology and Kattan nomograms for predicting biochemical recurrences free survival [2].  38 S. ESKICORAPCI ET AL. The prediction accuracy of nomograms should absolutely be assessed and validated internally and externally. However, the application of such nomograms may be nonsense without understanding relationship between the parameters. To better understanding the nomograms in prostate cancer, they should be classified according to categories for their use (Table 1). In this review, we discussed the prediction models as- sociated with radical prostatectomy (RP). 2.1. Nomograms for Prediction of Pathological Parameters after Rp (Table 2) Radical prostatectomy is frequently preferred treatment options for organ confined prostate cancer. In order to predict the pathology of the RP and the most suitable treatments for particular patients, several nomograms have been developed [2]. In these nomograms, the Pre- operative parameters are used such as PSA, Gleason score, clinical stage, cancer volume in biopsy and PSA density. 2.1.1. The Predictions for Pathological Stage (Partin Look-up Tables and Others) In 1987, Oesterling et al. developed a multiple logistic regression analysis to predict the pathological stage by using prostate acid phosphatase (PAP) , clinical stage and Gleason grade for 275 patients and it was the first publication in this topic [11]. Later, Narayan et al. set up the probability graphics by using the clinical stage, PSA, Gleason grade and transrectal ultrasonography [12]. Sub- sequently, the “Partin” look-up tables were developed to predict the pathological stage are the frequently used models. The Partin tables are first formulated through the pa- tients’ data at Johns Hopkins University in 1993 [3]. The Partin Tables were updated in 1997, 2001 and 2007 [13-15]. The aim of the Partin Tables is to predict the pathological stage using 3 pre-operative parameters as clinical stage (TNM classification), Gleason grade and serum PSA. In clinical practice, it is performed in order to determine the probabilities for organ-confined dis- eases, seminal vesicular and lymph node involvement. The prediction accuracy of any nomogram should be tested through validation (approval) processes, is con- ducted by the other data sets or populations. The Partin tables were validated by Blute et al. at Mayo Clinic in 2000 and by Greafen et al. on European patients in 2003. They stated that use of Partin tables is appropriate for both groups [16,17]. Also, Augustin et al. firstly com- pared the two Partin tables (1997 and 2001) and vali- dated in 2004 [18]. The validation of 2007 Partin tables was accomplished by using SEER (Surveillance Epide- miology and End Results) database of American Na- tional Cancer Institute in early 2010. They found that the discrimination power of Partin tables for seminal vesical and lymph node involvement was high but is limited for predicting extracapsular extension and localized disease [19]. Owing to rising PSA screening over the years in the world, increased number of organ confined cancer was diagnosed due to early detection of prostate cancer in our Table 1. Classification of nomograms for prediction of radica l p rostatectomy relate d outc o mes. 1) Nomograms for predicting pathological parameters and stages after RP The predictions for pathological stage (Partin tables and others) Nomograms to predict the organ confined diseases and extracapsular involvement Nomograms for the prediction of SV invasion and lymph node involvement Nomograms to predict the surgical margin status Nomograms to predict the Gleason score upgrade Nomograms to predict the location of the tumor (peripheral zone and transitional zone) and tumor volume Nomograms to predict clinically insignificant cancers 2) Nomograms to predict PSA recurrence/disease free/general survival after RP Nomograms with the data prior to RP Nomograms with the data after RP 3) Nomograms to predict Prostate Cancer Specific Mortality After RP 4) Nomograms to predict the quality of life after RP RP: Radical Prostatectomy; SV: Seminal Vesicle. Copyright © 2011 SciRes. OJU 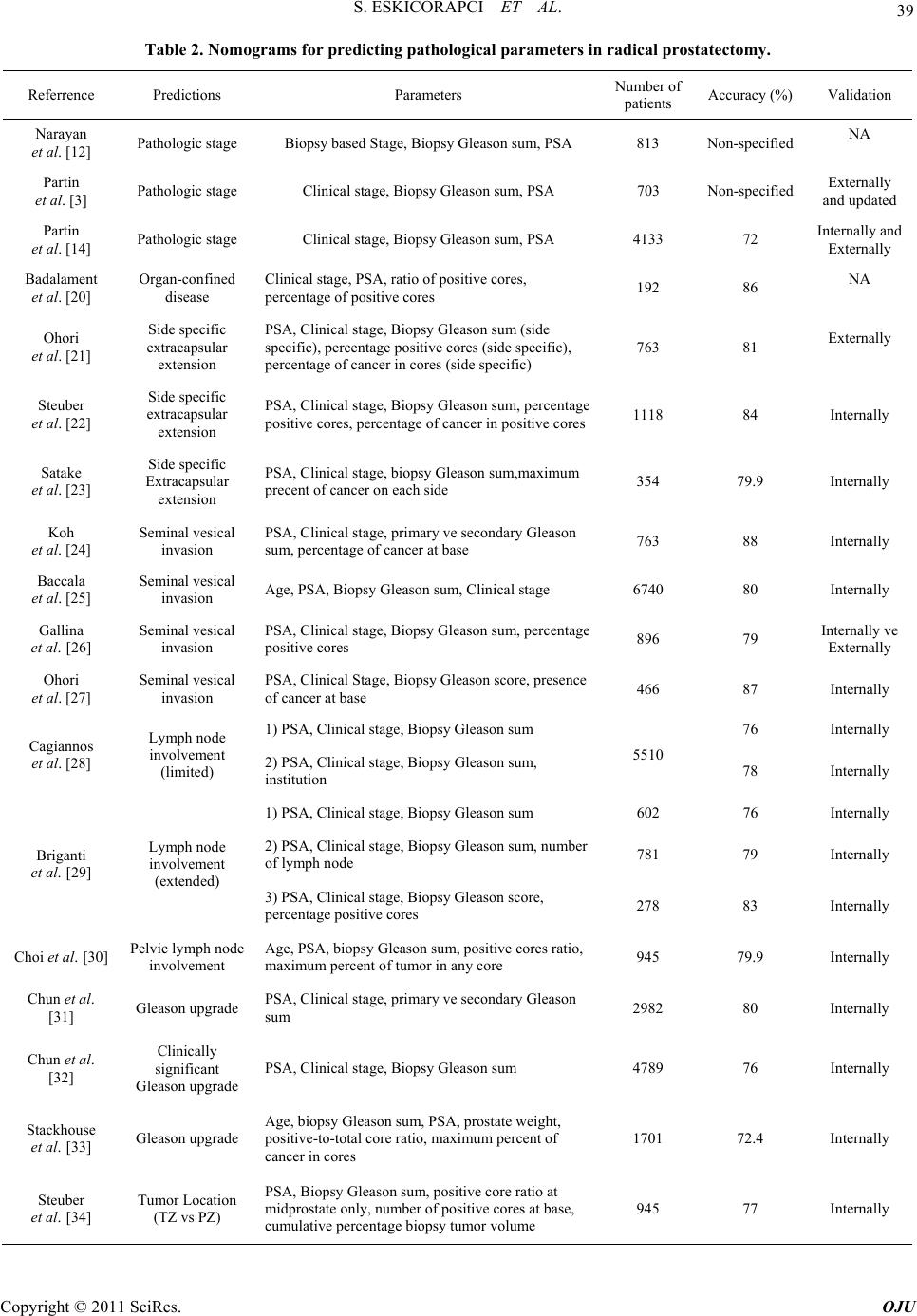 S. ESKICORAPCI ET AL. 39 Table 2. Nomograms for predicting pathological parameters in radical prostatectomy. Referrence Predictions Parameters Number of patients Accuracy (%) Validation Narayan et al. [12] Pathologic stage Biopsy based Stage, Biopsy Gleason sum, PSA 813 Non-specified NA Partin et al. [3] Pathologic stage Clinical stage, Biopsy Gleason sum, PSA 703 Non-specified Externally and updated Partin et al. [14] Pathologic stage Clinical stage, Biopsy Gleason sum, PSA 4133 72 Internally and Externally Badalament et al. [20] Organ-confined disease Clinical stage, PSA, ratio of positive cores, percentage of positive cores 192 86 NA Ohori et al. [21] Side specific extracapsular extension PSA, Clinical stage, Biopsy Gleason sum (side specific), percentage positive cores (side specific), percentage of cancer in cores (side specific) 763 81 Externally Steuber et al. [22] Side specific extracapsular extension PSA, Clinical stage, Biopsy Gleason sum, percentage positive cores, percentage of cancer in positive cores 1118 84 Internally Satake et al. [23] Side specific Extracapsular extension PSA, Clinical stage, biopsy Gleason sum,maximum precent of cancer on each side 354 79.9 Internally Koh et al. [24] Seminal vesical invasion PSA, Clinical stage, primary ve secondary Gleason sum, percentage of cancer at base 763 88 Internally Baccala et al. [25] Seminal vesical invasion Age, PSA, Biopsy Gleason sum, Clinical stage 6740 80 Internally Gallina et al. [26] Seminal vesical invasion PSA, Clinical stage, Biopsy Gleason sum, percentage positive cores 896 79 Internally ve Externally Ohori et al. [27] Seminal vesical invasion PSA, Clinical Stage, Biopsy Gleason score, presence of cancer at base 466 87 Internally 1) PSA, Clinical stage, Biopsy Gleason sum 76 Internally Cagiannos et al. [28] Lymph node involvement (limited) 2) PSA, Clinical stage, Biopsy Gleason sum, institution 5510 78 Internally 1) PSA, Clinical stage, Biopsy Gleason sum 602 76 Internally 2) PSA, Clinical stage, Biopsy Gleason sum, number of lymph node 781 79 Internally Briganti et al. [29] Lymph node involvement (extended) 3) PSA, Clinical stage, Biopsy Gleason score, percentage positive cores 278 83 Internally Choi et al. [30] Pelvic lymph node involvement Age, PSA, biopsy Gleason sum, positive cores ratio, maximum percent of tumor in any core 945 79.9 Internally Chun et al. [31] Gleason upgrade PSA, Clinical stage, primary ve secondary Gleason sum 2982 80 Internally Chun et al. [32] Clinically significant Gleason upgrade PSA, Clinical stage, Biopsy Gleason sum 4789 76 Internally Stackhouse et al. [33] Gleason upgrade Age, biopsy Gleason sum, PSA, prostate weight, positive-to-total core ratio, maximum percent of cancer in cores 1701 72.4 Internally Steuber et al. [34] Tumor Location (TZ vs PZ) PSA, Biopsy Gleason sum, positive core ratio at midprostate only, number of positive cores at base, cumulative percentage biopsy tumor volume 945 77 Internally Copyright © 2011 SciRes. OJU  S. ESKICORAPCI ET AL. Copyright © 2011 SciRes. OJU 40 Peller et al. [35] Tumor volume Biopsy Gleason sum, number positive sextant cores and PSA 102 Non-specified NA 1) PSA, primary ve secondary Gleason sum 64 Internally 2) PSA, TRUS volume, primary ve secondary Gleason sum, percentage positive core 74 Internally Kattan et al. [36] Clinically indolent cancer (tumor volume < 0.5 cm3, organ confined and gleason grad e < 4) 3) PSA, Clinical stage, TRUS volume, primary ve secondary Gleason sum, milimeter of the positive core, milimeter of the negative core 409 79 Internally country as same. Validation of the Partin nomograms has been conducted by Eskiçorapçı et al. with the participa- tion of 1043 patients from 13 different centers in Turkish population [37]. In conclusion, the urologists should be keeping in mind that the Partin tables are only beneficial for predicting the pathological stage but not prognosis or biochemical recurrences. 2.1.2. Nomograms to Predict the Organ Confined Diseases and Extracapsular Involvement Badalament et al. have developed a formula which cal- culates the probability for organ confined disease by us- ing Gleason grade, nuclear grade, PSA and tumor in- volvement rates [20]. Later, the models which was cal- culating the probability of extracapsular involvement by using the Gleason grade, age, PSA and tumor involve- ment rates have been established and some of them were validated [38]. These models are not widely used be- cause of complexity of the parameters (nuclear grade, total tumor involvement rate etc.). Partin tables may pre- dict the extracapsular involvement but it fails to locate the effected side. Therefore, Ohori et al. and Steuber et al. developed the specific prediction nomograms for side specific extracapsular involvement [21,22]. 2.1.3. Nomograms for the Prediction of SV Invasion and Lymph Node Involvement The prediction of seminal vesicle and lymph node in- volvement is very important because these patients have generally worse prognosis and the success rate of radical surgery or radiotherapy is very low. Predictions for these patients have advantages in order to select the proper adjuvant treatment. Many researchers have been devel- oped the models to predict the seminal vesicle and lymph node involvement [24-26]. However, these models could not take place in clinical practice due to diagnosing the diseases at earlier stages and founding more comprehen- sive prediction models like Partin tables. On the other hand, Ohori et al. recently developed a nomogram which predicts seminal involvement by using PSA, clinical stage, Gleason sum and cancer at the base. It was stated the accuracy of the nomogram was 87% [27]. The results of this nomogram are promising. Eventually, Cagiannos et al. developed a prediction model for limited lymph node dissection and Briganti et al. developed for extended lymph node dissection. These nomograms may be helpful to make a decision for se- lecting the patients who need lymphadenectomy [28,29]. 2.1.4. Nomograms to Predict the Surgical Margin Status The positivity of surgical margin is an important prog- nostic parameter for the prediction of PSA relapse after RP. However, none of the nomograms predicting the surgical margin has been validated to date and they are not widely used [39,40]. 2.1.5. Nomograms to Predict the Gleason Score Upgrade in RP Gleason grade of RP is generally higher than the biopsy Gleason grade. D’Amico et al. developed a nomogram to predict the Gleason score upgrade and has recently been validated [7,31]. In addition, Chun et al. have developed a model to predict the high increases in Gleason grade with their nomograms and was internally validated [32]. Stackhouse et al. conducted a nomogram by using age, PSA, prostate volume, biopsy Gleason sum, ratio of pos- itive biopsy core and maximum percentage of cancer in cores. The accuracy of nomogram was 72.4% [33]. Ca- pitonio et al. developed their nomograms by using PSA, clinical stage, primary and secondary Gleason score in biopsy. The concordance index (c-index) was calculated as 74.89% [41]. These nomograms may be used espe- cially for cryotheraphy, HIFU (high intensity focused ultrasonography) and active surveillance. 2.1.6. Nomograms to Predict the Location of the Tumor (Peripheral Zone and Transitional Zone) and Tumor Volume The fact that organ confined disease rate of the transi- tional zone prostate cancer is higher despite the high PSA levels. Steuber et al. have developed a nomogram for predicting the transitional zone prostate cancer which c-index was 77% [34]. Peller et al. have developed ano- ther nomogram to predict the tumor volume in the pros- tate. However, this nomogram could not be widely used in view of including small number of patients and data of sextant biopsy [35].  S. ESKICORAPCI ET AL. 41 2.1.7. Nomograms to Predict Clinically Insignificant Cancers The most of prostate cancers are clinically insignificant. Besides the nomograms predicting the clinically insig- nificant prostate cancers, the three nomograms were de- veloped by Kattan et al. is widely used. The nomograms are based on the criteria of Epstein et al. [36]. These nomograms may be useful for the elderly patients with high co-morbidity which require especially conservative approach. 2.2. Nomograms to Predict Biochemical Recurrence, Disease F ree and General Survival after RP (Table 3) 2.2.1. Nomograms with the Data Prior to RP After the Partin tables which were widely accepted for predicting the pathological stage, nomograms have been developed for prediction of survival which is the primary end point for prostate cancer. The most frequently used nomogram is the pre-operative Kattan nomogram, was firstly developed in 1998 [4]. The Kattan nomogram represents 5 years biochemical recurrence free survival rates by constituting PSA, clinical stage, Gleason grade. Kattan nomogram seems to have some advantages such as easy to apply, predicts progression free survival and defines the requirement of adjuvant treatments. The ac- curacy of the nomogram is increased by adding İn- ter- lökin-6 soluble receptor and transforming growth factor beta-1 [42]. In 2006, a new version of Kattan nomogram which predicts 10-years survival was published [43]. In our country, the validation of Kattan nomogram is re- cently accomplished by Eskicorapci et al. In this study the two pre-operative Kattan nomograms (developed in 1998 and 2006) validated and c-index was found as 68% and 70%, respectively [44]. The prediction model was developed by D’Amico et al. is similar to the Kattan nomogram [45]. This model predicts 10-years biochemical recurrences free survival with pre-operative PSA, Gleason grade and tumor stage. The patients are divided into three groups: 1) Low risk: Stage T1c - T2a, PSA ≤ 10 ng/mL and Gleason grade ≤ 6 (10 years progression free survival is 83%) 2) Medium risk: Phase T2b, PSA > 10 ng/mL ve < 20 ng/mL veya Gleason grade = 7 (10 years progression free survival is 46%) 3) High risk: Phase T2c, PSA ≥ 20 ng/mL veya Glea- son grade ≥ 8) (10 years progression free survival is 29%) Both nomograms (D’Amico and Kattan) predict the PSA progression but not mortality. The life survival of most patients is high despite PSA recurrence. These no- mograms are useful to identify requirement of adjuvant treatment, predict disease free survival and select the patients for clinical trial. 2.2.2. Nomograms with the Data after RP In 1999, Kattan et al. developed a nomogram to predict 5 years survival by comprising PSA, Gleason grade, cap- sular invasion, surgical margin status, seminal vesicle invasion and lymph node involvement. (56) These no- mograms have been validated and widely used [46]. Stephenson et al. represents a new nomogram to predict 10 years progression free survival with additional pa- rameters [47]. Moul et al. drew up tables predicting 3-5-7 year’s survival without recurrence in 2001 [48]. This table is involved with PSA, race, Gleason score of RP and pathological stage. The other researchers namely Han et al., Bauer et al., Blute et al. and D’Amico et al. conducted similar models [49-52]. Recently, Morieira et al. designed a study to determine whether the Postoperative nomograms are affected by race with comparison of 7 nomograms. They stated all nomograms have similar performance regardless of their racial characteristics [53]. In addition, a study conducted to determine the effects of lowered PSA at diagnosis with rising PSA screening over the years leads to the clinical stage migration. They found it does not reduce Postoperative Kattan nomogram prediction accuracy [54]. Furthermore, the nomograms were developed for pre- dicting early (2 years) and aggressive (9 - 12 months) recurrence after radical prostatectomy. Walz et al. set up a nomogram to predict early recurrence with 6 parame- ters and c-index was found as 82% [55]. Schroeck et al. developed a nomogram with 8 variables for predicting aggressive biochemical recurrence and compared with nine nomograms. They stated their nomogram is superior for determining aggressive recurrence [56]. Afterwards, they recalibrated and externally validated their nomo- gram [57]. 2.2.3. Nom o grams to Predic t Prostate Cancer Specific Mortality af ter RP Prostate cancer related mortality after RP is another im- portant issue for prediction models. Stephenson et al. set up a nomogram to predict 15 years survival and c-index was found as 82% [58]. Indeed, Porter et al. developed a nomogram with constituting age, pathological stage, pa- thological Gleason sum, performing lymph node dissec- tion and adjuvant radiotherapy data to determine 20-year disease-free survival after RP and c-index was 76.3% [59]. 2.2.4. Nomograms to Predict the Quality of Life after RP A lthough cancer specific survival has always been the Copyright © 2011 SciRes. OJU 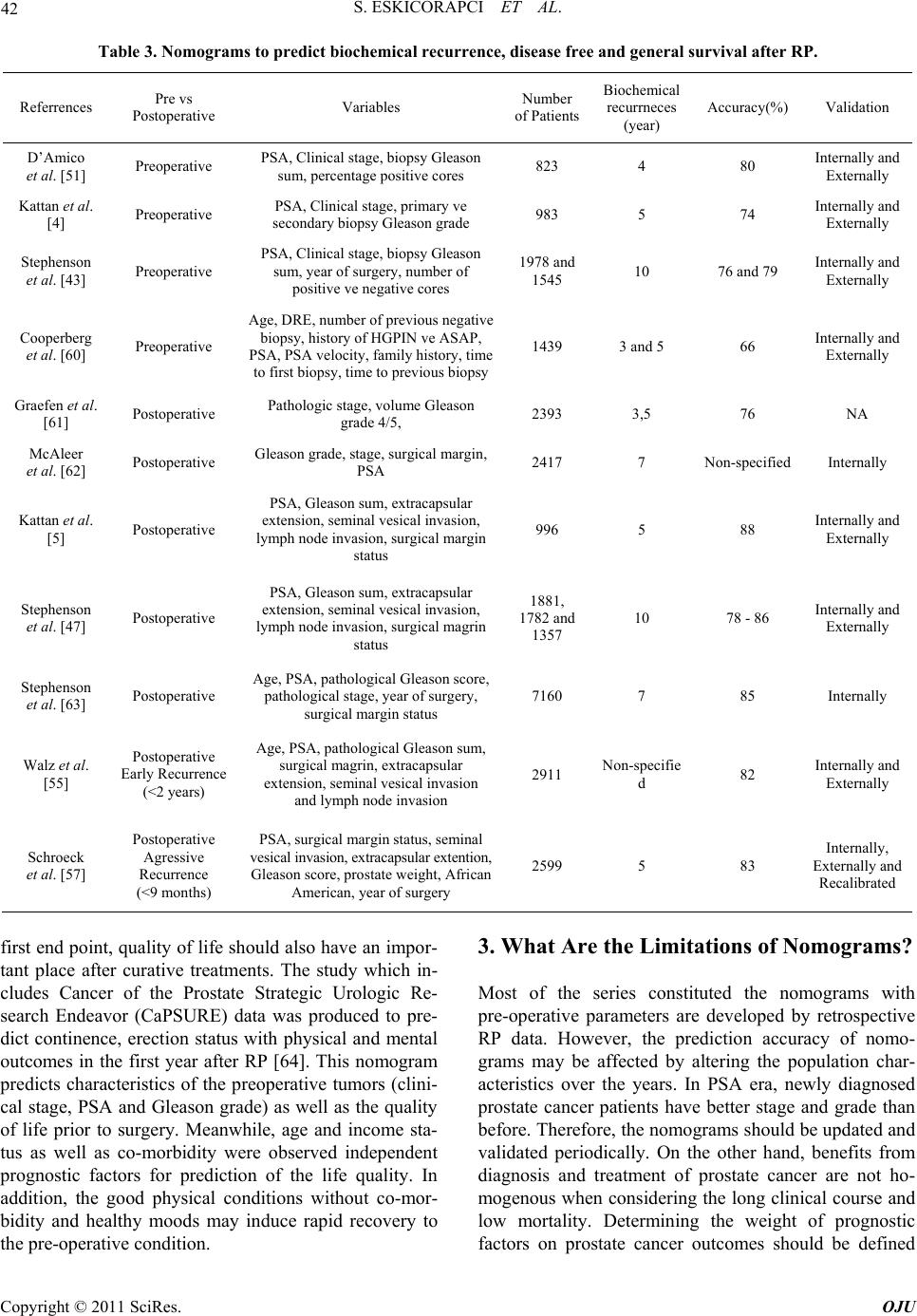 S. ESKICORAPCI ET AL. Copyright © 2011 SciRes. OJU 42 Table 3. Nomograms to predict biochemical recurrence, disease free and general survival after RP. Referrences Pre vs Postoperative Variables Number of Patients Biochemical recurrneces (year) Accuracy(%) Validation D’Amico et al. [51] Preoperative PSA, Clinical stage, biopsy Gleason sum, percentage positive cores 823 4 80 Internally and Externally Kattan et al. [4] Preoperative PSA, Clinical stage, primary ve secondary biopsy Gleason grade 983 5 74 Internally and Externally Stephenson et al. [43] Preoperative PSA, Clinical stage, biopsy Gleason sum, year of surgery, number of positive ve negative cores 1978 and 1545 10 76 and 79 Internally and Externally Cooperberg et al. [60] Preoperative Age, DRE, number of previous negative biopsy, history of HGPIN ve ASAP, PSA, PSA velocity, family history, time to first biopsy, time to previous biopsy 1439 3 and 5 66 Internally and Externally Graefen et al. [61] Postoperative Pathologic stage, volume Gleason grade 4/5, 2393 3,5 76 NA McAleer et al. [62] Postoperative Gleason grade, stage, surgical margin, PSA 2417 7 Non-specified Internally Kattan et al. [5] Postoperative PSA, Gleason sum, extracapsular extension, seminal vesical invasion, lymph node invasion, surgical margin status 996 5 88 Internally and Externally Stephenson et al. [47] Postoperative PSA, Gleason sum, extracapsular extension, seminal vesical invasion, lymph node invasion, surgical magrin status 1881, 1782 and 1357 10 78 - 86 Internally and Externally Stephenson et al. [63] Postoperative Age, PSA, pathological Gleason score, pathological stage, year of surgery, surgical margin status 7160 7 85 Internally Walz et al. [55] Postoperative Early Recurrence (<2 years) Age, PSA, pathological Gleason sum, surgical magrin, extracapsular extension, seminal vesical invasion and lymph node invasion 2911 Non-specifie d 82 Internally and Externally Schroeck et al. [57] Postoperative Agressive Recurrence (<9 months) PSA, surgical margin status, seminal vesical invasion, extracapsular extention, Gleason score, prostate weight, African American, year of surgery 2599 5 83 Internally, Externally and Recalibrated first end point, quality of life should also have an impor- tant place after curative treatments. The study which in- cludes Cancer of the Prostate Strategic Urologic Re- search Endeavor (CaPSURE) data was produced to pre- dict continence, erection status with physical and mental outcomes in the first year after RP [64]. This nomogram predicts characteristics of the preoperative tumors (clini- cal stage, PSA and Gleason grade) as well as the quality of life prior to surgery. Meanwhile, age and income sta- tus as well as co-morbidity were observed independent prognostic factors for prediction of the life quality. In addition, the good physical conditions without co-mor- bidity and healthy moods may induce rapid recovery to the pre-operative condition. 3. What Are the Limitations of Nomograms? Most of the series constituted the nomograms with pre-operative parameters are developed by retrospective RP data. However, the prediction accuracy of nomo- grams may be affected by altering the population char- acteristics over the years. In PSA era, newly diagnosed prostate cancer patients have better stage and grade than before. Therefore, the nomograms should be updated and validated periodically. On the other hand, benefits from diagnosis and treatment of prostate cancer are not ho- mogenous when considering the long clinical course and low mortality. Determining the weight of prognostic factors on prostate cancer outcomes should be defined  S. ESKICORAPCI ET AL. 43 individually and in prediction model at the same time. For this purpose, Kattan nomograms and Albertsen ta- bles are widely used [65,66]. To date, any model has perfect prediction performance. Additionally, some risk factors affecting the prognosis are not included in several nomograms. However, the models cannot achieve 100% accuracy even if all factors add into the nomograms. To increase the accuracy of nomograms, new biomarkers and imaging techniques have been investigated [42,67]. 4. Conclusions Predicting the clinical course of cancer is challenging for all patients. The urologists are willing to predict the pa- thological stages and possible scenarios after curative interventions. Therefore, the prognostic factors and no- mograms are the frequently applied sources. PSA, Glea- son grade and clinical stage are considered to be the most important prognostic factors. In addition, the pathologi- cal parameters are remarkable prognostic criteria. The Partin tables for predicting the radical prostatectomy pathology and Kattan nomograms for predicting the bio- chemical recurrences free survival rates are the most frequently used nomograms. Today, these nomograms should not replace the clinical decisions but they give significant information for the patients’ prognosis, treat- ment selection and follow up. 5. References [1] P. Boyle and J. Ferlay, “Cancer Incidence and Mortality in Europe, 2004,” Annals of Oncology, Vol. 16, No. 3, 2005, pp. 481-488. doi:10.1093/annonc/mdi098 [2] S. F. Shariat, P. I. Karakiewicz, V. Margulis and M. W. Kattan, “Inventory of Prostate Cancer Predictive Tools,” Current Opinion in Urology, Vol. 18, 2008, No. 3, pp. 279-296. doi:10.1097/MOU.0b013e3282f9b3e5 [3] A. W. Partin, J. Yoo, H. B. Carter, J. D. Pearson, D. W. Chan, J. I. Epstein and P. C. Walsh, “The Use of Prostate Specific Antigen, Clinical Stage and Gleason Score to Predict Pathological Stage in Men with Localized Pros- tate Cancer,” Journal of Urology, Vol. 150, 1993, pp. 110-114. [4] M. W. Kattan, J. A. Eastham, A. M. Stapleton, T. M. Wheeler and P. T. Scardino, “A Preoperative Nomogram for Disease Recurrence Following Radical Prostatectomy for Prostate Cancer,” Journal of the National Cancer In- stitute, Vol. 90, No. 10, 1998, pp. 766-771. doi:10.1093/jnci/90.10.766 [5] M. W. Kattan, T. M. Wheeler and P. T. Scardino, “Post- operative Nomogram for Disease Recurrence after Radi- cal Prostatectomy for Prostate Cancer,” Journal of Clini- cal Oncology, Vol. 17, No. 5, 1999, pp. 1499-1507. [6] P. B. Snow, D. S. Smith and W. J. Catalona, “Artificial Neural Networks in the Diagnosis and Prognosis of Pros- tate Cancer: A Pilot Study,” Journal of Urology, Vol. 152, 1994, pp. 1923-1926. [7] A. V. D’Amico, R. Whittington, S. B. Malkowicz, D. Schultz, M. Schnall, J. E. Tomaszewski and A. Wein, “A Multivariate Analysis of Clinical and Pathological Fac- tors That Predict for Prostate Specific Antigen Failure af- ter Radical Prostatectomy for Prostate Cancer,” Journal of Urology, Vol. 154, No. 1, 1995, pp. 131-138. [8] M. Graefen, A. Haese, U. Pichlmeier, P. G. Hammerer, J. Noldus, K. Butz, A. Erbersdobler, R. P. Henke, U. Michl, S. Fernandez and H. Huland, “A Validated Strategy for Side Specific Prediction of Organ Confined Prostate Cancer: A Tool to Select for Nerve Sparing Radical Prostatectomy,” Journal of Urology, Vol. 165, No. 3, 2001, pp. 857-863. doi:10.1016/S0022-5347(05)66544-5 [9] F. K. Chun, P. I. Karakiewicz, A. Briganti, J. Walz, M. W. Kattan, H. Huland and M. Graefen, “A Critical Appraisal of Logistic Regression-Based Nomograms, Artificial Neural Networks, Classification and Regression-Tree Models, Look-Up Tables and Risk-Group Stratification Models for Prostate Cancer,” British Journal of Urology International, Vol. 99, No. 4, 2007, pp. 794-800. doi:10.1111/j.1464-410X.2006.06694.x [10] M. W. Kattan, “Comparison of Cox Regression with Other Methods for Determining Prediction Models and Nomograms,” Journal of Urology, Vol. 170, No. 6, 2003, pp. S6-S9. doi:10.1097/01.ju.0000094764.56269.2d [11] J. E. Oesterling, C. B. Brendler, J. I. Epstein, A. W. Kimball Jr. and P. C. Walsh, “Correlation of Clinical Stage, Serum Prostatic Acid Phosphatase and Preopera- tive Gleason Grade with Final Pathological Stage in 275 Patients with Clinically Localized Adenocarcinoma of the Prostate,” Journal of Urology, Vol. 138, No. 1, 1987, pp. 92-98. [12] P. Narayan, V. Gajendran, S. P. Taylor, A. Tewari, J. C. Presti Jr., R. Leidich, R. Lo, K. Palmer, K. Shinohara and J. T. Spaulding, “The Role of Transrectal Ultrasound- Guided Biopsy-Based Staging, Preoperative Serum Pros- tate-Specific Antigen, and Biopsy Gleason Score in Pre- diction of Final Pathologic Diagnosis in Prostate Cancer,” Urology, Vol. 46, No. 2, 1995, pp. 205-212. doi:10.1016/S0090-4295(99)80195-2 [13] D. V. Makarov, B. J. Trock, E. B. Humphreys, L. A. Mangold, P. C. Walsh, J. I. Epstein and A. W. Partin, “Updated Nomogram to Predict Pathologic Stage of Prostate Cancer Given Prostate-Specific Antigen Level, Clinical Stage, and Biopsy Gleason Score (Partin Tables) Based on Cases from 2000 to 2005,” Urology, Vol. 69, No. 6, 2007, pp. 1095-1101. doi:10.1016/j.urology.2007.03.042 [14] A. W. Partin, M. W. Kattan, E. N. Subong, P. C. Walsh, K. J. Wojno, J. E. Oesterling, P. T. Scardino and J. D. Pearson, “Combination of Prostate-Specific Antigen, Clinical Stage, and Gleason Score to Predict Pathological Stage of Localized Prostate Cancer. A Multi-institutional Update,” Journal of the American Medical Association, Vol. 277, No. 18, 1997, pp. 1445-1451. Copyright © 2011 SciRes. OJU  44 S. ESKICORAPCI ET AL. doi:10.1001/jama.277.18.1445 [15] A. W. Partin, L. A. Mangold, D. M. Lamm, P. C. Walsh, J. I. Epstein and J. D. Pearson, “Contemporary Update of Prostate Cancer Staging Nomograms (Partin Tables) for the New Millennium,” Urology, Vol. 58, No. 6, 2001, pp. 843-848. doi:10.1016/S0090-4295(01)01441-8 [16] M. L. Blute, E. J. Bergstralh, A. W. Partin, P. C. Walsh, M. W. Kattan, P. T. Scardino, J. E. Montie, J. D. Pearson, J. M. Slezak and H. Zincke, “Validation of Partin Tables for Predicting Pathological Stage of Clinically Localized Prostate Cancer,” Journal of Urology, Vol. 164, No. 5, 2000, pp. 1591-1595. do:10.1016/S0022-5347(05)67035-8 [17] M. Graefen, H. Augustin, P. I. Karakiewicz, P. G. Ham- merer, A. Haese, J. Palisaar, J. Blonski, S. Fernandez, A. Erbersdobler and H. Huland, “Can Predictive Models for Prostate Cancer Patients Derived in the United States of America be Utilized in European Patients? A Validation Study of the Partin Tables,” European Urology, Vol. 43, 2003, pp. 6-10. doi:10.1016/S0302-2838(02)00497-9 [18] H. Augustin, T. Eggert, S. Wenske, P. I. Karakiewicz, J. Palisaar, F. Daghofer, H. Huland and M. Graefen, “Comparison of Accuracy between the Partin Tables of 1997 and 2001 to Predict Final Pathological Stage in Clinically Localized Prostate Cancer,” Journal of Urol- ogy, Vol. 171, No. 1, 2004, pp. 177-181. doi:10.1097/01.ju.0000099827.77355.a7 [19] J. B. Yu, D. V. Makarov, R. Sharma, R. E. Peschel, A. W. Partin and C. P. Gross, “Validation of the Partin Nomo- gram for Prostate Cancer in a National Sample,” Journal of Urology, Vol. 183, No. 1, 2010, pp. 105-111. doi:10.1016/j.juro.2009.08.143 [20] R. A. Badalament, M. C. Miller, P. A. Peller, D. C. Young, D. K. Bahn, P. Kochie, G. J. O'Dowd and R. W. Veltri, “An Algorithm for Predicting Nonorgan Confined Prostate Cancer Using the Results Obtained from Sextant Core Biopsies with Prostate Specific Antigen Level,” Journal of Urology, Vol. 156, No. 4, 1996, pp. 1375- 1380. doi:10.1016/S0022-5347(01)65590-3 [21] M. Ohori, M. W. Kattan, H. Koh, N. Maru, K. M. Slawin, S. Shariat, M. Muramoto, V. E. Reuter, T. M. Wheeler, and P. T. Scardino, “Predicting the Presence and Side of Extracapsular Extension: A Nomogram for Staging Pros- tate Cancer,” Journal of Urology, Vol. 171, No. 5, 2004, pp. 1844-1849. [22] T. Steuber, M. Graefen, A. Haese, A. Erbersdobler, F. K. Chun, T. Schlom, P. Perrotte, H. Huland and P. I. Kara- kiewicz, “Validation of a Nomogram for Prediction of Side Specific Extracapsular Extension at Radical Prosta- tectomy,” Journal of Urology, Vol. 175, No. 3, 2006, pp. 939-944. doi:10.1016/S0022-5347(05)00342-3 [23] N. Satake, M. Ohori, C. Yu, M. W. Kattan, Y. Ohno, A. Miyakawa, T. Hatano and M. Tachibana, “Development and Internal Validation of a Nomogram Predicting Ex- tracapsular Extension in Radical Prostatectomy Speci- mens,” International Journal of Urology, Vol. 17, No. 3, 2010, pp. 267-272. doi:10.1111/j.1442-2042.2010.02452.x [24] H. Koh, M. W. Kattan, P. T. Scardino, K. Suyama, N. Maru, K. Slawin, T. M. Wheeler and M. Ohori, “A No- mogram to Predict Seminal Vesicle Invasion by the Ex- tent and Location of Cancer in Systematic Biopsy Re- sults,” Journal of Urology, Vol. 170, No. 4, 2003, pp. 1203-1208. doi:10.1097/01.ju.0000085074.62960.7b [25] A. Baccala Jr., A. M. Reuther, F. J. Bianco Jr., P. T. Scardino, M. W. Kattan and E. A. Klein, “Complete Re- section of Seminal Vesicles at Radical Prostatectomy Results in Substantial Long-Term Disease-Free Survival: Multi-Institutional Study of 6740 Patients,” Urology, Vol. 69, No. 3, 2007, pp. 536-540. doi:10.1016/j.urology.2006.12.013 [26] A. Gallina, F. K. Chun, A. Briganti, S. F. Shariat, F. Montorsi, A. Salonia, A. Erbersdobler, P. Rigatti, L. Va- liquette, H. Huland, M. Graefen and P. I. Karakiewicz, “Development and Split-Sample Validation of a Nomo- gram Predicting the Probability of Seminal Vesicle Inva- sion at Radical Prostatectomy,” European Urology, Vol. 52, No. 1, 2007, pp. 98-105. doi:10.1016/j.eururo.2007.01.060 [27] M. Ohori, M. W. Kattan, C. Yu, K. Matsumoto, T. Satoh, J. Ishii, A. Miyakawa, A. Irie, M. Iwamura and M. Ta- chibana, “Nomogram to Predict Seminal Vesicle Invasion Using the Status of Cancer at the Base of the Prostate on Systematic Biopsy,” International Journal of Urology, Vol. 17, No. 6, 2010, pp. 534-540. doi:10.1111/j.1442-2042.2010.02513.x [28] I. Cagiannos, P. Karakiewicz, J. A. Eastham, M. Ohori, F. Rabbani, C. Gerigk, V. Reuter, M. Graefen, P. G. Ham- merer, A. Erbersdobler, H. Huland, P. Kupelian, E. Klein, D. I. Quinn, S. M. Henshall, J. J. Grygiel, R. L. Suther- land, P. D. Stricker, C. G. Morash, P. T. Scardino and M. W. Kattan, “A Preoperative Nomogram Identifying De- creased Risk of Positive Pelvic Lymph Nodes in Patients with Prostate Cancer,” Journal of Urology, Vol. 170, No. 5, 2003, pp. 1798-1803. doi:10.1097/01.ju.0000091805.98960.13 [29] A. Briganti, F. K. Chun, A. Salonia, A. Gallina, E. Farina, L. F. Da Pozzo, P. Rigatti, F. Montorsi and P. I. Karakie- wicz, “Validation of a Nomogram Predicting the Prob- ability of Lymph Node Invasion Based on the Extent of Pelvic Lymphadenectomy in Patients with Clinically Lo- calized Prostate Cancer,” British Journal of Urology In- ternational, Vol. 98, No. 4, 2006, pp. 788-793. doi:10.1111/j.1464-410X.2006.06318.x [30] Y. D. Choi, H. S. Yu, K. H. Choi, et al., “Development of Preoperative Nomogram for Predicting Pelvic Lymph Node Invasion in Patients Undergoing Radical Prostatec- tomy,” European Urology, Vol. 9, 2010, p. 590. [31] F. K. Chun, T. Steuber, A. Erbersdobler, E. Currlin, J. Walz, T. Schlomm, A. Haese, H. Heinzer, M. McCor- mack, H. Huland, M. Graefen and P. I. Karakiewicz, “Development and Internal Validation of a Nomogram Predicting the Probability of Prostate Cancer Gleason Sum Upgrading between Biopsy and Radical Prostatec- tomy Pathology,” European Urology, Vol. 49, No. 5, 2006, pp. 820-826. doi:10.1016/j.eururo.2005.11.007 [32] F. K. Chun, A. Briganti, S. F. Shariat, M. Graefen, F. Copyright © 2011 SciRes. OJU  S. ESKICORAPCI ET AL. 45 Montorsi, A. Erbersdobler, T. Steuber, A. Salonia, E. Currlin, V. Scattoni, M. G. Friedrich, T. Schlomm, A. Haese, U. Michl, R. Colombo, H. Heinzer, L. Valiquette, P. Rigatti, C. G. Roehrborn, H. Huland and P. I. Kara- kiewicz, “Significant Upgrading Affects a Third of Men Diagnosed with Prostate Cancer: Predictive Nomogram and Internal Validation,” British Journal of Urology In- ternational, Vol. 98, No. 2, 2006, pp. 329-334. doi:10.1111/j.1464-410X.2006.06262.x [33] D. A. Stackhouse, L. Sun, F. R. Schroeck, J. Jaya- chandran, A. A. Caire, C. O. Acholo, C. N. Robertson, D. M. Albala, T. J. Polascik, C. F. Donatucci, K. E. Maloney and J. W. Moul, “Factors Predicting Prostatic Biopsy Gleason Sum under Grading,” Journal of Urology, Vol. 182, 2009, pp. 118-122. doi:10.1016/j.juro.2009.02.127 [34] T. Steuber, F. K. Chun, A. Erbersdobler, A. Briganti, A. Haese, M. Graefen, T. Schlomm, L. Valiquette, H. Hu- land and P. I. Karakiewicz, “Development and Internal Validation of Preoperative Transition Zone Prostate Cancer Nomogram,” Urology, Vol. 68, No. 6, 2006, pp. 1295-1300. doi:10.1016/j.urology.2006.08.1066 [35] P. A. Peller, D. C. Young, D. P. Marmaduke, W. L. Marsh and R. A. Badalament, “Sextant Prostate Biopsies. A Histopathologic Correlation with Radical Prostatec- tomy Specimens,” Cancer, Vol. 75, No. 2, 1995, pp. 530- 538. doi:10.1002/1097-0142(19950115)75:2<530::AID-CNC R2820750216>3.0.CO;2-Y [36] M. W. Kattan, J. A. Eastham, T. M. Wheeler, N. Maru, P. T. Scardino, A. Erbersdobler, M. Graefen, H. Huland, H. Koh, S. F. Shariat, K. M. Slawin and M. Ohori, “Coun- seling Men with Prostate Cancer: A Nomogram for Pre- dicting the Presence of Small, Moderately Differentiated, Confined Tumors,” Journal of Urology, Vol. 170, No. 5, 2003, pp. 1792-1797. doi:10.1097/01.ju.0000091806.70171.41 [37] S. Y. Eskicorapci, E. Karabulut, L. Turkeri, S. Baltaci, C. Cal, G. Toktas, H. Akpinar, G. Ozer, S. Sozen, R. Tokuc, M. Lekili, A. Soylu, S. Albayrak, H. Sahin, R. Alpar and H. Ozen, “Validation of 2001 Partin Tables in Turkey: A Multicenter Study,” European Urology, Vol. 47, No. 2, 2005, pp. 185-189. doi:10.1016/j.eururo.2004.08.002 [38] D. G. Bostwick, J. Qian, E. Bergstralh, P. Dundore, J. Dugan, R. P. Myers and J. E. Oesterling, “Prediction of Capsular Perforation and Seminal Vesicle Invasion in Prostate Cancer,” Journal of Urology, Vol. 155, No. 4, 1996, pp. 1361-1367. doi:10.1016/S0022-5347(01)66267-0 [39] D. A. Ackerman, J. M. Barry, R. A. Wicklund, N. Olson and B. A. Lowe, “Analysis of Risk Factors Associated with Prostate Cancer Extension to the Surgical Margin and Pelvic Node Metastasis at Radical Prostatectomy,” Journal of Urology, Vol. 150, No. 6, 1993, pp. 1845- 1850. [40] F. Rabbani, A. Bastar and W. R. Fair, “Site Specific Pre- dictors of Positive Margins at Radical Prostatectomy: An Argument for Risk Based Modification of Technique,” Journal of Urology, Vol. 160, No. 5, 1998, pp. 1727- 1733. doi:10.1016/S0022-5347(01)62394-2 [41] U. Capitanio, P. I. Karakiewicz, C. Jeldres, A. Briganti, A. Gallina, N. Suardi, A. Cestari, G. Guazzoni, A. Salonia and F. Montorsi, “The Probability of Gleason Score Up- grading between Biopsy and Radical Prostatectomy Can Be Accurately Predicted,” International Journal of Urology, Vol. 16, No. 5, 2009, pp. 526-529. doi:10.1111/j.1442-2042.2009.02270.x [42] M. W. Kattan, S. F. Shariat, B. Andrews, K. Zhu, E. Canto, K. Matsumoto, M. Muramoto, P. T. Scardino, M. Ohori, T. M. Wheeler and K. M. Slawin, “The Addition of Interleukin-6 Soluble Receptor and Transforming Growth Factor Beta1 Improves a Preoperative Nomo- gram for Predicting Biochemical Progression in Patients with Clinically Localized Prostate Cancer,” Journal of Clinical Oncology, Vol. 21, No. 19, 2003, pp. 3573-3579. doi:10.1200/JCO.2003.12.037 [43] A. J. Stephenson, P. T. Scardino, J. A. Eastham, F. J. Bianco Jr., Z. A. Dotan, P. A. Fearn and M. W. Kattan, “Preoperative Nomogram Predicting the 10-Year Prob- ability of Prostate Cancer Recurrence after Radical Pros- tatectomy,” Journal of the National Cancer Institute, Vol. 98, No. 10, 2006, pp. 715-717.doi:10.1093/jnci/djj190 [44] S. Y. Eskicorapci, L. Turkeri, E. Karabulut, C. Cal, H. Akpinar, S. Baltaci, K. Baykal, M. W. Kattan and H. Ozen, “Validation of Two Preoperative Kattan Nomo- grams Predicting Recurrence after Radical Prostatectomy for Localized Prostate Cancer in Turkey: A Multicenter Study of the Uro-Oncology Society,” Urology, Vol. 74, No. 6, 2009, pp. 1289-1295. doi:10.1016/j.urology.2009.03.019 [45] A. V. D'Amico, “Combined-Modality Staging for Local- ized Adenocarcinoma of the Prostate,” Oncology (Willis- ton Park), Vol. 15, No. 8, 2001, pp. 1049-1059. [46] M. Graefen, P. I. Karakiewicz, I. Cagiannos, et al., “Va- lidation Study of the Accuracy of a Postoperative Nomo- gram for Recurrence after Radical Prostatectomy for Lo- calized Prostate Cancer,” Journal of Clinical Oncology, Vol. 20, No. 20, 2002, pp. 951-956. doi:10.1200/JCO.20.4.951 [47] A. J. Stephenson, P. T. Scardino, J. A. Eastham, et al., “Postoperative Nomogram Predicting the 10-Year Prob- ability of Prostate Cancer Recurrence after Radical Pros- tatectomy,” Journal of Clinical Oncology, Vol. 23, No. 28, 2005, pp. 7005-7012. doi:10.1200/JCO.2005.01.867 [48] J. W. Moul, R. R. Connelly, D. P. Lubeck, J. J. Bauer, L. Sun, S. C. Flanders, G. D. Grossfeld and P. R. Carroll, “Predicting Risk of Prostate Specific Antigen Recurrence after Radical Prostatectomy with the Center for Prostate Disease Research and Cancer of the Prostate Strategic Urologic Research Endeavor Databases,” Journal of Urology, Vol. 166, No. 4, 2001, pp. 1322-1327. doi;10.1016/S0022-5347(05)65761-8 [49] J. J. Bauer, R. R. Connelly, I. A. Seterhenn, J. Deausen, S. Srivastava, D. G. McLeod and J. W. Moul, “Biostatistical Modeling Using Traditional Preoperative and Pathologi- cal Prognostic Variables in the Selection of Men at High Risk for Disease Recurrence after Radical Prostatectomy Copyright © 2011 SciRes. OJU  46 S. ESKICORAPCI ET AL. for Prostate Cancer,” Journal of Urology, Vol. 159, No. 3, 1998, pp. 929-933. doi:10.1016/S0022-5347(01)63773-X [50] M. L. Blute, E. J. Bergstralh, A. Iocca, B. Scherer and H. Zincke, “Use of Gleason Score, Prostate Specific Antigen, Seminal Vesicle and Margin Status to Predict Biochemi- cal Failure after Radical Prostatectomy,” Journal of Urology, Vol. 165, No. 1, 2001, pp. 119-125. doi:10.1097/00005392-200101000-00030 [51] A. V. D’Amico, R. Whittington, S. B. Malkowicz, J. Fondurulia, M. H. Chen, J. E. Tomaszewski and A. Wein, “The Combination of Preoperative Prostate Specific An- tigen and Postoperative Pathological Findings to Predict Prostate Specific Antigen Outcome in Clinically Local- ized Prostate Cancer,” Journal of Urology, Vol. 160, No. 6, 1998, pp. 2096-2101. [52] M. Han, A. W. Partin, M. Zahurak, S. Piantadosi, J. I. Epstein and P. C. Walsh, “Biochemical (Prostate Specific Antigen) Recurrence Probability Following Radical Pros- tatectomy for Clinically Localized Prostate Cancer,” Jou- rnal of Urology, Vol. 169, No. 2, 2003, pp. 517-523. doi:10.1016/S0022-5347(05)63946-8 [53] D. M. Moreira, J. C. Presti Jr., W. J. Aronson, M. K. Ter- ris, C. J. Kane, C. L. Amling, L. L. Sun, J. W. Moul and S. J. Freedland, “The Effect of Race on the Discrimina- tory Accuracy of Models to Predict Biochemical Recur- rence after Radical Prostatectomy: Results from the Shared Equal Access Regional Cancer Hospital and Duke Prostate Center Databases,” Prostate Cancer and Pros- tatic Diseases, Vol. 13, No. 1, 2010, pp. 87-93. doi:10.1038/pcan.2009.48 [54] R. Thanigasalam, K. K. Rasiah, P. D. Stricker, A. M. Haynes, S. I. Sutherland, R. L. Sutherland, S. M. Hen- shall and L. G. Horvath, “Stage Migration in Localized Prostate Cancer Has No Effect on the Post-Radical Pros- tatectomy Kattan Nomogram,” British Journal of Urol- ogy International, Vol. 105, No. 5, 2010, pp. 642-647. doi:10.1111/j.1464-410X.2009.08842.x [55] J. Walz, F. K. Chun, E. A. Klein, A. Reuther, F. Saad, M. Graefen, H. Huland and P. I. Karakiewicz, “Nomogram Predicting the Probability of Early Recurrence after Rad- ical Prostatectomy for Prostate Cancer,” Journal of Urology, Vol. 181, No. 2, 2009, pp. 601-608. doi:10.1016/j.juro.2008.10.033 [56] F. R. Schroeck, W. J. Aronson, J. C. Presti Jr., M. K. Terris, C. J. Kane, C. L. Amling and S. J. Freedland, “Do Nomograms Predict Aggressive Recurrence after Radical Prostatectomy More Accurately than Biochemical Re- currence Alone?” British Journal of Urology Interna- tional, Vol. 103, No. 5, 2009, pp. 603-608. doi:10.1111/j.1464-410X.2008.08118.x [57] F. R. Schroeck, M. W. Kattan, J. W. Moul, W. J. Aronson, J. C. Presti Jr., M. K. Terris, C. J. Kane, C. L. Amling, L. Sun and S. J. Freedland, “Re-calibration and External Validation of an Existing Nomogram to Predict Aggres- sive Recurrences after Radical Prostatectomy,” British Journal of Urology International, Vol. 105, No. 12, 2010, pp. 1654-1659. doi:10.1111/j.1464-410X.2009.09060.x [58] A. J. Stephenson, M. W. Kattan, J. A. Eastham, F. J. Bi- anco Jr., O. Yossepowitch, A. J. Vickers, E. A. Klein, D. P. Wood and P. T. Scardino, “Prostate Cancer-Specific Mortality after Radical Prostatectomy for Patients Treated in the Prostate-Specific Antigen Era,” Journal of Clinical Oncology, Vol. 27, No. 26, 2009, pp. 4300-4305. doi:10.1200/JCO.2008.18.2501 [59] C. R. Porter, N. Suardi, U. Capitanio, G. C. Hutterer, K. Kodama, R. P. Gibbons, R. Correa Jr., P. Perrotte, F. Montorsi and P. I. Karakiewicz, “A Nomogram Predict- ing Prostate Cancer-Specific Mortality after Radical Prostatectomy,” Urologia Internationalis, Vol. 84, No. 2, 2010, pp. 132-140. doi:10.1159/000277588 [60] M. R. Cooperberg, D. J. Pasta, E. P. Elkin, M. S. Litwin, D. M. Latini, J. Du Chane and P. R. Carroll, “The Uni- versity of California, San Francisco Cancer of the Pros- tate Risk Assessment Score: A Straightforward and Reli- able Preoperative Predictor of Disease Recurrence after Radical Prostatectomy,” Journal of Urology, Vol. 173, No. 6, 2005, pp. 1938-1942. doi:10.1097/01.ju.0000158155.33890.e7 [61] M. Graefen, J. Noldus, U. Pichlmeier, A. Haese, P. Hammerer, S. Fernandez, S. Conrad, R. Henke, E. Hu- land and H. Huland, “Early Prostate-Specific Antigen Relapse after Radical Retropubic Prostatectomy: Predic- tion on the Basis of Preoperative and Postoperative Tu- mor Characteristics,” European Urology, Vol. 36, No. 1, 1999, pp. 21-30. doi:10.1159/000019922 [62] S. J. McAleer, D. Schultz, R. Whittington, S. B. Malko- wicz, A. Renshaw, A. Wein, J. P. Richie and A. V. D’Amico, “PSA Outcome Following Radical Prostatec- tomy for Patients with Localized Prostate Cancer Strati- fied by Prostatectomy Findings and the Preoperative PSA Level,” Urologic Oncology, Vol. 23, No. 5, 2005, pp. 311-317. doi:10.1016/j.urolonc.2004.12.013 [63] A. J. Stephenson, D. P. Wood, M. W. Kattan, E. A. Klein, P. T. Scardino, J. A. Eastham and B. S. Carver, “Location, Extent and Number of Positive Surgical Margins Do Not Improve Accuracy of Predicting Prostate Cancer Recur- rence after Radical Prostatectomy,” Journal of Urology, Vol. 182, No. 4, 2009, pp. 1357-1363. doi:10.1016/j.juro.2009.06.046 [64] J. C. Hu, E. P. Elkin, D. J. Pasta, D. P. Lubeck, M. W. Kattan, P. R. Carroll and M. S. Litwin, “Predicting Qual- ity of Life after Radical Prostatectomy: Results from CaPSURE,” Journal of Urology, Vol. 171, No. 2, 2004, pp. 703-708. doi:10.1097/01.ju.0000107964.61300.f6 [65] P. C. Albertsen, J. A. Hanley, D. F. Gleason and M. J. Barry, “Competing Risk Analysis of Men Aged 55 to 74 Years at Diagnosis Managed Conservatively for Clini- cally Localized Prostate Cancer,” Journal of the Ameri- can Medical Association, Vol. 280, No. 11, 1998, pp. 975-980. doi:10.1001/jama.280.11.975 [66] M. W. Kattan, D. Giri, K. S. Panageas, A. Hummer, M. Cranor, K. J. Van Zee, C. A. Hudis, L. Norton, P. I. Bor- gen and L. K. Tan, “A Tool for Predicting Breast Carci- noma Mortality in Women Who Do Not Receive Adju- vant Therapy,” Cancer, Vol. 101, No. 11, 2004, pp. 2509- 2515. doi;10.1002/cncr.20635 Copyright © 2011 SciRes. OJU  S. ESKICORAPCI ET AL. Copyright © 2011 SciRes. OJU 47 [67] L. Wang, H. Hricak, M. W. Kattan, H. N. Chen, K. Ku- roiwa, H. F. Eisenberg and P. T. Scardino, “Prediction of Seminal Vesicle Invasion in Prostate Cancer: Incremental Value of Adding Endorectal MR Imaging to the Kattan Nomogram,” Radiology, Vol. 242, No. 1, 2007, pp. 182- 188.doi:10.1148/radiol.2421051254
|