Paper Menu >>
Journal Menu >>
 Materials Sciences and Applicatio n, 2011, 2, 1139-1142 doi:10.4236/msa.2011.28154 Published Online August 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA 1139 Alloying Behaviour of CuPd Liquid Alloy Bhrigunandan Prasad Singh1, Devendra Adhikari1*, Indu Shekhar Jha2, Jitendra Kumar3, Ram Prasad Koirala2 1University Department of Physics, T. M. Bhag. University, Bihar, India; 2Department of Physics, M. M. A. M. Campus, Biratnagar Tribhuvan University, Biratnagar, Nepal; 3Metals and Ceramics Div., Research Institute, University of Dayton, Dayton, USA. Email: *adksbdev@yahoo.com Received April 20th, 2011; revised May 23rd, 2011; accepted June 16th, 2011. ABSTRACT The mixing properties of CuPd alloy in molten state at 1350 K have been studied by assuming strong interaction be- tween Cu and Pd atoms. Regular associated solution model has been used for the study. The asymmetry in integral ex- cess free energy of mixing, heat of mixing and entropy of mixing has been well explained. Keywords: Strong Interaction, Entropy of Mixing, Mole Fraction, Electronegativity, Size Factor 1. Introduction The knowledge of thermodynamic properties of binary liquid alloys is necessary for the design and development of reliable materials for high temperature application. Moreover, the properties of alloys in the melt are helpful to understand the alloying behaviour of alloys in solid state. Thus the determination of thermodynamic func- tions for alloys in liquid state has been the subjects of active research for many years. Growing technological interest to the nonperiodicity in the atomic arrangement of disordered materials has led to an increasing need for a better description of their atomic scale structures. In this paper, we intend to study the thermodynamic properties of CuPd alloys in liquid state at 1350 K on the basis of regular associated solution model. Regular associated solution model has been proved to study the thermodynamic and structural prop- erties of weakly, moderately and highly interacting liquid alloys [1-6] by assuming the formation of complex. Such assumptions have been used in different models [7-12]. The phase diagram, which is the fundamental source to understand the formation of complex, manifests the presence of Cu3Pd, Cu4Pd and CuPd intermediate phases in solid state [13]. It is well known fact that the size fac- tor and electronegativity difference of the constituent metals play an important role on the alloying behaviour of the binary alloys. The size factor, which has been con- sidered to explain the anomalous behaviour of liquid alloy [14], is small (= 1.25) and does not seem responsi- ble for asymmetry in such alloys. The electronegativity difference of the constituent metals is also important for making alloys. Researchers [15] have argued that when metals of very different electronegativity are alloyed, a change from metallic behaviour to, at least, partly ionic behaviour is expected. But the electronegativity differ- ence of Pd and Cu (= 0.25) is not large enough to account the alloying behaviour of CuPd alloy. Thus, I have as- sumed strong interaction between Cu and Pd atoms is responsible for the the alloying behaviour of CuPd alloy in liquid state. The Cu and Pd atoms are considered to be energetically favoured to form chemical complexes Cu3Pd. The basic formalism of regular associated solu- tion model with the assumption of strong interaction is presented in Section 2. 2. Regular Associated Solution Model Suppose type A and type B metals are mixed in the melt to form alloy AB. According to regular associated solu- tion model, the melt consists of three species namely unassociated A-atoms, unassociated B-atoms and com- plex AB. Considering a solution of atoms of A and 2 atoms of B, the formation of complex re- quires 1AApB 1 n Ap nnB nn pn and 2 B ApB for conser- vation of mass in the partially associated solution, where AB and ApB are concentration of unassociated A-atoms, B- atoms and complex respectively where p is small integer. When there is association, the thermody- namic behaviour of complexes A and B components is governed by their true mole fractions A nnn ,nn,n x , B x and ApB x rather than their gross mole fraction 1 x and2 x , where  Alloying Behaviour of CuPd Liquid Alloy 1140 1 1 12 n xnn , 2 2 12 n xnn A A ABApB n xnnn , B B ABApB n xnnn and ApB ApB ABApB n xnnn . Using above relations we can show 12AApB x xpxx , 22 (1 ) B ApB x xpxx . Following Lele and Ramchandrarao [2], the equilib- rium constant for the reaction A pB pA B is given by 12 13 23 ln ln1 11 P AB BBA ApB ApBA AApBB B xx kpxxx xRT pxx xxpx x RT RT (1) and the integral excess free energy x s G is given by 12 13 ln ln B ApB xx x RT k 23 112 2 1 1 ln ln 1 lnln 1 xs A BA ApBApB ApB AABB ApB ApB ApB ApB Gxxxx px RT xxxx x px x RT xxxxpx (2) where 12 , 13 and 23 are interaction energies for the species A, B; A, ApB and B, ApB respectively, T the temperature and R stands for the universal gas constant. The pairwise interaction energies and equilibrium con- stant are determined following Lele and Ramchandrarao [2] as follows: In a regular associated solution11 AA x x and 22 B B xx , where 1 and 2 are respective gross activity coefficients of components 1 and 2. Thus 1 1 lnlnln A A x x (3a) and 2 2 lnln ln B B x x (3b) Following the technique of Lee and Ramchandrarao [2] the pairwise interaction energies, the equilibrium con- stants and the activity coefficients at infinite dilution can be written as 012 1 ln RT (4a) 12 13 12 exp oo oo kRT (4b) where1 o and 2 o are activity coefficients of compo- nent A and that of B at zero concentrations. Solving equations (2a) and (2b) we obtain 21 13 2 ln1 ln1 BBB BA ApB aa xxxx 12 B x xR RT x T (5) 12 23 2 ln1 ln1 AAA AB ApB aa xxxx 12 A x xR RT x T (6) Using equations (4), (12) and (13), we can derive 13 1 212 12 1 ln ln ln ln A ApB A p B ApB BApB xa kRT xx x aa xxRTx a (7) Now heat of mixing and entropy of mixing can be re- lated to the free energy of mixing with the standard thermodynamic relations as follows ,TP G HGTT (8) H G ST (9) 3. Results and Discussion We have calculated the concentration of complex, equi- librium constant and pairwise interaction energies fol- lowing the method employed by Lele and Ramchancra- rao [2] and D. Adhikari et al. [3,5,6] using Eqs. (3-7). The equilibrium constant and interaction energies for the alloy Cu3Pd in liquid state at 1350 K are found to be k = 0.125, 12RT = –0.467, 13 RT = –1.9 and 23 RT = –3.47 The calculated and observed value of integral excess free energy of mixing is in excellent agreement (Figure 1). The calculated integral excess free energy of mixing is minimum (– 6.57 kJ·mol–1) at xCu = 0.7 which almost matches with the observed value [13]. The observed asymmetry in integral excess free energy of mixing is well explained by our theoretical model. We have observed that if the interaction energies are supposed to be independent of temperature, i.e., 12 0 T Copyright © 2011 SciRes. MSA 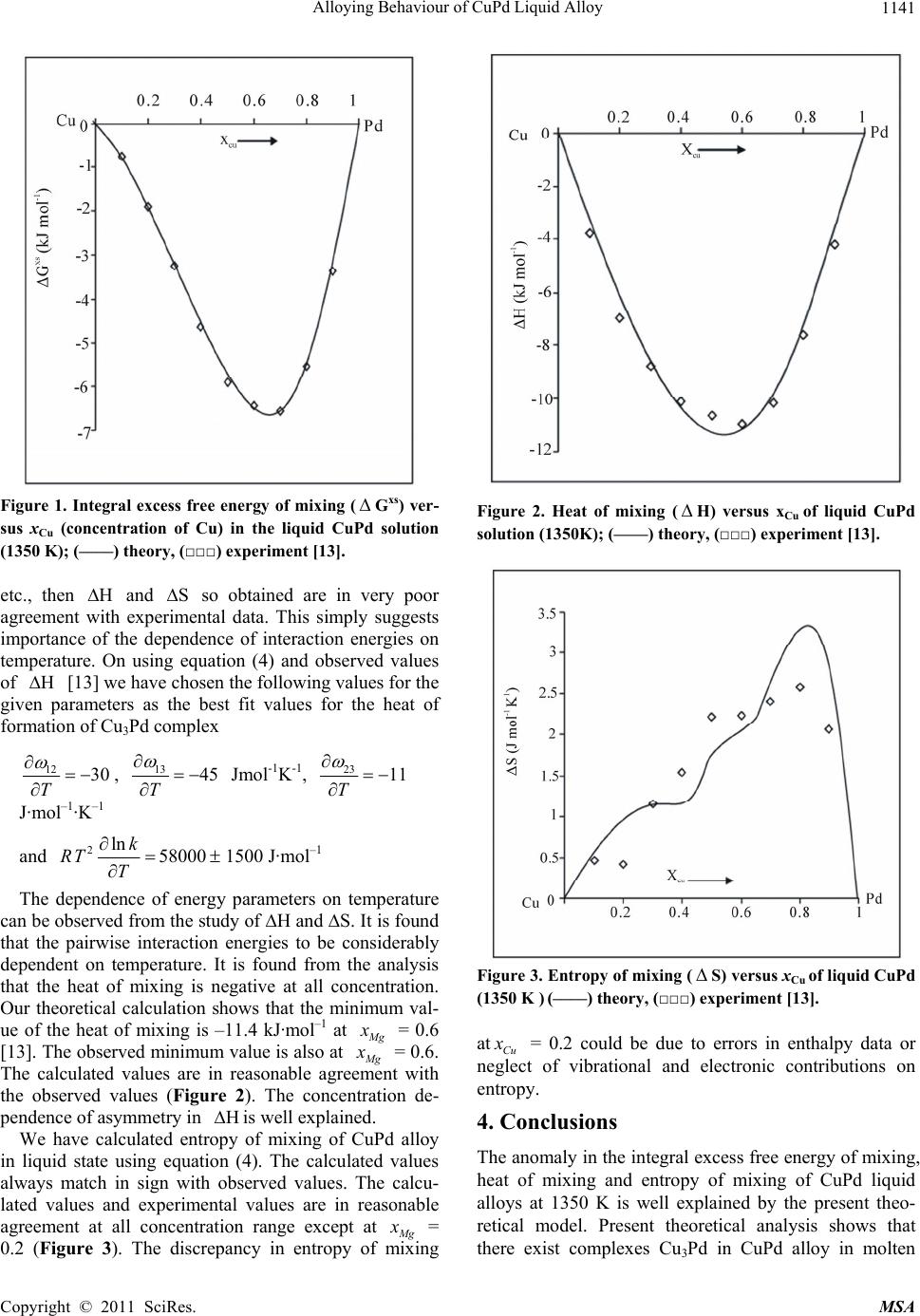 Alloying Behaviour of CuPd Liquid Alloy1141 Figure 1. Integral excess free energy of mixing ( Gxs) ver- sus xCu (concentration of Cu) in the liquid CuPd solution (1350 K); (––––) theory, (□□□) experiment [13]. etc., then and so obtained are in very poor agreement with experimental data. This simply suggests importance of the dependence of interaction energies on temperature. On using equation (4) and observed values of [13] we have chosen the following values for the given parameters as the best fit values for the heat of formation of Cu3Pd complex HS H 12 30 T , 13 45 T Jmol-1K-1, 2311 T J·mol–1·K–1 and 2lnk RT T 58000 1500 J·mol–1 The dependence of energy parameters on temperature can be observed from the study of ∆H and ∆S. It is found that the pairwise interaction energies to be considerably dependent on temperature. It is found from the analysis that the heat of mixing is negative at all concentration. Our theoretical calculation shows that the minimum val- ue of the heat of mixing is –11.4 kJ·mol–1 at M g x = 0.6 [13]. The observed minimum value is also at M g x = 0.6. The calculated values are in reasonable agreement with the observed values (Figure 2). The concentration de- pendence of asymmetry in is well explained. H We have calculated entropy of mixing of CuPd alloy in liquid state using equation (4). The calculated values always match in sign with observed values. The calcu- lated values and experimental values are in reasonable agreement at all concentration range except at M g x = 0.2 (Figure 3). The discrepancy in entropy of mixing Figure 2. Heat of mixing ( H) versus xCu of liquid CuPd solution (1350K); (––––) theory, (□□□) experiment [13]. Figure 3. Entropy of mixing (S) versus xCu of liquid CuPd (1350 K ) (––––) theory, (□□□) experiment [13]. at Cu x = 0.2 could be due to errors in enthalpy data or neglect of vibrational and electronic contributions on entropy. 4. Conclusions The anomaly in the integral excess free energy of mixing, heat of mixing and entropy of mixing of CuPd liquid alloys at 1350 K is well explained by the present theo- retical model. Present theoretical analysis shows that there exist complexes Cu3Pd in CuPd alloy in molten Copyright © 2011 SciRes. MSA  Alloying Behaviour of CuPd Liquid Alloy Copyright © 2011 SciRes. MSA 1142 state and the pairwise interaction energies are tempera- ture dependent. REFERENCES [1] B. P. Singh, D. Adhikari and I. S. Jha, “Concentration Dependence of the Structure and Thermodynamic Proper- ties of Silver-Antimony Alloys,” Journal Non-Crystals Solids, Vol. 356, No. 3-4, 2010, pp. 1730-1734. doi:10.1016/j.jnoncrysol.2010.06.004 [2] S. Lele and P. Ramchandrarao, “Estimation of Complex Concentration in a Regular Associated Solution,” Metal- lurgical Transactions B, Vol. 12B, 1981, pp. 659-666. doi:10.1007/BF02654134 [3] D. Adhikari, I. S. Jha and B. P. Singh, “Thermodynamic and Microscopic Structure of Liquid Cu-Sn Alloys,” Phy- sica B, Vol. 405, No. 7, 2010, pp. 1861-1865. doi:10.1016/j.physb.2010.01.064 [4] A. S. Jordan, “A Theory of Regular Associated Solution Applied to the Liquidus Curves of the Zn-Te and Cd-Te Systems,” Metallurgical Transactions, Vol. 1, 1970, pp. 239-249. [5] D. Adhikari, B. P. Singh, I. S. Jha and B. K. Singh, “Thermodynamic Properties and Microscopic Structure of Liquid Cd-Na Alloys by Estimating Complex Concentra- tion in a Regular Associated Solution,” Journal Molecu- lar Liquids, Vol. 156, No. 2-3, 2010, pp. 115-119. doi:10.1016/j.molliq.2010.05.020 [6] D. Adhikari, I. S. Jha and B. P. Singh, “Structural Asymmetry in Liquid Fe-Si Alloys,” Philosophical Mag- azine, Vol. 90, No. 20, 2010, pp. 2687-2694. doi:10.1080/14786431003745302 [7] V. Milekhine, M. I. Onsoien, J. K. Solberg and T. Skal- and, “Mechanical Properties of FeSi (ε), FeSi2 (ζα) and Mg2Si,” Intermetallics, Vol. 10, No. 8, 2002, pp. 743-750. doi:10.1016/S0966-9795(02)00046-8 [8] A. B. Bhatia and D. E. Thornton, “Structural Aspects of the Electrical Resistivity of Binary Alloys,” Physics Re- viwes B, Vol. 8, No. 2, 1970, pp. 3004-3012. doi:10.1103/PhysRevB.2.3004 [9] K. Hoshino and W. H. Young, “Entropy of Mixing of Compound Forming Liquid Binary Alloys,” Journal Physics F, Vol. 10, No. 7, 1980, pp. 1365-1376. doi:10.1088/0305-4608/10/7/006 [10] S. P. McAlister and E. D. Crozier, “The Concentra- tion-Concentration Correlation Function in the Long- Wavelength Limit for Binary Liquid Alloys,” Journal of Physics C, Vol. 7, No. 19, 1974, p. 3509. [11] I. S. Jha, R. N. Singh, P. L. Shrivastava and N. R. Mitra, “Stability of HgNa and HgK Liquid Alloys,” Philosophi- cal Magazine, Vol. 61, No. 1, 1990, pp. 15-24. doi:10.1080/13642819008208649 [12] R. N. Singh and F. Sommer, “Segregation and Immisci- bility in Liquid Binary Alloys,” Reports Progress Physics, Vol. 60, No. 1, 1997, p. 57. doi:10.1088/0034-4885/60/1/003 [13] R. Hultgren, P. D. Desai, D. T. Hawkins, M. Gleiser and K. K. Kelley, “Selected Values of the Thermodynamic Properties of Binary Alloys,” ASM, Metal Park, 1973. [14] A. B. Bhatia and N. H. March, “Size Effects, Peaks in Concentration Fluctuations and Liquidus Curves of Na-Cs,” Journal Physics F: Meterial Physics, Vol. 5, No. 6, 1975, p. 1100. doi:10.1088/0305-4608/5/6/011 [15] W. B. Pearson, “The Crystals Chemistry and Physics of Metals,” Wiley-International Science, 1972. |

