 Journal of Cancer Therapy, 2011, 2, 342-353 doi:10.4236/jct.2011.23047 Published Online August 2011 (http://www.SciRP.org/journal/jct) Copyright © 2011 SciRes. JCT Randomized Trial Comparing Cyclophosphamide, Methotrexate, and 5-Fluorouracil (CMF) Regimen with Rotational CMFEV Regimen (E = Epirubicin, V = Vincristine) as Adjuvant Chemotherapy in Moderate Risk Operable Breast Carcinoma* Giorgio Cocconi1, Corrado Boni2, Maurizio Tonato3, Rodolfo Passalacqua1, Mariantonietta Colozza3, Anna M. Mosconi3, Giancarlo Bisagni2, Ermanno Rondini2, Lina Rodinò4, Amalia Carpi4, Francesco Di Costanzo5, Mauro Brugia5, Giuseppe Attardo6, Luigi Acito7, Riccardo Rossetti8, Maria Bella1, Roberta Camisa1, Francesco Cardinale9, Beatrice Dozin9 1Medical Oncology Division, Azienda Ospedaliera Universitaria, Parma, Italy; 2Medical Oncology Service, Azienda Ospedaliera, Reggio Emilia, Italy; 3Medical Oncology Division, Azienda Ospedaliera Universitaria, Perugia, Italy; 4Medical Oncology Service, Azienda Sanitaria Locale, Piacenza, Italy; 5Medical Oncology Service, Azienda Sanitaria Locale, Terni, Italy; 6Medical Oncology Unit, Azienda Ospedaliera, Vigevano, Italy; 7Medical Oncology Division, Azienda Sanitaria Locale, Fermo, Italy; 8Miedical Oncology Unit, Marciano, Italy; 9Epidemiologia Clinica, Istituto Nazionale per la Ricerca sul Cancro, Genova, Italy. Email: Giorgio.cocconi@tin.it Received April 29th, 2011; revised June 2nd, 2011; accepted June 10th, 2011. ABSTRACT Objectives: The CMFEV (cyclophosphamide, methotrexate, 5-fluorouracil, epirubicin, vincristine) regimen is an inno- vative schedule, designed by our Group, aimed at administering five partially or totally no cross-resistant cytotoxic agents in breast carcinoma. It was randomly compared to CMF (cyclophosphamide, methotrexate, 5-fluorouracil) as primary treatmen t in operab le disea se and demons trated a short-term sign ifican t increase in clinical comp lete response rate and a long-term significant locoregional relapse-free survival in premenopausal patients. So, it seemed worth comparing this regimen with CMF as adjuvant chemotherapy in moderate risk operable breast carcinoma. Methods: Four hundred and eighty-nine patients with stage I or II moderate risk breast carcinoma were randomized to receive CMF or CMFEV regimen for 6 cycles after surgery. Main end points were overall survival (OS), invasive disease-free survival (IDFS) and recurrence-free interval (RFI), as estimated by Kaplan-Meier analyses and log-rank tests. Results: At a median observation time of 7.3 years (range 5.4 months-10.3 years), no significant differences in OS and IDFS were observed between the two arms. Deaths from breast carcinoma were more frequent with CMF (58.5%) than with CMFEV regimen (41.7%) as well as recurrences from breast carcinoma (58.8% with CMF and 41.2% with CMFEV). These differences were not statistically significant. Conclusion: CMFEV appears more effective than CMF in prevent- ing recurrences from primary disease in patients with moderate risk stage I-II breast carcinoma. The lack of statistical significance of th e ob served differen ces was p roba b ly du e to the limited number o f patients en rolled which rend ered the study underpowdered. Keywords: Breast Carcinoma, Adjuvant Chemotherapy, CMF Regimen, Epirubicin, Vincristine, Second Mali gna ncy 1. Introduction The role of adjuvant systemic therapy in early stage re- sectable breast carcinoma has been established in a number of prospective randomized studies, and its sig- nificant contribution in reducing the odds of relapse and death has been clearly validated by the worldwide over- view [1]. *On behalf of the Italian Oncology Group for Clinical Research (GOIRC), Parma, Italy. The Medical Oncology Units of Palermo, Foligno, Todi and Grosseto contributed with only a few cases. The Milan Cancer Institute research group activated the first studies demonstrating and confirming the long  Randomized Trial Comparing Cyclophosphamide, Methotrexate, and 5-Fluorouracil (CMF) Regimen with 343 Rotational CMFEV Regimen (E = Epirubicin, V = Vincristine) as Adjuvant Chemotherapy in Moderate Risk Operable Breast Carcinoma term efficacy of the combination of cyclophosphamide, methotrexate, 5-fluorouracil (CMF), which became a classical chemotherapy regimen [2,3]. Since the first re- port, this combination has been modified in a number of ways, but mainly by the addition of other drugs, such anthracycline, sometimes vincristine and, lately, taxanes [4-8]. The CMFEV (cyclophosphamide, methotrexate, 5- fluorouracil, epirubicin, vincristine) regimen is an inno- vative schedule, compared to CMF, aimed at administer- ing five partially or totally no cross-resistant cytotoxic agents. It was first designed and tested by our Group as a means of late intensification after CMF in metastatic breast carcinoma [9] and then in the neoadjuvant setting of operable breast carcinoma [10,11]. Its rotational strat- egy is different from that of alternating or sequential schemes as the five agents are administered at full dose but, in order to avoid excessive toxicity and consequent dose reductions, each cycle involves the administration of only four drugs, always including vincristine (V) and epirubicin (E). The regimen is organized in such a way that only two, among the three potentially myelotoxic drugs of the CMF regimen, are rotatively included in the schedule (CMEV, CFEV, MFEV). The planned dosages of C, M and F in each cycle were therefore either 100% or 0%. The aim of this prospective randomized study was to compare the classical CMF regimen with the CMFEV rotational regimen, both administered postoperatively for 6 cycles, in patients with operable breast carcinoma at a moderate risk of relapse (1 to 3 positive axillary nodes or axillary node negative with at least one biological or morphological risk factor). 2. Patients and Methods 2.1. Eligibility Criteria The main eligibility criteria were 1) histologically proven operable breast carcinoma recently submitted to a poten- tially curative surgery; 2) 1 to 3 axillary positive nodes or negative axillary nodes with at least one biological risk factor (estrogen and progesterone receptor negative and/or high proliferative activity (Ki 67, or another pro- liferative index, higher than 15%) and/or histological grade 3; 3) age ≤70 years; 4) absence of previous or concomitant contralateral breast carcinoma or of previ- ous or concomitant different malignant neoplasm; 5) ab- sence of distant metastases following a complete staging including physical examination, chest X-ray, bone scan, liver echography or computed tomography; adequate bone marrow, kidney, liver, and heart function. Patients with clinical stage III tumours (T3N1; or T4 any N; or any T N2) were not eligible. 2.2. Study Design and Treatment This was a multi-institutional study carried out by the Medical Oncology Units of Parma, Reggio Emilia, Pe- rugia, Piacenza, Terni, Vigevano, Fermo and Marsciano of the Italian Oncology Group for Clinical Research (GOIRC). The Medical Oncology Units of Palermo, Fo- ligno, Todi and Grosseto contributed with a few cases. The study design was approved by the ethical com- mittees of the participating institutions and conducted in accordance with the International Good Clinical Practice Guidelines. All patients gave their written informed con- sent before enrolment in the study. The patients were centrally randomized via phone call to the coordinating office of the GOIRC in Parma. Allocation was made within strata defined by institution, menopausal status (premenopausal vs. postmenopausal), tumour diameter (T1, T2, T3), hormonal receptor status (negative or posi- tive), axillary nodal status (positive or negative). Patients were assigned to receive 6 cycles of CMF regimen or 6 cycles of CMFEV rotational regimen. Doses and schedules of the CMF combination and of the CMFEV rotational combination are reported in Table 1. Postmenopausal patients, independently from their es- trogen receptor (ER) and/or progesterone receptor (PgR) status, received oral tamoxifen 20 mg per day for 5 years, starting at the end of the chemotherapy treatment. Premenopausal patients received this treatment with oral tamoxifen only if they had their estrogen and/or proges- terone receptor status positive. Blood chemistry and liver function tests were repeated on day 1 of each cycle, and complete blood counts were obtained on day 1 and 8. Treatment was delayed of one week if the white blood cell (WBC) count was lower than 4000 and/or platelet count was lower than 120,000. On day 1 after the one week delay, and on day 8, the dosages of C, M, F, and E were reduced by 30% when the WBC ranged from 3900 to 3600 and/or the platelet count ranged from 119,000 to 100,000, and by 50% when the WBC ranged from 3500 to 2500 and/or the platelet count ranged from 99,000 to 70,000. No drugs were administered when the WBC was less than 2500 and/or the platelet count was less than 70,000. No dose reductions were planned for vincristine. The adjuvant chemotherapy program had to begin no later than 6 weeks from the initial surgery. Radiation therapy, whenever indicated, had to start after the con- clusion of chemotherapy program. Treatment with tamoxifen (20 mg/day for 5 years), Copyright © 2011 SciRes. JCT 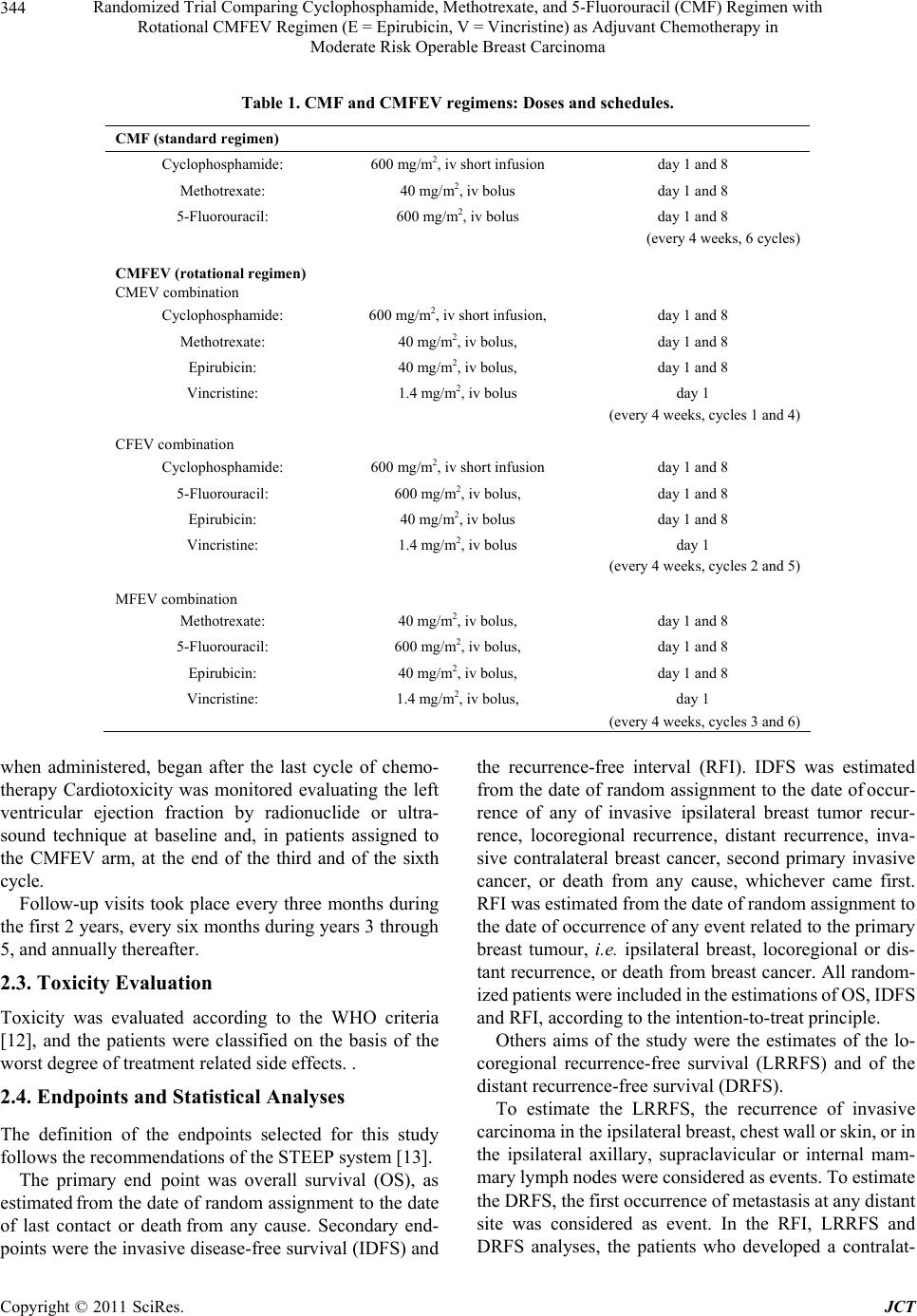 Randomized Trial Comparing Cyclophosphamide, Methotrexate, and 5-Fluorouracil (CMF) Regimen with 344 Rotational CMFEV Regimen (E = Epirubicin, V = Vincristine) as Adjuvant Chemotherapy in Moderate Risk Operable Breast Carcinoma Table 1. CMF and CMFEV re gimens: Doses and schedules. CMF (standard regimen) Cyclophosphamide: 600 mg/m2, iv short infusion day 1 and 8 Methotrexate: 40 mg/m2, iv bolus day 1 and 8 5-Fluorouracil: 600 mg/m2, iv bolus day 1 and 8 (every 4 weeks, 6 cycles) CMFEV (rotational regimen) CMEV combination Cyclophosphamide: 600 mg/m2, iv short infusion, day 1 and 8 Methotrexate: 40 mg/m2, iv bolus, day 1 and 8 Epirubicin: 40 mg/m2, iv bolus, day 1 and 8 Vincristine: 1.4 mg/m2, iv bolus day 1 (every 4 weeks, cycles 1 and 4) CFEV combination Cyclophosphamide: 600 mg/m2, iv short infusion day 1 and 8 5-Fluorouracil: 600 mg/m2, iv bolus, day 1 and 8 Epirubicin: 40 mg/m2, iv bolus day 1 and 8 Vincristine: 1.4 mg/m2, iv bolus day 1 (every 4 weeks, cycles 2 and 5) MFEV combination Methotrexate: 40 mg/m2, iv bolus, day 1 and 8 5-Fluorouracil: 600 mg/m2, iv bolus, day 1 and 8 Epirubicin: 40 mg/m2, iv bolus, day 1 and 8 Vincristine: 1.4 mg/m2, iv bolus, day 1 (every 4 weeks, cycles 3 and 6) when administered, began after the last cycle of chemo- therapy Cardiotoxicity was monitored evaluating the left ventricular ejection fraction by radionuclide or ultra- sound technique at baseline and, in patients assigned to the CMFEV arm, at the end of the third and of the sixth cycle. Follow-up visits took place every three months during the first 2 years, every six months during years 3 through 5, and annually thereafter. 2.3. Toxicity Evaluation Toxicity was evaluated according to the WHO criteria [12], and the patients were classified on the basis of the worst degree of treatment related side effects. . 2.4. Endpoints and Statistical Analyses The definition of the endpoints selected for this study follows the recommendations of the STEEP system [13]. The primary end point was overall survival (OS), as estimated from the date of random assignment to the date of last contact or death from any cause. Secondary end- points were the invasive disease-free survival (IDFS) and the recurrence-free interval (RFI). IDFS was estimated from the date of random assignment to the date of occur- rence of any of invasive ipsilateral breast tumor recur- rence, locoregional recurrence, distant recurrence, inva- sive contralateral breast cancer, second primary invasive cancer, or death from any cause, whichever came first. RFI was estimated from the date of random assignment to the date of occurrence of any event related to the primary breast tumour, i.e. ipsilateral breast, locoregional or dis- tant recurrence, or death from breast cancer. All random- ized patients were included in the estimations of OS, IDFS and RFI, according to the intention-to-treat principle. Others aims of the study were the estimates of the lo- coregional recurrence-free survival (LRRFS) and of the distant recurrence-free survival (DRFS). To estimate the LRRFS, the recurrence of invasive carcinoma in the ipsilateral breast, chest wall or skin, or in the ipsilateral axillary, supraclavicular or internal mam- mary lymph nodes were considered as events. To estimate the DRFS, the first occurrence of metastasis at any distant site was considered as event. In the RFI, LRRFS and DRFS analyses, the patients who developed a contralat- Copyright © 2011 SciRes. JCT 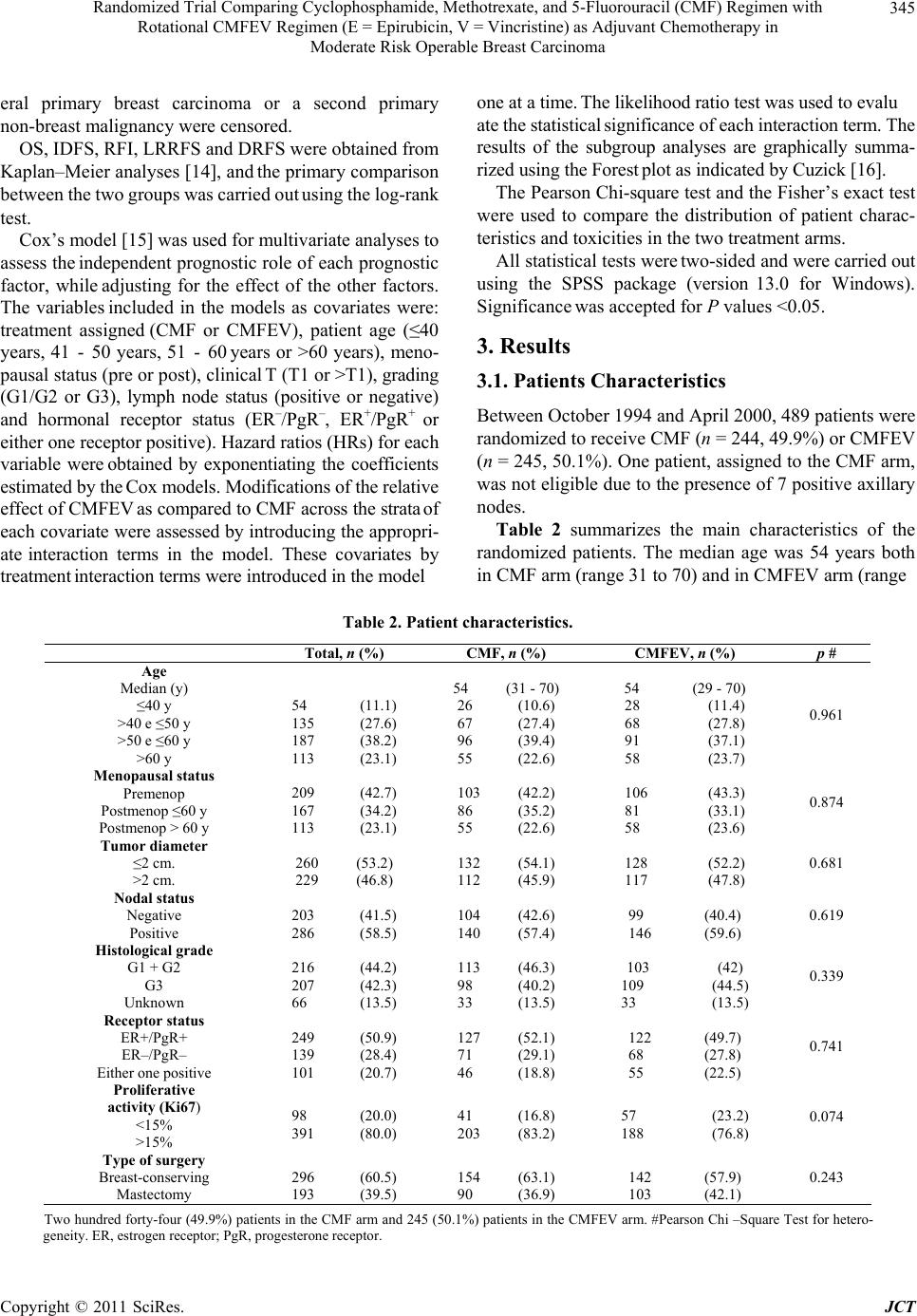 Randomized Trial Comparing Cyclophosphamide, Methotrexate, and 5-Fluorouracil (CMF) Regimen with 345 Rotational CMFEV Regimen (E = Epirubicin, V = Vincristine) as Adjuvant Chemotherapy in Moderate Risk Operable Breast Carcinoma one at a time. The likelihood ratio test was used to evalu eral primary breast carcinoma or a second primary non-breast malignancy were censored. ate the statistical significance of each interaction term. The results of the subgroup analyses are graphically summa- rized using the Forest plot as indicated by Cuzick [16]. OS, IDFS, RFI, LRRFS and DRFS were obtained from Kaplan–Meier analyses [14], and the primary comparison between the two groups was carried out using the log-rank test. The Pearson Chi-square test and the Fisher’s exact test were used to compare the distribution of patient charac- teristics and toxicities in the two treatment arms. Cox’s model [15] was used for multivariate analyses to assess the independent prognostic role of each prognostic factor, while adjusting for the effect of the other factors. The variables included in the models as covariates were: treatment assigned (CMF or CMFEV), patient age (≤40 years, 41 - 50 years, 51 - 60 years or >60 years), meno- pausal status (pre or post), clinical T (T1 or >T1), grading (G1/G2 or G3), lymph node status (positive or negative) and hormonal receptor status (ER–/PgR–, ER+/PgR+ or either one receptor positive). Hazard ratios (HRs) for each variable were obtained by exponentiating the coefficients estimated by the Cox models. Modifications of the relative effect of CMFEV as compared to CMF across the strata of each covariate were assessed by introducing the appropri- ate interaction terms in the model. These covariates by treatment interaction terms were introduced in the model All statistical tests were two-sided and were carried out using the SPSS package (version 13.0 for Windows). Significance was accepted for P values <0.05. 3. Results 3.1. Patients Characteristics Between October 1994 and April 2000, 489 patients were randomized to receive CMF (n = 244, 49.9%) or CMFEV (n = 245, 50.1%). One patient, assigned to the CMF arm, was not eligible due to the presence of 7 positive axillary nodes. Table 2 summarizes the main characteristics of the randomized patients. The median age was 54 years both in CMF arm (range 31 to 70) and in CMFEV arm (range Table 2. Patient characteristics. Total, n (%) CMF, n (%) CMFEV, n (%) p # Age Median (y) ≤40 y >40 e ≤50 y >50 e ≤60 y >60 y 54 (11.1) 135 (27.6) 187 (38.2) 113 (23.1) 54 (31 - 70) 26 (10.6) 67 (27.4) 96 (39.4) 55 (22.6) 54 (29 - 70) 28 (11.4) 68 (27.8) 91 (37.1) 58 (23.7) 0.961 Menopausal status Premenop Postmenop ≤60 y Postmenop > 60 y 209 (42.7) 167 (34.2) 113 (23.1) 103 (42.2) 86 (35.2) 55 (22.6) 106 (43.3) 81 (33.1) 58 (23.6) 0.874 Tumor diameter ≤2 cm. >2 cm. 260 (53.2) 229 (46.8) 132 (54.1) 112 (45.9) 128 (52.2) 117 (47.8) 0.681 Nodal status Negative Positive 203 (41.5) 286 (58.5) 104 (42.6) 140 (57.4) 99 (40.4) 146 (59.6) 0.619 Histological grade G1 + G2 G3 Unknown 216 (44.2) 207 (42.3) 66 (13.5) 113 (46.3) 98 (40.2) 33 (13.5) 103 (42) 109 (44.5) 33 (13.5) 0.339 Receptor status ER+/PgR+ ER–/PgR– Either one positive 249 (50.9) 139 (28.4) 101 (20.7) 127 (52.1) 71 (29.1) 46 (18.8) 122 (49.7) 68 (27.8) 55 (22.5) 0.741 Proliferative activity (Ki67) <15% >15% 98 (20.0) 391 (80.0) 41 (16.8) 203 (83.2) 57 (23.2) 188 (76.8) 0.074 Type of surgery Breast-conserving Mastectomy 296 (60.5) 193 (39.5) 154 (63.1) 90 (36.9) 142 (57.9) 103 (42.1) 0.243 Two hundred forty-four (49.9%) patients in the CMF arm and 245 (50.1%) patients in the CMFEV arm. #Pearson Chi –Square Test for hetero- geneity. ER, estrogen receptor; PgR, progesterone receptor. Copyright © 2011 SciRes. JCT  Randomized Trial Comparing Cyclophosphamide, Methotrexate, and 5-Fluorouracil (CMF) Regimen with 346 Rotational CMFEV Regimen (E = Epirubicin, V = Vincristine) as Adjuvant Chemotherapy in Moderate Risk Operable Breast Carcinoma 29 to 70). Two hundred and nine (42.7%) patients were premenopausal; overall, 280 (57.3%) patients were postmenopausal; among those, 167 (34.2%) were <60 years and 113 (23.1%) were >60 years. Two hundred and sixty (53.2%) patients had tumour diameter <2 cm and 229 (46.8%) >2 cm. Two hundred and three (41.5%) patients were node-negative and 286 (58.5%) patients were node-positive. Histological grade G1 or G2 were found in 216 (44.2%) patients and G3 in 207 (42.3%) patients. ER and PgR were both positive in 249 (50.9%) patients and both negative in 139 (28.4%) patients. Either one of the receptors was positive in 101 (20.7%) of the cases. Tumour proliferative activity was low in 98 (20.0%) patients and moderate/high in 391 (80.0%) pa- tients. Two hundred ninety-six (60.5%) patients received breast conserving surgery, and the remaining 193 (39.5%) underwent mastectomy. No remarkable differences in the patient characteristic distribution between the two study arms were seen (all p > 0.05). 3.2. Survival and Events The distribution of all events (death, recurrence or new malignancy) between the two arms is summarized in Table 3. Sample size estimates, at the time of the design of this trial, were based on unrealistic and outdated projections about survival and effects of the experimental treatment (e.g. 5-year OS = 65%). Posterior power estimates, based on the number of events actually observed, indicate that the study, with 120 relapses, had a power of 89% to de- tect HR’s of 0.6 for IDFS. With 63 deaths, the study had standard power (80%) to detect only risk reductions in excess of 50%. 3.2.1. Overall Survival The cut-off date for follow-up was July 31, 2006. The median observation time from random assignment to death or censoring was 7.31 years (range: 5.4 months -10.3 years). At the end of the observation period, 426 patients (87.1 %) were alive, with a median follow-up of 7.68 years (7.73 years for the CMF arm and 7.64 years for the CMFEV arm). Among those alive patients, 36 (85.4%) were disease-free and 62 (14.6%) were not. Overall, 63 deaths occurred, 33 (52.4%) in the CMF arm and 30 (47.6%) in the CMFEV arm. Among these deaths, 48 (76.2%) were a consequence of the primary breast tumor and 15 (23.8%) were due to other causes. No sig- nificant difference in OS was seen between the two arms (Figure 1, log rank p = 0.687). Cumulative OS at 5 years was 93.0% (95% CI 91.2 - 94.4) in the CMFEV arm and 92.6% (95% CI 90.8 - 94.1) in the CMF arm. At 10 years, these values decreased to 80.5% (95% CI 77.9 - 82.4) in the CMFEV arm and 82.3 (95% CI 79.8 - 84.5) in the CMF arm. 3.2.2. Invasive Disease-Free Survival Overall, 120 events were observed, 61(50.8%) in the CMF arm and 59 (49.2%) in the CMFEV arm. Among these events, 1 case of death from primary breast cancer without relapse was observed. The 120 events thus in- cluded 90 locoregional or distant recurrences and 30 cases of second malingnacy or contralateral breast cancer. IDFS, as estimated for all these events, was not statisti- cally different between the two arms (log rank p = 0.892, not shown). 3.2.3. Recurrence-Free Interval, Locoregional Recurrence-Free Survival and Distant Recurrence-Free Survival When considering the RFI, which evaluates only the events related to the primary breast tumor, we observed that the rate of recurrence was higher in the CMF arm than in the CMFEV arm. Of the 90 recurrences, 53 (58.9%) occurred in the CMF arm and only 37 (41.1%) Table 3. Events according to treatment. EVENT CMF arm n (%) CMFEV arm n (%)TOTAL n (%) Death from breast cancer from other cause total 28 (58.5) 5 (33.3) 33 (52.4) 20 (41.7) 10 (66.7) 30 (47.6) 48 (76.2) 15 (23.8) 63 (100.0) Recurrence locoregional distant ¶ total 15 (62.5) 38 (57.5) 53 (58.9) 9 (37.5) 28 (42.4) 37 (41.1) 24 (26.7) 66 (73.3) 90 (100.0) Second malignancies contralateral breast endometrium leukemia others § total 2 (33.3) 3 (50.0) 0 - 3 (20.0) 8 (26.7) 4 (66.7) 3 (50.0) 3 (100.0) 12 (80.0) 22 (73.3) 6 (20.0) 6 (20.0) 3 (10.0) 15 (50.0) 30 (100.0) ¶ including the one patient who died without revealed recurrence; § including ovary Copyright © 2011 SciRes. JCT 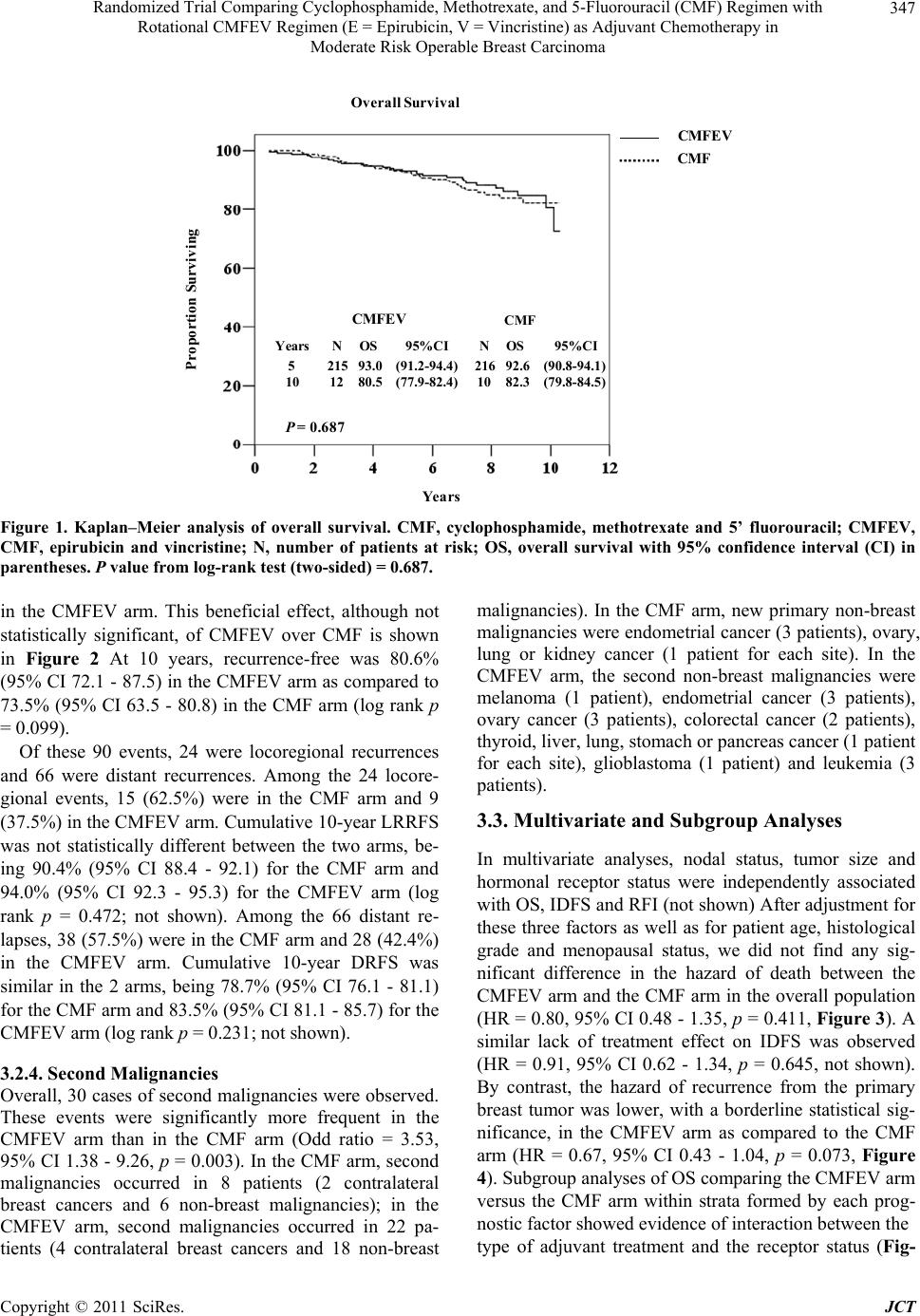 Randomized Trial Comparing Cyclophosphamide, Methotrexate, and 5-Fluorouracil (CMF) Regimen with 347 Rotational CMFEV Regimen (E = Epirubicin, V = Vincristine) as Adjuvant Chemotherapy in Moderate Risk Operable Breast Carcinoma OS 95%CIOSYears 5 10 N 215 12 93.0 80.5 (91.2-94.4) (77.9-82.4) CMF EV N 216 10 92.6 82.3 95%CI (90.8-94.1) (79.8-84.5) CMF P= 0.687 CMFEV CMF Overall Survival Years Proportion Surviving Figure 1. Kaplan–Meier analysis of overall survival. CMF, cyclophosphamide, methotrexate and 5’ fluorouracil; CMFEV, CMF, epirubicin and vincristine; N, number of patients at risk; OS, overall survival with 95% confidence interval (CI) in parentheses. P value from log-rank test (two-sided) = 0.687. in the CMFEV arm. This beneficial effect, although not statistically significant, of CMFEV over CMF is shown in Figure 2 At 10 years, recurrence-free was 80.6% (95% CI 72.1 - 87.5) in the CMFEV arm as compared to 73.5% (95% CI 63.5 - 80.8) in the CMF arm (log rank p = 0.099). Of these 90 events, 24 were locoregional recurrences and 66 were distant recurrences. Among the 24 locore- gional events, 15 (62.5%) were in the CMF arm and 9 (37.5%) in the CMFEV arm. Cumulative 10-year LRRFS was not statistically different between the two arms, be- ing 90.4% (95% CI 88.4 - 92.1) for the CMF arm and 94.0% (95% CI 92.3 - 95.3) for the CMFEV arm (log rank p = 0.472; not shown). Among the 66 distant re- lapses, 38 (57.5%) were in the CMF arm and 28 (42.4%) in the CMFEV arm. Cumulative 10-year DRFS was similar in the 2 arms, being 78.7% (95% CI 76.1 - 81.1) for the CMF arm and 83.5% (95% CI 81.1 - 85.7) for the CMFEV arm (log rank p = 0.231; not shown). 3.2.4. Second Malignan cies Overall, 30 cases of second malignancies were observed. These events were significantly more frequent in the CMFEV arm than in the CMF arm (Odd ratio = 3.53, 95% CI 1.38 - 9.26, p = 0.003). In the CMF arm, second malignancies occurred in 8 patients (2 contralateral breast cancers and 6 non-breast malignancies); in the CMFEV arm, second malignancies occurred in 22 pa- tients (4 contralateral breast cancers and 18 non-breast malignancies). In the CMF arm, new primary non-breast malignancies were endometrial cancer (3 patients), ovary, lung or kidney cancer (1 patient for each site). In the CMFEV arm, the second non-breast malignancies were melanoma (1 patient), endometrial cancer (3 patients), ovary cancer (3 patients), colorectal cancer (2 patients), thyroid, liver, lung, stomach or pancreas cancer (1 patient for each site), glioblastoma (1 patient) and leukemia (3 patients). 3.3. Multivariate and Subgroup Analyses In multivariate analyses, nodal status, tumor size and hormonal receptor status were independently associated with OS, IDFS and RFI (not shown) After adjustment for these three factors as well as for patient age, histological grade and menopausal status, we did not find any sig- nificant difference in the hazard of death between the CMFEV arm and the CMF arm in the overall population (HR = 0.80, 95% CI 0.48 - 1.35, p = 0.411, Figure 3). A similar lack of treatment effect on IDFS was observed (HR = 0.91, 95% CI 0.62 - 1.34, p = 0.645, not shown). By contrast, the hazard of recurrence from the primary breast tumor was lower, with a borderline statistical sig- nificance, in the CMFEV arm as compared to the CMF arm (HR = 0.67, 95% CI 0.43 - 1.04, p = 0.073, Figure 4). Subgroup analyses of OS comparing the CMFEV arm versus the CMF arm within strata formed by each prog- nostic factor showed evidence of interaction between the type of adjuvant treatment and the receptor status (Fig- Copyright © 2011 SciRes. JCT 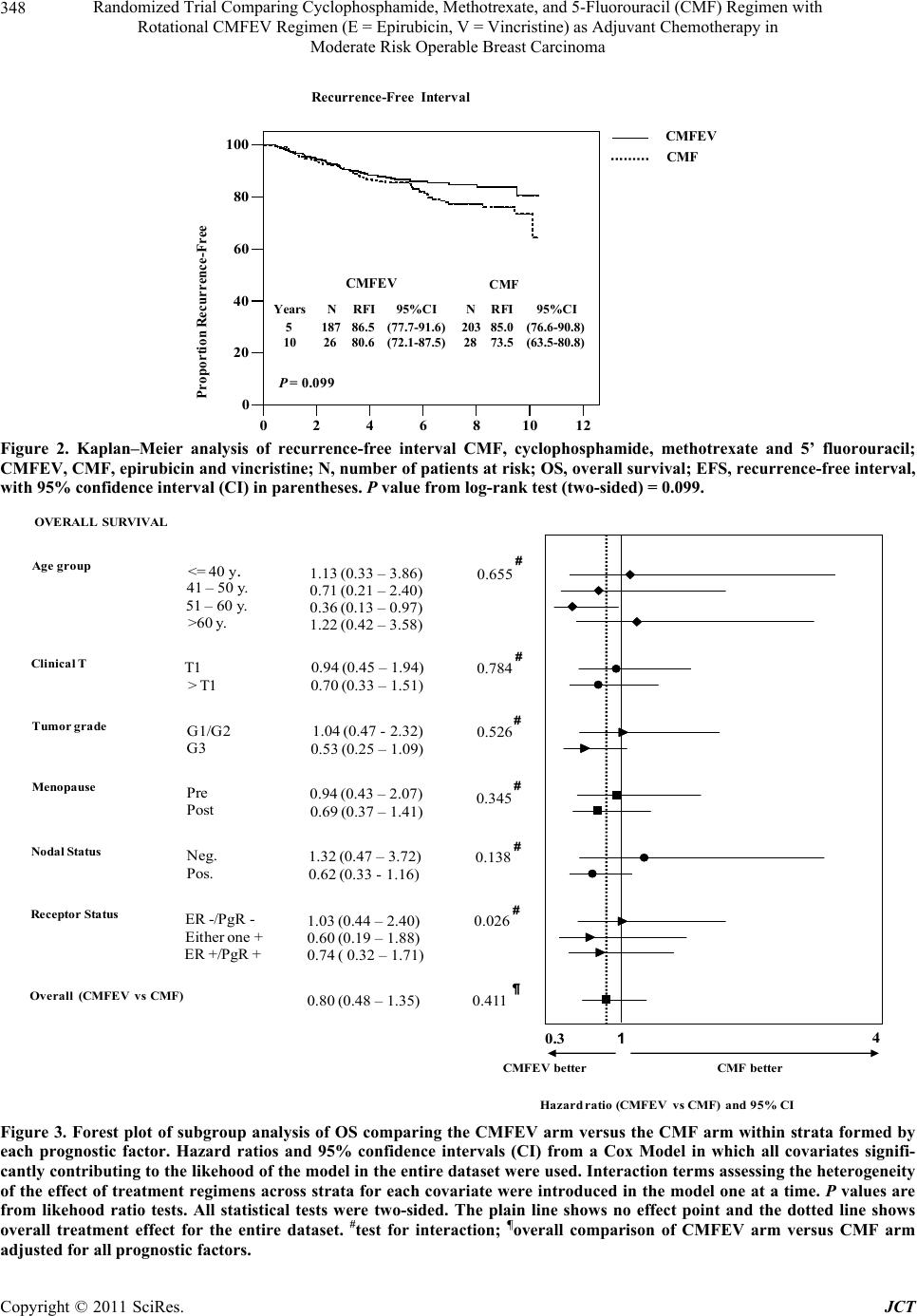 Randomized Trial Comparing Cyclophosphamide, Methotrexate, and 5-Fluorouracil (CMF) Regimen with 348 Rotational CMFEV Regimen (E = Epirubicin, V = Vincristine) as Adjuvant Chemotherapy in Moderate Risk Operable Breast Carcinoma 121086420 100 80 60 40 20 0 CMFEV CMF Recurrence Free Interval P= 0.099 RFI 95%CIRFIYears 5 10 N 86.5 80.6 (77.7-91.6) (72.1-87.5) CMFEV N 203 28 85.0 73.5 95%CI (76.6-90.8) (63.5-80.8) CMF 187 26 Proport ion Recurren ce-F ree Figure 2. Kaplan–Meier analysis of recurrence-free interval CMF, cyclophosphamide, methotrexate and 5’ fluorouracil; CMFEV, CMF, epirubicin and vincristine; N, number of patients at risk; OS, overall survival; EFS, recurrence-free interval, with 95% confidence interval (CI) in parentheses. P value from log-rank test (two-sided) = 0.099. C lin ic al T Ag e group Tu mor grad e Me nopause Nodal Status Receptor Status Overall (C MFEV vs CMF) <= 40 y . 41 –50 y. 51 –60 y. >60 y. T1 > T1 G1/G2 G3 Pre Post Neg. Pos. ER -/PgR - Either one + ER +/PgR + 0.655 0.784 0.526 0.345 0.138 0.026 1.03 (0.44 –2.40) 0.74 ( 0.32 – 1.71) 1.13 (0.33 –3.86) 0.71 (0.21 –2.40) 0.36 (0.13 –0.97) 1.22 (0.42 –3.58) 0.94 (0.45 –1.94) 0.70 (0.33 –1.51) 1.04 (0.47 -2.32) 0.53 (0.25 –1.09) 0.94 (0.43 –2.07) 0.69 (0.37 –1.41) 1.32 (0.47 –3.72) 0.62 (0.33 -1.16) 0.60 (0.19 –1.88) 0.80 (0.48 –1.35)0.411 # ¶ # # # # # CMFE V be tte rCMF be tte r H az ard ratio (CM FEV vs CMF) and 95% CI 0.3 14 OVE RAL L SURVIVAL Figure 3. Forest plot of subgroup analysis of OS comparing the CMFEV arm versus the CMF arm within strata formed by each prognostic factor. Hazard ratios and 95% confidence intervals (CI) from a Cox Model in which all covariates signifi- cantly contributing to the likehood of the model in the entire dataset were used. Interaction terms assessing the heterogeneity of the effect of treatment regimens across strata for each covariate were introduced in the model one at a time. P values are from likehood ratio tests. All statistical tests were two-sided. The plain line shows no effect point and the dotted line shows overall treatment effect for the entire dataset. #test for interaction; ¶overall comparison of CMFEV arm versus CMF arm adjusted for all prognostic factors. Copyright © 2011 SciRes. JCT  Randomized Trial Comparing Cyclophosphamide, Methotrexate, and 5-Fluorouracil (CMF) Regimen with 349 Rotational CMFEV Regimen (E = Epirubicin, V = Vincristine) as Adjuvant Chemotherapy in Moderate Risk Operable Breast Carcinoma CMF E V be tt e r 0.1 51 CMF be tte r C li n ica l T A g e gr oup Tu mor grade Menopaus e Nodal Status Rece ptor Status <= 40 y . 41 –50 y. 51 –60 y. >60 y. T1 > T1 G1/G2 G3 Pre Post Neg. Pos. ER -/PgR - Either one + ER +/PgR + 0.233 0.203 0.149 0.460 0.57 (0.15 –2.25) 0.65 (0.28 –1.53) 0.57 (0.26 –1.21) 0.88 (0.36 – 2.17) 0.64 (0.33 –1.25) 0.71 (0.40 –1.29) 0.88 (0.47 –1.63) 0.47 (0.25 –0.91) 0.60 (0.30 –1.19) 0.74 (0.41 –1.32) 1.06 (0.22 –5.01) 0.77 (0.31 –1.91) 1.03 (0.47 –2.24) 0.55 (0.22 –1.38) 0.48 ( 0.24 – 0.98) 0.67 (0.43 –1.04)0.073 0.002 # ¶ 0.338 # # # # # Hazard ratio CMFEV vs CMF and 95% CI Overall (CMFEV vs. CMF) RECURRENCE-FREE INTERVAL Figure 4. Forest plot of subgroup analysis of RFS comparing the CMFEV arm versus the CMF arm within strata formed by each prognostic factor. Hazard ratios and 95% confide nce intervals (CI) from a Cox Model in which all covariates significantly contributing to the likehood of the model in the entire dataset were used. Interaction terms assessing the heterogeneity of the effect of treatment regimens across strata for each covariate were introduced in the model one at a time. P values are from like- hood ratio tests. All statistical tests were two-sided. The plain line shows no effect point and the dotted line shows overall treat- ment effect for the entire dataset. #test for interaction; ¶overall comparison of CMFEV arm versus CMF arm adjusted for all prognostic factors. Table 4. Main toxicities. CMF arm CMFEV arm n%n% Hematological toxicities Hemoglobin G1/2 34147129 G3/4 7318 7 WBC G1/2 1044393 38 G3/4 17744 18 Platelets G1/2 4294 Non haematological toxicities Nausea/vomiting 1606617973 Epigastric pain 27114016 Constipation 62156 Oral 532210944 Mucositis 71296928 Skin 104156 Kidney 2121 Liver 30122912 Heart 156177 Asthenia 27114117 Mandibular pain 00146 Peripheral nervous system 1677430 Copyright © 2011 SciRes. JCT  Randomized Trial Comparing Cyclophosphamide, Methotrexate, and 5-Fluorouracil (CMF) Regimen with 350 Rotational CMFEV Regimen (E = Epirubicin, V = Vincristine) as Adjuvant Chemotherapy in Moderate Risk Operable Breast Carcinoma ure 3, p = 0.026). CMFEV regimen was more favourable when patients presented both estrogen and progesterone receptors positive, or either one receptor positive (HR = 0.74, 95% CI 0.32 - 1.71 and HR = 0.60, 95% CI 0.19 - 1.88, respectively). By contrast, no treatment effect was seen when both receptors were negative (HR = 1.03, 95% CI 0.44 - 2.40) Similar results were obtained for sub- group analyses of RFI (Figure 4, p = 0.002; HR = 0.48, 95% CI 0.24 - 0.98 for ER+/PgR+; HR = 0.55, 95% CI 0.22 - 1.38 for either one receptor positive; HR = 1.0, 95% CI 0.47-2.24 for ER-/PgR). Regarding IDFS sub- group analysis, no significant treatment effect with re- spect to positive receptors was observed (not shown). 3.4. Toxicities Table 4 reports the main toxicities according to treat- ment. With respect to haematological toxicities, there was a higher incidence of anaemia both grade 1 - 2 and 3 - 4 in the CMFEV arm than in the CMF arm (29% vs. 14%, p = < 0.001 and 7% vs. 3%, p = 0.02, respectively); there were no relevant differences between the two arms in terms of WBC and platelet toxicities. With respect to non-haematological toxicities, in the CMFEV arm, compared to the CMF arm, there was a more frequent occurrence of stomatitis (44% vs. 22%; p < 0.001), constipation (6% vs. 2%; p = 0.04), peripheral nervous system toxicity (30% vs. 7%; p < 0.001), man- dibular pain (6% vs. 0%). 4. Discussion 4.1. CMFEV Regimen versus CMF Regimen: General Considerations In the present study, our Group compared for the second time the conventional CMF regimen with the experi- mental CMFEV regimen in the treatment of non metas- tatic breast carcinoma. In the first occasion, the com- parison was developed in a neoadjuvant settings [10,11]; in the present second occasion, the comparison was de- veloped in an adjuvant settings. Therefore such com- parison presents, by itself, some similarities but also some substantial differences. Firstly, these differences are related to the therapeutic scheme utilized, which re- fers to the different treatment settings. Moreover, in the neoadjuvant settings, the comparison of the therapeutic results could be done both on a short term, based on the evaluation of objective therapeutic response [10], as well as on a long term, based on the outcome [11]. In the ad- juvant settings the comparison was possible only on a long term. In addition, in the present study, some results, which confirmed a superiority of the CMFEV regimen over the CMF regimen, were possibly disturbed and masked by an unexpected increase of second malignancies observed in the CMFEV arm. For all these reasons, we believe that in a first part of the discussion, it could be useful to summarize in a comparative way the number and the proportions of the main events (relapse and death) we observed in the pre- sent study when administering CMF or CMFEV. In a second part of the discussion, we will elaborate a com- parative evaluation of the main results observed in our first study of neoadjuvant chemotherapy and in the pre- sent study of adjuvant chemotherapy, in terms of efficacy, relationship with endocrine parameters, toxicities. In a third part, we will comment on the unexpected increase of second malignant tumors here observed with CMFEV. In the last part of the discussion, we will compare our results to those reported by other Authors within a com- prehensive evaluation of the adjuvant chemotherapy of operable breast carcinoma. 4.2. CMFEV Regimen versus CMF Regimen: Efficacy, Relationship with Endocrine Parameters and Toxicity The results of the present study showed that the recur- rence from the primary tumor was more frequent with CMF regimen (63 events, 58.2%) than with CMFEV regimen (38 events, 41.8%). At 10 years, RFI was 73.5% with CMF and 80.6% with CMFEV. Similarly, deaths due to primary tumor were more frequent in the CMF arm (28, 58.5%) compared to the CMFEV arm (20, 41.7%), even if the OS at 10 years was similar with ei- ther regimen (82.3% with CMF, 80.5% with CMFEV). These differences in terms of recurrence and death, although not statistically significant, offer evidence of a potential major efficacy of the CMFEV compared to the CMF regimen. This efficacy was, at least in part, more clearly showed in our previous study on neo-adjuvant chemotherapy where, on a short term evaluation, the rate of clinical responses, both complete (CR) and complete plus partial (PR), were significantly higher in the subset of pre-menopausal patients treated with CMFEV com- pared to those treated with CMF [10]. Similarly, on a long-term evaluation, again in the subset of pre-menopausal patients, the proportion of RFS tended to be higher and the proportion of LRRFS was significantly higher in the CMFEV arm compared to the CMF arm, thus mirroring the short-term response results [11]. The reason of the lack of statistical significant differ- ences observed in the present study and of only a few statistically significant differences observed in the pre- Copyright © 2011 SciRes. JCT  Randomized Trial Comparing Cyclophosphamide, Methotrexate, and 5-Fluorouracil (CMF) Regimen with 351 Rotational CMFEV Regimen (E = Epirubicin, V = Vincristine) as Adjuvant Chemotherapy in Moderate Risk Operable Breast Carcinoma ent study. vious study, is probably due to the fact that, in the two studies, different parameters were considered and esti- mated, and that the eligibility criteria, although similar, were not identical. In any case, both studies may have been under-powered to demonstrate a significant major efficacy of CMFEV over CMF. Interestingly, both the previous and the present studies showed statistically significant differences between the two chemotherapy regimens according to the menopausal status of the patients or to the biological characteristics of their tumors in terms of ER and/or PgR status. Indeed in the first study, the superiority of CMFEV regimen, as short term objective response, was seen only in pre- menopausal and not in post-menopausal patients; in ad- dition, in a multivariate analysis, a significant interaction was confirmed between the menopausal status and the type of treatment on the probability to obtain CR or CR plus PR [10]. In the present study, a significant correla- tion was observed between the estrogen and/or proges- terone receptor status and the type of regimen; CMFEV was more effective, in terms of OS and RFI, in patients with positive ER and/or PgR tumors but not in patients with both negative ER and PgR tumors. Considering those correlations, it appears that endocrine influences induced by the menopausal status of the patients and/or by the ER and/or PgR status of the tumors may deter- mine the comparative responses of CMF / CMFEV used either as neo-adjuvant or adjuvant chemotherapy in op- erable breast carcinoma. As to the main toxicities observed in the present study, the higher proportion of constipation, mandibular pain and peripheral nervous system toxicity on the CMFEV, compared to CMF regimen, could be due to the addition of vincristine. In the first study, a higher proportion of mild neurological side effects was also observed when administering CMFEV as compared to CMF [10]. The anaemia and the stomatitis could also be attributed to vincristine, or to the addition of epirubicin. Overall, the toxicities reported after the administration of CMFEV were rather well tolerated; only in a few patients, the treatment had to be shortened or vincristine administra- tion had to be discontinued. 4.3. CMFEV Regimen versus CMF Regimen: Incidence of Second Malignancies The significantly higher incidence of second malignan- cies with CMFEV as compared to CMF was unexpected and deserves some consideration as we did not observed this difference when using these regimens in a neo-adjuvant settings [10] This discrepancy may result either from the higher number of patients in the present study (489 versus 211) or from differences in the number of chemotherapy cycles delivered (4 cycles as neo-adjuvant therapy versus 6 cycles as adjuvant ther- apy). Moreover, epirubicin and vincristine were admin- istered in 3 and 4 cycles, respectively, in the previous study [10] compared to 6 cycles in the pres 4.4. CMFEV Regimen versus CMF Regimen: Comprehensive Evaluation of the Adjuvant Chemotherapy of Operable Breast Carcinoma Two overviews had reported that, in the adjuvant che- motherapy of operable breast cancer, anthracycline con- taining regimens were more effective than CMF in pre- venting recurrence and death [1,4]. This concept was particularly stressed by the former study that reported highly statistically significant differences in favour of the anthracycline-containing regimens as compared to CMF [1]. It has to be noted that this overview focused on the anthracycline-containing regimens FAC (fluorouracil, adriamycin/doxorubicin, cyclophosphamide) and FEC (fluorouracil, epirubicin, cyclophosphamide) while the anthracycline-containing regimens AC (adriamycin/, cyclophosphamide) and EC (epirubicin, cyclophos- phamide) were not considered [1]. According to the clas- sification adopted by us in 2003 [5], the administration of anthracycline in the AC and EC regimens was substan- tially “substitutive” as only one of the three CMF agents (cyclophosphamide) was maintained, while in the FAC and FEC regimens the administration of anthracycline was substantially “additive” as two of the three agents of CMF (cyclophosphamide and 5-fluorouracil) were main- tained. On the other hand, considering the results of single studies where more than 2000 patients were enrolled, comparisons of CMF with the AC regimen did not pro- vide evidences favouring AC [17,18], while comparisons of CMF with the FAC regimen or with the FEC regimen reported results significantly favouring these two anthra- cycline-containing combinations [19,20]. In this light, our CMFEV experimental regimen clearly belongs to the “additive” type of combinations but it exceeds FAC or FEC combinations as it involves the conservation of not only two, but of all three CMF agents, although in a rotational strategy. This considera- tion together with the results we obtained with the CMFEV regimen, both in the first study and in the pre- sent one, should encourage the conduction of further studies but the unexpected increase of second malignan- cies here observed upon CMFEV treatment suggests some cautions. In addition, at the present time, any dis- cussion about the adjuvant chemotherapy of breast car- Copyright © 2011 SciRes. JCT  Randomized Trial Comparing Cyclophosphamide, Methotrexate, and 5-Fluorouracil (CMF) Regimen with 352 Rotational CMFEV Regimen (E = Epirubicin, V = Vincristine) as Adjuvant Chemotherapy in Moderate Risk Operable Breast Carcinoma cinoma should not exclude the consideration of the addi- tion of taxanes to anthracyclines although results re- ported so far in this line do not sufficiently encourage this approach [21,22]. 4.5. Conclusions This study provides preliminary evidence suggesting a potentially higher efficacy of the CMFEV regimen as compared to the standard CMF regimen. This result is in line with the overall evidence demonstrating an increased efficacy of anthracycline-containing adjuvant regimens in breast cancer [1], but also lends to support the ration- ale of using a 5-drugs, rotational regimen. However, be- fore this rationale can be further explored in larger, more focused trials, the increased incidence of cancer at other sites here observed in the CMFEV arm, needs to be thoroughly considered. 5. Acknowledgements This work was supported by a grant from Associazione Italiana per la Ricerca sul Cancro (AIRC). The authors would like to thank dr. Paolo Bruzzi for his statistical help in the preparation of the manuscript. REFERENCES [1] Early Breast Cancer Trialists’ Collaborative Group, “Ef- fect of Chemotherapy and Hormonal Therapy for Early Breast Cancer on Recurrence and 15-Year Survival: An Overview of the Randomised Trials,” Lancet, Vol. 365, No. 9472, 2005, pp. 1687-1717. doi:10.1016/S0140-6736(05)66544-0 [2] G. Bonadonna, E. Brusamolino, P. Valagussa, A. Rossi, L. Brugnatelli, C. Brambilla, M. De Lena, G. Tancini, E. Bajetta, R. Musumeci and U. Veronesi, “Combination Chemotherapy as an Adjuvant Treatment in Operable Breast Cancer,” The New England Journal of Medicine, Vol. 294, No. 8, 1976, pp. 405-410. doi:10.1056/NEJM197602192940801 [3] G. Bonadonna, A. Moliterni, M. Zambetti, M. G. Dandone, S. Pilotti, L. Gianni and P. Valagussa, “30 Years’ Follow up of Randomised Studies of Adjuvant CMF in Operable Breast Cancer: Cohort Study,” British Medicine Journal, Vol. 330, No. 7485, 2005, pp. 205-206. doi:10.1136/bmj.38314.622095.8F [4] Early Breast Cancer Trialists Collaborative Group, “Poly- chemotherapy for Early Breast Cancer: An Overview of Randomised Trials,” Lancet, Vol. 352, No. 9132, 1998, No. 9132, pp. 930-942. doi:10.1016/S0140-6736(98)03301-7 [5] G. Cocconi, “Adjuvant Chemotherapy in Early Breast Cancer: Optimal and Suboptimal Anthracycline Containig Regimens,” Breast Cancer Research and Treatment, Vol. 80, No. 3, 2003, pp. 313-320. doi:10.1023/A:1024955408785 [6] R. B Weiss, D. C.Tormey, F. Holland, V. E. Weinberg, G. Lesnick, M. Perloff, G. Falkson and O. J. Glidewell, “A Randomised Trial of Postoperative Five-versus Three Drug Chemotherapy after Mastectomy; A Cancer and Leukemia Group B (CALGB) Study,” Recent Results Cancer Research, Vol. 80, No. 1, 1982, pp. 170-176. [7] D. C. Tormey, V. E. Weinberg, J. F. Holland, R. B. Weiss, O. J. Glidewell, M. Perloff, G. Falson, H. C. Falkson, P. H. Henry and L. A. Leone, “A Randomised Trial of Five and Three Drug Chemotherapy and Chemo-Immunotherapy in Women with Operable Node Positive Breast Cancer. A CALGB Study,” Journal of Clinical Oncology, Vol. 1, No. 2, 1983, pp. 138-145. [8] L. Gianni, J. Baselga, W, Elemann, V. G. Porta, V. Semi- glazov, A. Lluch, M. Zambetti, D. Sabadell, G. Raab, A. L. Cussac, A. Bozhok, A. Martinez-Agullo, M. Greco, M. Byakhov, J. J. Lopez, M. Mansutti, P. Valagussa and G. Bonadonna, “Phase III Trial Evaluating the Addition of Paclitaxel to Doxorubicin Followed by Cyclo-phosphamide, Methotrexate, and Fluorouracil, as Adjuvant or Primary Systemic Therapy European Coop- erative Trial in Operable Breast Cancer,” Journal of Clinical Oncology, Vol. 27, No. 15, 2009, pp. 2474-2481. doi:10.1200/JCO.2008.19.2567 [9] G. Cocconi, G. Bisagni, M. Bacchi, F. Buzzi, R. Canaletti, A. Carpi, G. Ceci, A. Colozza, V. De Lisi and R. Lottici “A Comparison of Continuation versus Late Intensifica- tion Followed by Discontinuation of Chemotherapy in Advanced Breast Cancer. A Prospective Randomized Trial of the Italian Oncology Group for Clinical Research (GOIRC),” Annals of Oncology, Vol. 1, No. 1, 1990, pp. 36-44. [10] G. Cocconi, B. Di Blasio, C. Boni, G. Bisagniu, G. Ceci, E. Rondini, M. Bella, F. Leonardi, L. Savoldi, R. Camisa and P. Bruzzi, “Randomized Trial Comparing Cyclo- phosphamide, Methotrexate, and 5-Fluorouracil (CMF) with Rotational CMF, Epirubicin and Vincristine as Pri- mary Chemotherapy in Operable Breast Cancer,” Cancer, Vol. 95, No. 2, 2002, pp. 228-235. doi:10.1002/cncr.10678 [11] G. Cocconi, B. Di Blasio, C. Boni, G. Bisagni, G. Ceci, E. Rondini, M. Bella, F. Leonardi, L. Savoldi, C. Vallisneri, R. Camisa and P. Bruzzi, “Primary Chemotherapy in Op- erable Breast Carcinoma Comparing CMF (Cyclophos- phamide, Methotreaxate, 5-Fluorouracil) with an Anthra- cycline Containing Regimen: Short-Term Responses Translated into Long-Term Outcomes,” Annals of On- cology, Vol. 16, No. 9, 2005, pp. 1469-1476. doi:10.1093/annonc/mdi278 [12] A. B. Miller, R. Hoogstraten, M. Staquet and A. Winkler, “Reporting Results of Cancer Treatment,” Cancer, Vol. 47, No. 1, 1981, pp. 207-214. doi:10.1002/1097-0142(19810101)47:1<207::AID-CNC R2820470134>3.0.CO;2-6 [13] C. A. Hudis, W. E. Barlow, J. P. Costantino, R. J. Gray, Copyright © 2011 SciRes. JCT  Randomized Trial Comparing Cyclophosphamide, Methotrexate, and 5-Fluorouracil (CMF) Regimen with Rotational CMFEV Regimen (E = Epirubicin, V = Vincristine) as Adjuvant Chemotherapy in Moderate Risk Operable Breast Carcinoma Copyright © 2011 SciRes. JCT 353 K. I. Pritchard, J. W. Chapman, J. A. Sparano, S. Huns- berger, R. A. Enos, R. D. Gelber and J. O. Zujewski, “Proposal for Standardized Definitions for Efficacy End Points in Adjuvant Breast Cancer Trials: The STEEP System,” Journal of Clinical Oncology, Vol. 25, No. 15, 2007, pp. 2127-2132. doi:10.1200/JCO.2006.10.3523 [14] E. L. Kaplan and P. Meier, “Nonparametric Estimation from Incomplete Observations,” Journal of the American Statistical Association, Vol. 53, No. 282, 1958, pp. 457-481. doi:10.2307/2281868 [15] D. R. Cox, “The Regression Analysis of Binary Se- quences,” Journal of the Royal Statistical Society, Vol. 20, No. 2, 1958, pp. 215-242. [16] J. Cuzick, “Forest Plots and the Interpretation of Sub- groups,” Lancet, Vol. 365, No. 9467, 2005, p. 1308. doi:10.1016/S0140-6736(05)61026-4 [17] B. Fisher, A. M. Brown, N. V. Dimitrov, R. Poisson, C. Redmond, R. G. Margolese, D. Bowman, N. Wolmark, D. L. Wickerham, C. G. Kardinal, H. Shibata, A. H. G. Paterson, C. M. Sutherland, N. J. Robert, P. J. Ager. L. Levy, J. Walter, T. Wozniak, E. R. Fisher and M. Deutsch, “Two Months of Doxorubicin-Cyclophosphamide with and without Reinduction Therapy Compared with 6 Months of Cyclophosphamide, Methotrexate, and Fluo- rouracil in Positive-Node Breast Cancer Patients with Tamoxifen Non-responsive Tumors: Results from the Na- tional Surgical Adjuvant Breast and Bowel Project B-15,” Journal of Clinical Oncology, Vol. 8, No. 9, 1990, pp. 1483-1496. [18] B. Fisher, S. Anderson, Chiu-Tan, N. Wolmark, D. L. Wickerham, E. R. Fisher, N. V. Dimitrov, J. N. Atkins, N. Abramson, S. Merajver, E. H. Romon, C. G. Kardinakl, H. R. Shibata, R. G. Margolese, E. H. Romond, C. G. Kardinal, H. R. Shibata, R. G. Margolese and W. B. Farrar, “Tamoxifen and Chemotherapy for Axillary Node-Negative, Estrogen Receptor-Negative Breast Can- cer: Findings from the National Surgical Adjuvant Breast and Bowel Project B-23,” Journal of Clinical Oncology, Vol. 19, No. 4, 2001, pp. 931-942. [19] M. Martin, A. Villar, A. Sole-Calvo, R. Gonzalez, B. Massuti, J. Lizon, C. Camps, A. Carrato, A. Casado, M. T. Candel, J. Albanell, J. Aranda, B. Munarriz, J. Campbell and E. Diaz-Rubio, “Doxorubicin in Combination with Fluorouracil and Cyclophosphamide (i.v. FAC Regimen day 1, 21) versus Methotrexate in Combination with Fluorouracil and Cyclophosphamide (i.v. CMF Regimen, day 1, 21) as Adjuvant Chemotherapy for Operable Breast Cancer: A Study of the GEICAM Group,” Annals of Oncology, Vol. 14, No. 6, pp. 833-842. doi:10.1093/annonc/mdg260 [20] H. T. Mouridsen, J. Andersen, M. Andersson, P. Dom- bernowsky, B. Ejilertsen, C, Rose, P. G. Sorensen, E. Sandberg, K. W. Andersen, M. B. Jensen, N. O. Bengston, J. Bergh and B. Nordenskjold, “Adjuvant Anthracycline in Breast Cancer Improves Outcome in Premenopausal Patients Following Substitution of Methotrexate in the CMF Combination with Epirubicin,” Proceed ASCO, Vol. 18, No. 68, 1999, p. 19. [21] P. Francis, J. Crown, A. Di Leo, M. Buyse, A. Balil, M. Andersson, B. Nordenskjold, I. Lang, R. Jakesz, D. Vo- robiof, J. Gutierrez, G. van Hazel, S. Dolci, S. Jamin, B. Bendahmane, R. D. Gelber, M. Castiglione-Gerstsch and M. Piccart-Gebhart, “Adjuvant Chemotherapy with Se- quential or Concurrent Anthracycline and Docetaxel: Breast International Group 02-98 Randomized Trial,” Journal of National Cancer Institution, Vol. 100, No. 2, 2008, pp. 121-133. doi:10.1093/jnci/djm287 [22] J. M. Albert, A. U. Budzar, R. Guzman, P. K. Allen, E. A. Strom, G. H. Perkins, W. A. Woodward, K. E. Hoffman, W. Tereffe, K. K. Hunt, T. A. Buchholz and G. L. Oh, “Prospective Randomized Trial of 5-Fluorouracil, Doxo- rubicin, and Cyclophosphamide (FAC) versus Paclitaxel and FAC (TFAC) in Patients with Operable Breast Can- cer: Impact of Taxane Chemotherapy on Locoregional Control,” Breast Cancer Research and Treatment, Vol. 128, No. 2, 2011, pp. 421-427.
|