S. Das et al. / Open Journal of Ecology 1 (2011) 35-40 39
Mic r obial growth of 3 so il segment in anaerobic c on dit ion
0
1
2
3
4
5
0 20406080
I ncubation Period (hour)
CFU X10
6
gm
-1
dry w e ight of soil
TopSoil
Midd leSoil
B ottomSoil
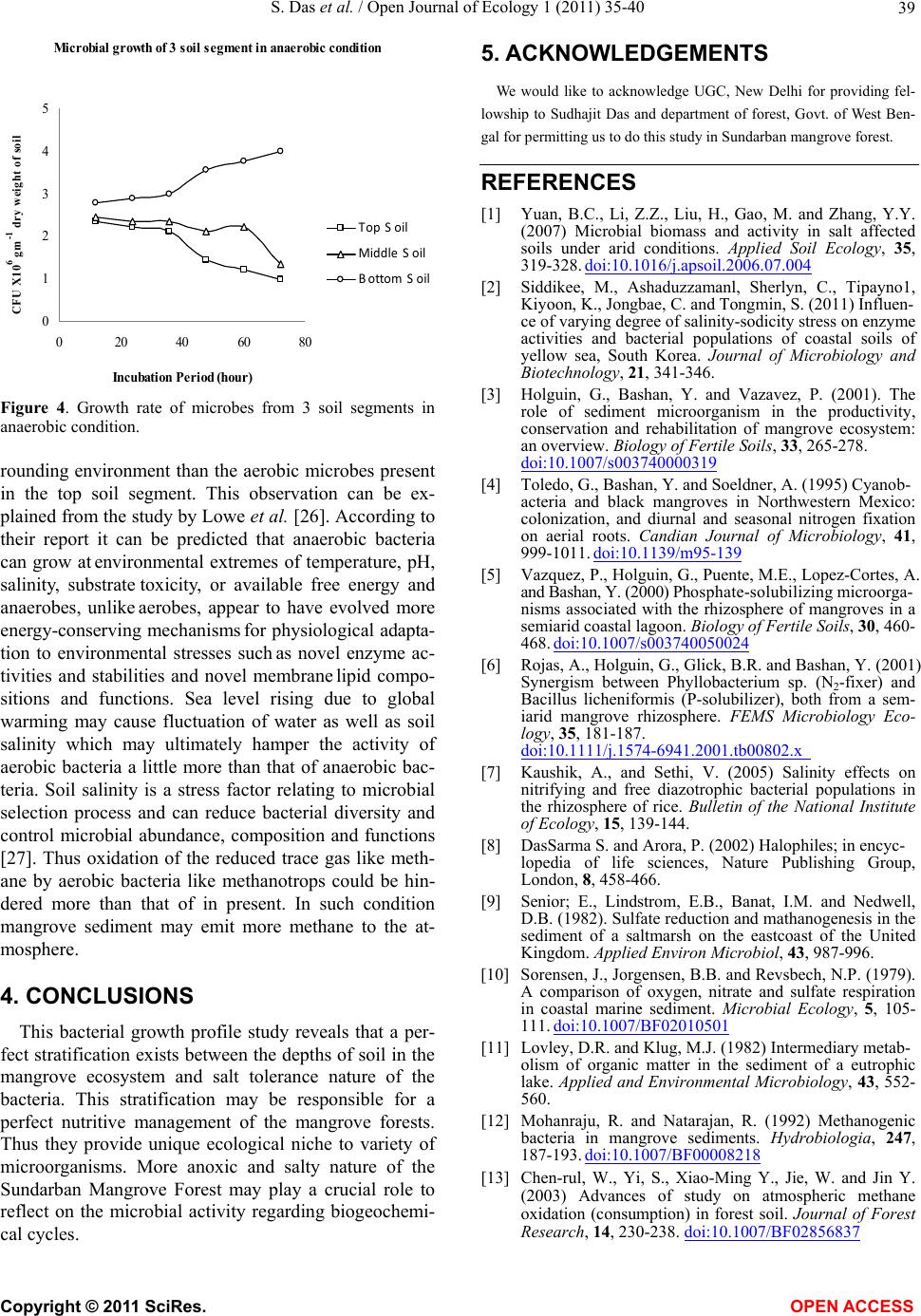
Figure 4. Growth rate of microbes from 3 soil segments in
anaerobic condition.
rounding environment than the aerobic microbes present
in the top soil segment. This observation can be ex-
plained from the study by Lowe et al. [26]. According to
their report it can be predicted that anaerobic bacteria
can grow at environmental extremes of temperature, pH,
salinity, substrate toxicity, or available free energy and
anaerobes, unlike aerobes, appear to have evolved more
energy-conserving mechanisms for physiological adapta-
tion to environmental stresses such as novel enzyme ac-
tivities and stabilities and novel membrane lipid compo-
sitions and functions. Sea level rising due to global
warming may cause fluctuation of water as well as soil
salinity which may ultimately hamper the activity of
aerobic bacteria a little more than that of anaerobic bac-
teria. Soil salinity is a stress factor relating to microbial
selection process and can reduce bacterial diversity and
control microbial abundance, composition and functions
[27]. Thus oxidation of the reduced trace gas like meth-
ane by aerobic bacteria like methanotrops could be hin-
dered more than that of in present. In such condition
mangrove sediment may emit more methane to the at-
mosphere.
4. CONCLUSIONS
This bacterial growth profile study reveals that a per-
fect stratification exists between the depths of soil in the
mangrove ecosystem and salt tolerance nature of the
bacteria. This stratification may be responsible for a
perfect nutritive management of the mangrove forests.
Thus they provide unique ecological niche to variety of
microorganisms. More anoxic and salty nature of the
Sundarban Mangrove Forest may play a crucial role to
reflect on the microbial activity regarding biogeochemi-
cal cycles.
5. ACKNOWLEDGEMENTS
We would like to acknowledge UGC, New Delhi for providing fel-
lowship to Sudhajit Das and department of forest, Govt. of West Ben-
gal for permitting us to do this study in Sundarban mangrove forest.
REFERENCES
[1] Yuan, B.C., Li, Z.Z., Liu, H., Gao, M. and Zhang, Y.Y.
(2007) Microbial biomass and activity in salt affected
soils under arid conditions. Applied Soil Ecology, 35,
319-328. doi:10.1016/j.apsoil.2006.07.004
[2] Siddikee, M., Ashaduzzamanl, Sherlyn, C., Tipayno1,
Kiyoon, K., Jongbae, C. and Tongmin, S. (2011) Influen-
ce of varying degree of salinity-sodicity stress on enzyme
activities and bacterial populations of coastal soils of
yellow sea, South Korea. Journal of Microbiology and
Biotechnology, 21, 341-346.
[3] Holguin, G., Bashan, Y. and Vazavez, P. (2001). The
role of sediment microorganism in the productivity,
conservation and rehabilitation of mangrove ecosystem:
an overview. Biology of Fe rtile Soils, 33, 265-278.
doi:10.1007/s003740000319
[4] Toledo, G., Bashan, Y. and Soeldner, A. (1995) Cyanob-
acteria and black mangroves in Northwestern Mexico:
colonization, and diurnal and seasonal nitrogen fixation
on aerial roots. Candian Journal of Microbiology, 41,
999-1011. doi:10.1139/m95-139
[5] Vazquez, P., Holguin, G., Puente, M.E., Lopez-Cortes, A.
a nd Bas ha n, Y. ( 200 0) Ph osphate-solub ilizing m icroorga-
nisms associated with the rhizosphere of mangroves in a
semiarid coastal lagoon. Biology of Fertile Soils, 30, 460-
468. doi:10.1007/s003740050024
[6] Rojas, A., Holguin, G., Glick, B.R. and Bashan, Y. (2001)
Synergism between Phyllobacterium sp. (N2-fixer) and
Bacillus licheniformis (P-solubilizer), both from a sem-
iarid mangrove rhizosphere. FEMS Microbiology Eco-
logy, 35, 181-187.
doi:10.1111/j.1574-6941.2001.tb00802.x
[7] Kaushik, A., and Sethi, V. (2005) Salinity effects on
nitrifying and free diazotrophic bacterial populations in
the rhizosphere of rice. Bulletin of the National Institute
of Ecology, 15, 139-144.
[8] DasSarma S. and Arora, P. (2002) Halophiles; in encyc-
lopedia of life sciences, Nature Publishing Group,
London, 8, 458-466.
[9] Senior; E., Lindstrom, E.B., Banat, I.M. and Nedwell,
D.B. (1982). Sulfate reduction and mathanogenesis in the
sediment of a saltmarsh on the eastcoast of the United
Kingdom. Applied Environ Microbiol, 43, 987-996.
[10] Sorensen, J., Jorgensen, B.B. and Revsbech, N.P. (1979).
A comparison of oxygen, nitrate and sulfate respiration
in coastal marine sediment. Microbial Ecology, 5, 105-
111. doi:10.1007/BF02010501
[11] Lovley, D.R. and Klug, M.J. (1982) Intermediary metab-
olism of organic matter in the sediment of a eutrophic
lake. Applied and Environmental Microbiology, 43, 552-
560.
[12] Mohanraju, R. and Natarajan, R. (1992) Methanogenic
bacteria in mangrove sediments. Hydrobiologia, 247,
187-193. doi:10.1007/BF00008218
[13] Chen-rul, W., Yi, S., Xiao-Ming Y., Jie, W. and Jin Y.
(2003) Advances of study on atmospheric methane
oxidation (consumption) in forest soil. Journal of Forest
Research, 14, 230-238. doi:10.1007/BF02856837
Copyright © 2011 SciRes. OPEN A CCESS