 Vol.3, No.8, 651-660 (2011) Natural Science http://dx.doi.org/10.4236/ns.2011.38089 Copyright © 2011 SciRes. OPEN ACCESS Reactivity of 1-methylisoquinoline synthesis of pyrazolyl triazoloisoquinoline and thiadiazolyl isoquinoline derivatives Hamdi Mahmoud Hassaneen*, Huwaida Mahmmed Elsayd Hassaneen, Yasmin Shafie Mohammed Department of Chemistry, Faculty of Science, Cairo University, Cairo, Egypt; *Corresponding Author: Hamdihass@gmail.com Received 18 May 2011; revised 10 June 2011; accepted 16 June 2011. ABSTRACT The reaction of 1-methylisoquinoline 1 with hy- drazonoyl halides 2 in ethanol in the presence of chitosan under microwave irradiation affords triazoloisoquinoline 4. Product 4 reacts with dimethylformamide-dimethylacetal to give ena- minones 7 which react with hydrazonoyl halides to give pyrazolyl triazoloisoquinoline 13. Also, 1-methylisoquinoline 1 reacts with arylisothio- cyanate to give thioanilide 15 which reacts with hydrazonoyl halides to give the corresponding thiadiazolyl isoquinoline derivatives 20, 24. Keywords: [1,2,4] Triazolo [3,4-a] Isoquinolines; Enaminones; Hydrazonoyl Halides; Cycloaddition Reactions; Chitosan; Thioanilides; [1,3,4] Thiadiazolylisoquinoline Derivatives 1. INTRODUCTION Within the class of fused isoquinoline with their car- diovascular [1], anti-inflammatory [2], and antidepres- sant activites [3], [1,2,4]triazolo [3,4-a]isoquinolines are of considerable pharmaceutical and agricultural interest [4-7]. Therefore, the synthesis of this ring system is an attractive goal. We have previously reported the synthe- ses of triazoloisoquinoline and fused isoquinoline com- pounds via reaction of 3,4-dihydro-6,7-dimethoxyisoq- uinoline derivatives with hydrazonoyl halides in chloro- form in the presence of triethylamine or in pyridine as catalyst and solvent [8-12]. The aim of the present study is to introduce a new synthetic method by replacing triethylamine in chloroform by the ecologically more acceptable catalyst chitosan [13,14] and under micro- wave irradiation to enhance reaction rates [15-19] for the synthesis of [1,2,4]triazolo[3,4-a]isoquinolines which were found to be useful precursors for the synthesis of new enaminones 7. The latter compounds 7 were used to prepare carbonylpyrazolyl triazoloisoquinoline deriva- tives 13. Also, we synthesis thiadiazolyl isoquinoline derivatives 20, 24 via a reaction of thioanilide 15 with hydrazonoyl halides 16. 2. EXPERIMENTAL The melting points were determined on a Stuart melt- ing point apparatus and are uncorrected. The IR spectra were recorded as KBr pellets using a FTIR unit Bruker- vector 22 spectrophotometer. The 1H NMR and 13C NMR spectra were recorded in CDCl3 and DMSO-d6 as solvents at 300 MHz on Varian Gemini NMR spectro- meter using TMS as internal standard. Chemical shifts are reported in δ units (ppm). Mass spectra were meas- ured on a Shimadzu GCMS-QP-1000 EX mass spec- trometer at 70 eV. Microwave used was CEM Discover labmateTM microwave apparatus (300 W with Chem- DriverTM software). The elemental analyses were per- formed at the Micro Analytical Center, Cairo University. 2.1. Synthesis of 1-(1-Aryl-8,9-Dimethoxy- 10b-Methyl-1,5,6,10b-Tetrahydro [1,2,4] Triazolo [3,4-a]Isquinlin-3-yl) Ethanone-4a-c Chitosan (0.1 g) was added to a solution of hydrazo- noyl chloride 2 (1 mmol) and 1-methyl-3,4-dihydro-6, 7-dimethoxyisoquinoline 1 (0.28 g, 1 mmol) in absolute ethanol (5 mL) at room temperature. The reaction mixture was irradiated under constant pressure (11.2 Bar, 80˚C) for 10 min at a power of 300 W. The hot solution was filtered to remove chitosan. After cooling, dilute HCl was added till pH became acidic and the solid was collected and crystallized from suitable solvent. The compounds prepared 4a-c with their physical data are listed in Tables 1 and 2.  H. M. Hassaneen et al. / Natural Science 3 (2011) 651-660 Copyright © 2011 SciRes. OPEN ACCESS 652 2.2. Synthesis of (E)-1-(1-Aryl-8,9-Dimethoxy- 10b-Methyl-1,5,6,10b-Tetrahydro [1,2,4] Triazolo-[3,4-a]Isoquinolin-3-yl)-3- Dimethylaminopropenone 7a-c A mixture of 1-(1-aryl-8,9-dimethoxy-10b-methyl-1, 5,6,10b-tetra-hyd ro[1,2 ,4]triazolo[ 3,4-a]isoquinolin-3-yl) ethanone 4 (5 mmoles) and DMF-DMA (3 mL) was re- fluxed for 4 h. The solid that precipitated was collected and crystallized from suitable solvent. The compounds prepared 7 a-c with their physical data are listed in Tables 1 and 2. 2.3. Synthesis of Pyrazolyl Triazoloisoquinoline Derivatives 13a-i To a solution of the appropriate hydrazonoyl chloride 2, 8, 9 (1 mmol) and enaminones 7 (1 mmol) in absolute ethanol (5 mL) was added chitosan (0.1 g) at room tem- perature. The reaction mixture was irradiated under con- stant pressure (11.2 Bar, 80˚C) for 10 min at a power of 300 W. The hot solution was filtered to remove chitosan. After cooling, dilute HCl was added till pH became acidic and the solid was collected and crystallized from suitable solvent. The compounds prepared 13a-i with their physical data are listed in Tables 1 and 2. 2.4. Synthesis of Thiadiazolyl Isoquinoline Derivatives 20a-j and 24a-g Equimolar quantities of thioanilides 15 and the appro- priate hydrazonoyl halides were dissolved in absolute ethanol (5 mL) in the presence of chitosan (0.1 g) at room temperature. The reaction mixture was irradiated under constant pressure (11.2 Bar, 80˚C) for 10 min at a power of 300 W. The hot solution was filtered to remove chitosan. After cooling, dilute HCl was added till pH became acidic and the solid was collected and crystal- lized from suitable solvent to give the corresponding 1, 3,4-thiadiazoles. The compounds prepared 20a-j and 24a-g with their physical data are listed in Tables 1 and 2. 3. RESULTS AND DISCUSSION The reaction of hydrazonoyl halides 2 with 1-methyl- 3, 4-dihydro-6, 7-dimethoxyisoquinoline 1 which has ac- tive group at C-1 was studied. The capacity of this dipolarophile 1 to behave as cy- clic ketimine 1 A or as a secondary enamine 1B has been discussed by many investigators [20,21]. Our aim point of interest whether addition of nitrilimines 3 occurred on the C = N double bond of ketimine structure 1 A or enamine double bond of 1 B (Figure 1). Thus, reaction of 1 with nitrilimines 3, generated in situ by treatment of hydrazonoyl chlorides 2 with chitosan [22] in ethanol under microwave irradiation, gave products whose ele- mental analyses were compatible with triazole deriva- tives 4, spyropyrazolines 5 or triazine derivatives 6. The structures 5 and 6 were discarded on the basis of 1H NMR evidences. For example, structure 5 will reveal two singlet signals assignable to CH2 and NH protons, while the other isomeric structure 6 is expected to reveal doublet and triplet assignable to C1-CH2 and C11b-CH protons. Such signals were absent in the 1H NMR spec- tra of the isolated products from reaction of 2 with 1. Instead of these signals, the 1H NMR spectra of the latter products showed one singlet signal at δ 2.08 ppm. The presence of such signal is compatible with the assigned structure 4. Indeed the proton resonance of the moiety -N = C(CH3)- appears in the 1H NMR spectra of the di- polarophile 1 at δ 2.3 ppm [23]. This resonance was shifted to higher field in the 1H NMR spectra (2.08 ppm) of the cycloadducts 4 indicating the conversion of such moiety to the saturated moiety -N-C(CH3)- due to cycloaddition. Based on these findings the products iso- lated from the reaction mixture were assigned structure 4 (Figure 1). Refluxing of 4a with DMFDMA for 4 h afforded a compound 7a which analyzed correctly for C24H28N4O3 (Figure 2). Similarly compounds 4b, c were also pre0 pared by reaction of the corresponding 3-acetylisoquino- line derivatives with DMFDMA. The structures of the products 7 were fully established on the basis of spectral (MS, IR, 1H NMR and 13C NMR) and elemental analy- ses. For example, the 1H NMR spectrum of 7a showed two singlet signals at δ 2.96 and 3.72 ppm characteristic for -N(Me)2 group, two doublet signals at δ about 5.75 and 7.64 ppm with coupling constant J = 13 Hz assign- able to the two olefinic protons. The value of coupling constant is compatible with the E-configuration [24] de- picted in Figure 2. Also its 13C NMR spectrum showed two signals at δ 37.31 and 45.48 ppm assignable to -N(Me)2 group [25], in addition to the signals of other carbon atoms. Reaction of enaminones 7 with nitrilimines 10, gener- ated in situ by the action of chitosan on the correspond- ing α-ketohydrazonoyl halides 2, 8, 9 in refluxed ethanol gave, in each case, one isolable product as evidenced by TLC analysis and 1H NMR spectra of the crude reaction mixture (Figure 3). All the isolated cycloadducts gave satisfactory ele- mental analyses and mass spectral data which were con- sistent with either one of the two isomeric structures 13 or 14. Structure 14 was ruled out on the basis of 1H NMR spectra. For example, in the pyrazole ring system C (4) is the most electron rich carbon, thus, H (4) in is expected to appear at higher field at δ 6.31 ppm. On the 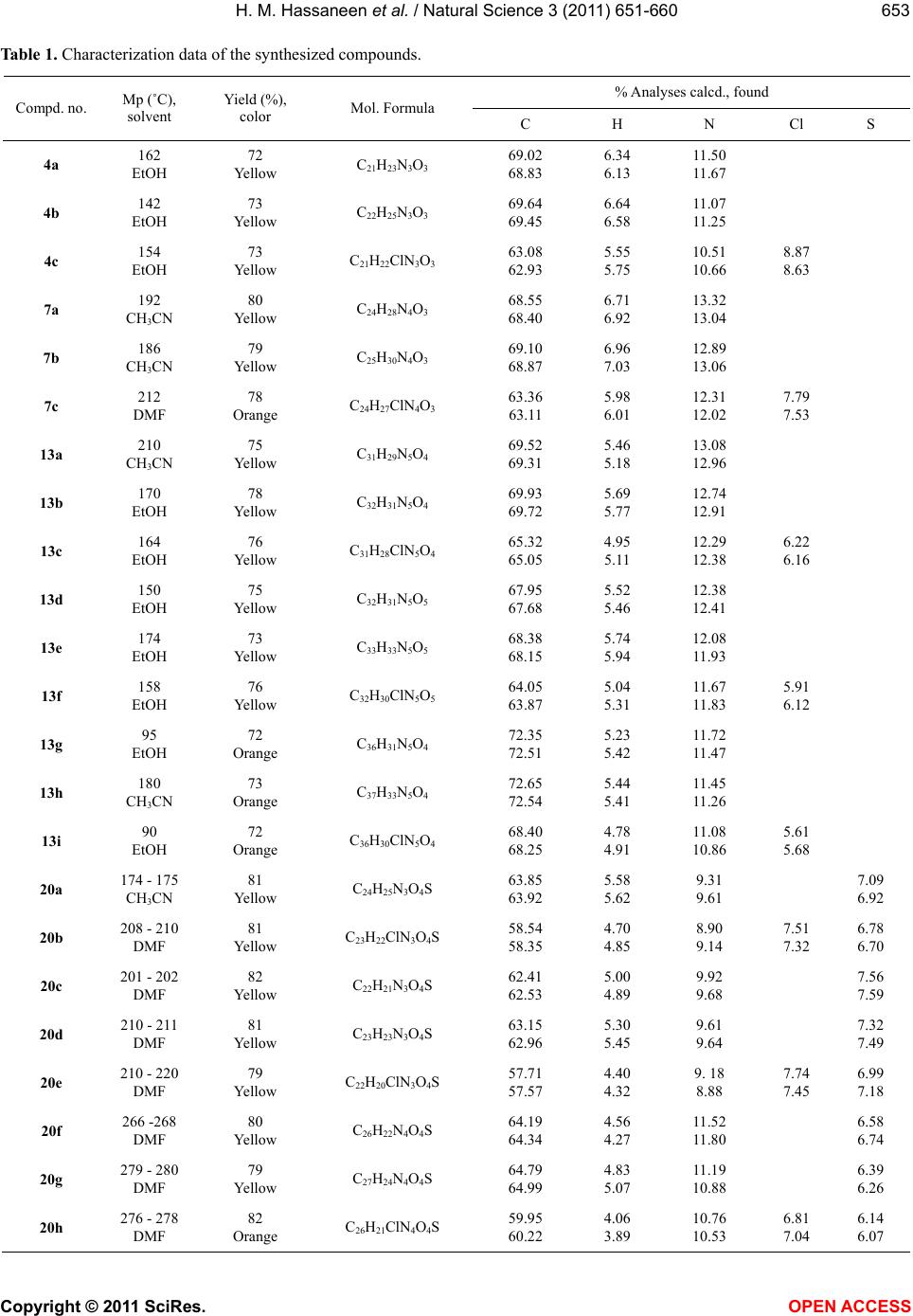 H. M. Hassaneen et al. / Natural Science 3 (2011) 651-660 Copyright © 2011 SciRes. OPEN ACCESS 653653 Table 1. Characterization data of the synthesized compounds. % Analyses calcd., found Compd. no. Mp (˚C), solvent Yield (%), color Mol. Formula C H N Cl S 4a 162 EtOH 72 Yellow C21H23N3O3 69.02 68.83 6.34 6.13 11.50 11.67 4b 142 EtOH 73 Yellow C22H25N3O3 69.64 69.45 6.64 6.58 11.07 11.25 4c 154 EtOH 73 Yellow C21H22ClN3O3 63.08 62.93 5.55 5.75 10.51 10.66 8.87 8.63 7a 192 CH3CN 80 Yellow C24H28N4O3 68.55 68.40 6.71 6.92 13.32 13.04 7b 186 CH3CN 79 Yellow C25H30N4O3 69.10 68.87 6.96 7.03 12.89 13.06 7c 212 DMF 78 Orange C24H27ClN4O3 63.36 63.11 5.98 6.01 12.31 12.02 7.79 7.53 13a 210 CH3CN 75 Yellow C31H29N5O4 69.52 69.31 5.46 5.18 13.08 12.96 13b 170 EtOH 78 Yellow C32H31N5O4 69.93 69.72 5.69 5.77 12.74 12.91 13c 164 EtOH 76 Yellow C31H28ClN5O4 65.32 65.05 4.95 5.11 12.29 12.38 6.22 6.16 13d 150 EtOH 75 Yellow C32H31N5O5 67.95 67.68 5.52 5.46 12.38 12.41 13e 174 EtOH 73 Yellow C33H33N5O5 68.38 68.15 5.74 5.94 12.08 11.93 13f 158 EtOH 76 Yellow C32H30ClN5O5 64.05 63.87 5.04 5.31 11.67 11.83 5.91 6.12 13g 95 EtOH 72 Orange C36H31N5O4 72.35 72.51 5.23 5.42 11.72 11.47 13h 180 CH3CN 73 Orange C37H33N5O4 72.65 72.54 5.44 5.41 11.45 11.26 13i 90 EtOH 72 Orange C36H30ClN5O4 68.40 68.25 4.78 4.91 11.08 10.86 5.61 5.68 20a 174 - 175 CH3CN 81 Yellow C24H25N3O4S 63.85 63.92 5.58 5.62 9.31 9.61 7.09 6.92 20b 208 - 210 DMF 81 Yellow C23H22ClN3O4S 58.54 58.35 4.70 4.85 8.90 9.14 7.51 7.32 6.78 6.70 20c 201 - 202 DMF 82 Yellow C22H21N3O4S 62.41 62.53 5.00 4.89 9.92 9.68 7.56 7.59 20d 210 - 211 DMF 81 Yellow C23H23N3O4S 63.15 62.96 5.30 5.45 9.61 9.64 7.32 7.49 20e 210 - 220 DMF 79 Yellow C22H20ClN3O4S 57.71 57.57 4.40 4.32 9. 18 8.88 7.74 7.45 6.99 7.18 20f 266 -268 DMF 80 Yellow C26H22N4O4S 64.19 64.34 4.56 4.27 11.52 11.80 6.58 6.74 20g 279 - 280 DMF 79 Yellow C27H24N4O4S 64.79 64.99 4.83 5.07 11.19 10.88 6.39 6.26 20h 276 - 278 DMF 82 Orange C26H21ClN4O4S 59.95 60.22 4.06 3.89 10.76 10.53 6.81 7.04 6.14 6.07 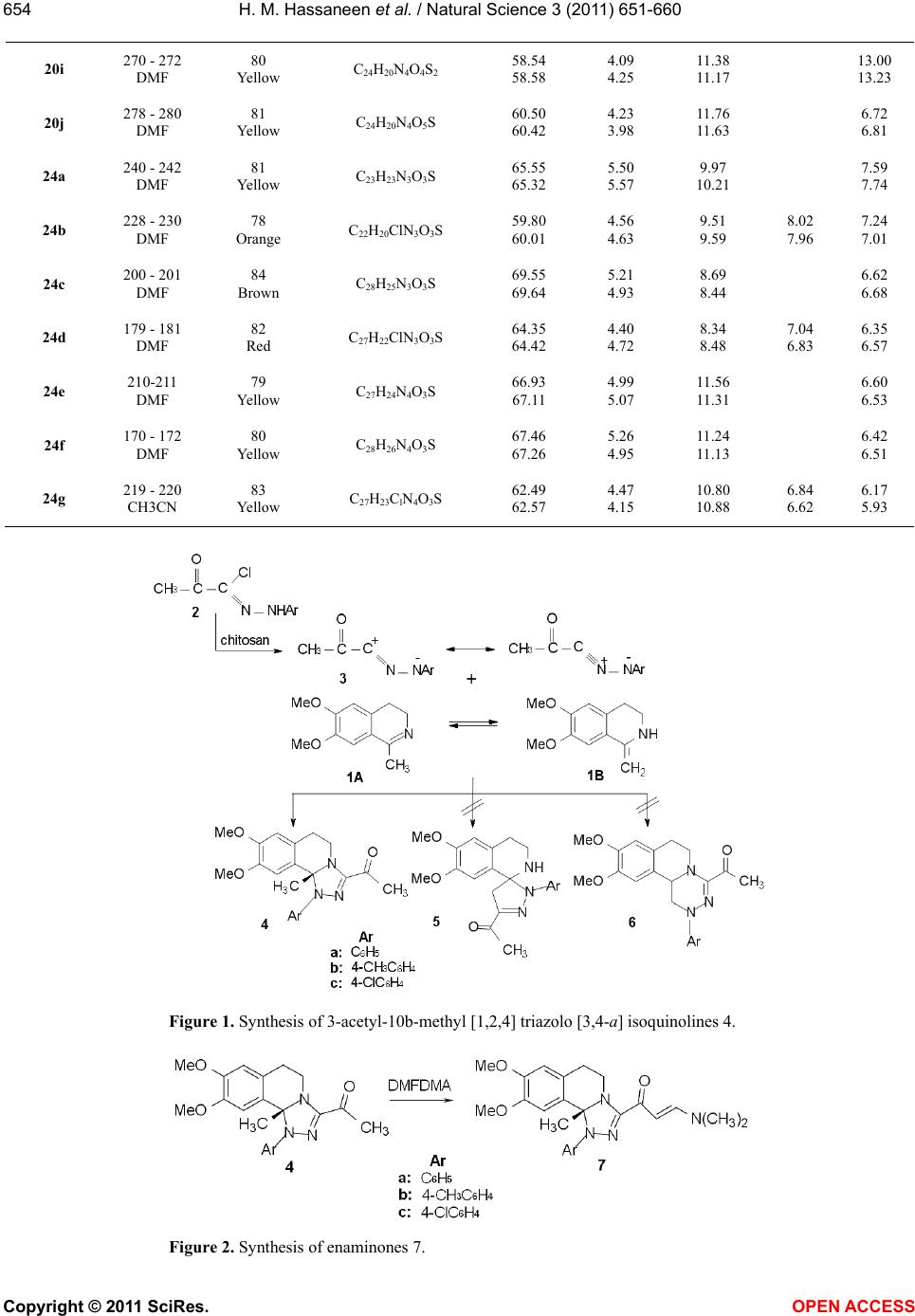 H. M. Hassaneen et al. / Natural Science 3 (2011) 651-660 Copyright © 2011 SciRes. OPEN ACCESS 654 20i 270 - 272 DMF 80 Yellow C24H20N4O4S2 58.54 58.58 4.09 4.25 11.38 11.17 13.00 13.23 20j 278 - 280 DMF 81 Yellow C24H20N4O5S 60.50 60.42 4.23 3.98 11.76 11.63 6.72 6.81 24a 240 - 242 DMF 81 Yellow C23H23N3O3S 65.55 65.32 5.50 5.57 9.97 10.21 7.59 7.74 24b 228 - 230 DMF 78 Orange C22H20ClN3O3S 59.80 60.01 4.56 4.63 9.51 9.59 8.02 7.96 7.24 7.01 24c 200 - 201 DMF 84 Brown C28H25N3O3S 69.55 69.64 5.21 4.93 8.69 8.44 6.62 6.68 24d 179 - 181 DMF 82 Red C27H22ClN3O3S 64.35 64.42 4.40 4.72 8.34 8.48 7.04 6.83 6.35 6.57 24e 210-211 DMF 79 Yellow C27H24N4O3S 66.93 67.11 4.99 5.07 11.56 11.31 6.60 6.53 24f 170 - 172 DMF 80 Yellow C28H26N4O3S 67.46 67.26 5.26 4.95 11.24 11.13 6.42 6.51 24g 219 - 220 CH3CN 83 Yellow C27H23ClN4O3S 62.49 62.57 4.47 4.15 10.80 10.88 6.84 6.62 6.17 5.93 Figure 1. Synthesis of 3-acetyl-10b-methyl [1,2,4] triazolo [3,4-a] isoquinolines 4. Figure 2. Synthesis of enaminones 7.  H. M. Hassaneen et al. / Natural Science 3 (2011) 651-660 Copyright © 2011 SciRes. OPEN ACCESS 655655 Table 2. Spectra of the synthesized compounds. Compd. no. Spectral data (IR, 1H NMR, 13C NMR and MS) 4a IR (KBr) ν 1670 (C=O) cm–1; 1H NMR (CDCl3) δ 2.08 (s, 3H), 2.37 (m, 1H), 2.38 (s, 3H), 2.80 (m, 1H), 3.18 (s, 3H), 3.2 (m, 1H), 3.73 (s, 3H), 4.60 (m, 1H), 5.85 (s, 1H), 6.40 (s, 1H), 7.13 - 7.28 (m, 5H) ppm; 13C NMR (CDCl3) δ 26.21, 27.32, 29.24, 40.22, 54.9, 55.35, 90.09, 109.20, 111.04, 123.94, 125.09, 127.16, 127.69, 128.70, 143.19, 146.04, 147.75, 147.97, 189.88 ppm; MS, m/z (%): 365 (M+, 10.9), 334 (100.0), 103 (22.0), 90 (38.4), 76 (30.8). 4b IR (KBr) ν 1662 (C=O) cm–1; 1H NMR (CDCl3) δ 2.05 (s, 3H), 2.29 (s, 3H), 2.34 (m, 1H), 2.38 (s, 3H), 2.84 (m, 1H), 3.14 (m, 1H), 3.23 (s, 3H), 3.75 (s, 3H), 4.65 (m, 1H), 5.79 (s, 1H), 6.41 (s, 1H), 7.09 - 7.12 (m, 4H) ppm; 13C NMR (CDCl3) δ 20.61, 26.24, 27.40, 29.20, 40.35, 54.76, 55.40, 90.21, 109.45, 111.02, 124.39, 127.11, 127.86, 129.28, 135.16, 140.60, 145.96, 147.68, 147.95, 189.92 ppm; MS, m/z (%): 364 (M+-15, 100.0), 104 (33.4), 90 (20.2). 4c IR (KBr) ν 1666 (C=O) cm–1; 1H NMR (CDCl3) δ 2.03 (s, 3H), 2.35 (m, 1H), 2.39 (s, 3H), 2.83 (m, 1H), 3.13 (m, 1H), 3.27 (s, 3H), 3.74 (s, 3H), 4.60 (m, 1H), 5.86 (s, 1H), 6.41 (s, 1H), 7.14-7.25 (m, 4H) ppm; 13C NMR (CDCl3) δ 26.51, 27.70, 29.17, 40.61, 55.17, 55.63, 90.24, 109.39, 111.42, 125.16, 127.13, 128.17, 128.93, 130.49, 142.18, 146.44, 148.29, 148.40, 190.13 ppm; MS, m/z (%): 399 (M+, 1.8), 384 (M+-15, 100.0), 90 (8.6). 7a IR (KBr) ν 1642 (C=O) cm–1; 1H NMR (CDCl3) δ 2.04 (s, 3H), 2.38 (m, 1H), 2.80 (s, 3H), 2.88 (m, 1H), 2.96 (s, 3H), 3.16 (s, 3H), 3.19 (m, 1H), 3.72 (s, 3H), 4.80 (m, 1H), 5.75 (d, 1H), 5.89 (s, 1H), 6.38 (s, 1H), 7.04 - 7.24 (m, 5H), 7.64 (d, 1H) ppm; 13C NMR (CDCl3) δ 27.85, 29.10, 37.31, 40.42, 45.48, 54.95, 55.37, 88.63, 93.13, 109.52, 110.93, 123.80, 124.29, 128.02, 128.04, 128.50, 144.36, 145.88, 147.67, 150.13, 152.23, 179.19 ppm; MS, m/z (%): 420 (M+, 3.2), 405 (M+-15, 87.9), 322 (11.0), 98 (100.0). 7b IR (KBr) ν 1640 (C=O) cm–1; 1H NMR (CDCl3) δ 1.99 (s, 3H), 2.26 (s, 3H), 2.35 (m, 1H), 2.80 (s, 3H), 2.87 (m, 1H), 2.95 (s, 3H), 3.16 (m, 1H), 3.20 (s, 3H), 3.71 (s, 3H), 4.80 (m, 1H), 5.74 (d, 1H), 5.81 (s, 1H), 6.37 (s, 1H), 7.01-7.10 (m, 4H), 7.63 (d, 1H) ppm; 13C NMR (CDCl3) δ 20.77, 28.10, 29.25, 37.38, 42.71, 45.49, 54.96, 55.58, 88.96, 93.40, 109.94, 111.08, 124.49, 128.13, 128.38, 129.25, 134.42, 141.99, 145.97, 147.82, 150.18, 152.37, 179.46 ppm; MS, m/z (%): 434 (M+, 2.0), 419 (M+-15, 46.7), 336 (10.5), 98 (100.0). 7c IR (KBr) ν 1640 (C=O) cm–1; 1H NMR (CDCl3) δ 1.98 (s, 3H), 2.37 (m, 1H), 2.79 (s, 3H), 2.87 (m, 1H), 2.94 (s, 3H), 3.15 (m, 1H), 3.28 (s, 3H), 3.71 (s, 3H), 4.67 (m, 1H), 5.75 (d, 1H), 5.90 (s, 1H), 6.38 (s, 1H), 7.13-7.19 (m, 4H), 7.63 (d, 1H) ppm; 13C NMR (CDCl3) δ 28.17, 28.99, 37.42, 40.77, 44.98, 55.18, 55.61, 88.71, 93.22, 109.68, 111.30, 124.97, 127.99, 128.48, 128.67, 129.48, 143.35, 146.25, 148.08, 150.81, 152.68, 179.17 ppm; MS, m/z (%): 454 (M+, 1.9), 439 (M+-15, 29.2), 285 (31.5), 98 (100.0). 13a IR (KBr) ν 1692 (C=O), 1645 (C=O) cm–1; 1H NMR (CDCl3) δ 2.13 (s, 3H), 2.52 (m, 1H), 2.63 (s, 3H), 3.24 (s, 3H), 3.38 (m, 1H), 3.41 (m, 1H), 3.81 (s, 3H), 4.79 (m, 1H), 5.87 (s, 1H), 6.53 (s, 1H), 7.15 - 7.68 (m, 10H), 8.33 (s, 1H) ppm; 13C NMR (CDCl3) δ 27.93, 28.12, 29.65, 40.92, 55.23, 55.64, 90.27, 109.58, 111.46, 119.85, 122.74, 124.95, 125.70, 127.27, 127.88, 128.77, 128.91, 129.54, 131.01, 138.98, 143.35, 146.17, 148.24, 149.04, 151.56, 178.11, 193.99 ppm; MS, m/z (%): 535 (M+, 1.8), 520 (M+-15, 100.0), 303 (21.1), 213 (13.9), 77 (33.8). 13b IR (KBr) ν 1694 (C=O), 1645 (C=O) cm–1; 1H NMR (CDCl3) δ 2.10 (s, 3H), 2.31 (s, 3H), 2.52 (m, 1H), 2.63 (s, 3H), 3.26 (s, 3H), 3.30 (m, 1H), 3.34 (m, 1H), 3.83 (s, 3H), 4.80 (m, 1H), 5.82 (s, 1H), 6.54 (s, 1H), 7.01 - 7.69 (m, 9H), 8.34 (s, 1H) ppm; 13C NMR (CDCl3) δ 20.85, 27.97, 28.14, 29.59, 40.97, 55.04, 55.63, 90.32, 109.75, 111.38, 119.89, 122.77, 125.34, 127.20, 127.87, 128.86, 129.43, 129.54, 131.04, 135.78, 139.02, 140.71, 146.05, 148.17, 148.86, 151.60, 178.01, 194.10 ppm; MS, m/z (%): 549 (M+, 1.1), 534 (M+-15, 100.0), 317 (13.2), 213 (23.9), 91 (11.8). 13c IR (KBr) ν 1692 (C=O), 1650 (C=O) cm–1; 1H NMR (CDCl3) δ 2.08 (s, 3H), 2.52 (m, 1H), 2.62 (s, 3H), 3.31 (m, 1H), 3.33 (m, 1H), 3.35 (s, 3H), 3.82 (s, 3H), 4.78 (m, 1H), 5.90 (s, 1H), 6.55 (s, 1H), 7.06 - 7.69 (m, 9H), 8.32 (s, 1H) ppm; MS, m/z (%): 581 (M+, 1.0), 557 (17.1), 556 (46.1), 555 (45.9), 554 (100.0), 337 (15.0), 213 (43.8), 98 (13.6). 13d IR (KBr) ν 1728 (C=O), 1635 (C=O) cm–1; 1H NMR (CDCl3) δ 1.31 (t, 3H), 2.17 (s, 3H), 2.45 (m, 1H), 3.20 (m, 1H), 3.24 (s, 3H), 3.30 (m, 1H), 3.80 (s, 3H), 4.36 (q, 2H), 4.81 (m, 1H), 5.95 (s, 1H), 6.50 (s, 1H), 7.16-7.69 (m, 10H), 8.44 (s, 1H) ppm; 13C NMR (CDCl3) δ 14.13, 27.82, 29.57, 40.66, 55.22, 55.69, 61.52, 90.21, 109.37, 111.37, 120.17, 123.14, 124.02, 125.36, 127.64, 127.94, 128.27, 129.01, 129.46, 131.10, 138.94, 144.05, 145.92, 147.65, 148.81, 149.22, 162.54, 176.33 ppm; MS, m/z (%): 550 (M+-15, 100.0), 474 (30.1), 103 (45.1), 76 (82.4), 56 (47.6). 13e IR (KBr) ν 1720 (C=O), 1639 (C=O) cm–1; 1H NMR (CDCl3) δ 1.32 (t, 3H), 2.12 (s, 3H), 2.30 (s, 3H), 2.49 (m, 1H), 3.18 (m, 1H), 3.25 (s, 3H), 3.13 (m, 1H), 3.79 (s, 3H), 4.36 (q, 2H), 4.77 (m, 1H), 5.88 (s, 1H), 6.48 (s, 1H), 7.10 - 7.67(m, 9H), 8.43 (s, 3H) ppm;  H. M. Hassaneen et al. / Natural Science 3 (2011) 651-660 Copyright © 2011 SciRes. OPEN ACCESS 656 13f IR (KBr) ν 1720 (C=O), 1639 (C=O) cm–1; 1H NMR (CDCl3) δ 1.31 (t, 3H), 2.12 (s, 3H), 2.46 (m, 1H), 3.09 (m, 1H), 3.18 (m, 1H), 3.35 (s, 3H), 3.79 (s, 3H), 4.39 (q, 2H), 4.72 (m, 1H), 5.98 (s, 1H), 6.51 (s, 1H), 7.15 - 7.71 (m, 9H), 8.42 (s, 1H) ppm; MS, m/z (%): 584 (M+-15, 100.0), 148 (21.1), 127 (22.1), 103 (61.5), 76 (67.1). 13g IR (KBr) ν 1675 (C=O), 1631 (C=O) cm–1; 1H NMR (CDCl3) δ 2.10 (s, 3H), 2.44 (m, 1H), 3.21 (s, 3H), 3.24 (m, 1H), 3.28 (m, 1H), 3.82 (s, 3H), 4.77 (m, 1H), 5.83 (s, 1H), 6.51 (s, 1H), 7.02-8.04 (m, 15H), 8.61 (s, 1H) ppm; 13C NMR (CDCl3) δ 27.75, 29.40, 40.52, 55.06, 55.59, 89.90, 109.26, 111.36, 119.79, 123.67, 124.39, 125.29, 127.28, 127.72, 128.19, 128.33, 128.73, 129.43, 130.15, 130.86, 133.13, 136.51, 138.88, 143.03, 146.07, 148.12, 148.54, 152.01, 176.64, 189.11 ppm; MS, m/z (%): 582 (M+-15, 100.0), 104 (81.6), 76 (94.3). 13h IR (KBr) ν 1678 (C=O), 1624 (C=O) cm–1; 1H NMR (CDCl3) δ 2.07 (s, 3H), 2.29 (s, 3H), 2.44 (m, 1H), 3.20 (m, 1H), 3.23 (s, 3H), 3.28 (m, 1H), 3.83 (s, 3H), 4.78 (m, 1H), 5.79 (s, 1H), 6.51 (s, 1H), 6.90 - 8.04 (m, 14H), 8.62 (s, 1H) ppm; 13C NMR (CDCl3) δ 20.78, 27.83, 29.39, 40.65, 54.95, 55.66, 90.03, 109.51, 111.34, 119.89, 123.72, 124.83, 127.26, 127.75, 128.25, 128.51, 129.33, 129.48, 130.21, 130.97, 133.15, 135.41, 136.61, 139.00, 140.51, 146.02, 148.13, 148.40, 152.10, 176.55, 189.29 ppm; MS, m/z (%): 611 (M+, 1.1), 596 (M+-15, 100.0), 324 (49.7), 104 (21.7), 76 (25.7). 13i IR (KBr) ν 1678 (C=O), 1639 (C=O) cm–1; 1H NMR (CDCl3) δ 2.04 (s, 3H), 2.50 (m, 1H), 3.20 (m, 1H), 3.30 (s, 3H), 3.36 (m, 1H), 3.83 (s, 3H), 4.79 (m, 1H), 5.83 (s, 1H), 6.53 (s, 1H), 6.87 - 8.03 (m, 14H), 8.57 (s, 1H) ppm; MS, m/z (%): 632 (M+, 2.9), 617 (M+-15, 100.0), 275 (21.8), 104 (59.2), 76 (44.3). 20a IR (KBr) ν 1732 (C=O) cm–1; 1H NMR (CDCl3) δ 1.41 (t, 3H), 2.43 (s, 3H), 2.66 (t, 2H), 3.79 (s, 3H), 3.84 (t, 2H), 3.88 (s, 3H), 4.44 (q, 2H), 6.22 (s, 1H), 6.69 (s, 1H), 6.81 (s, 1H), 7.30 - 7.48 (m, 4H) ppm; 13C NMR (CDCl3) δ 14.18, 21.14, 26.92, 45.84, 55.81, 56.43, 62.19, 85.56, 108.46, 110.49, 122.59, 125.39, 130.06, 131.78, 136.88, 138.71, 143.14, 147.37, 149.93, 150.56, 157.96, 160.06 ppm; MS, m/z (%): 451 (M+, 8.9), 293 (100.0), 91 (18.3). 20b IR (KBr) ν 1734 (C=O) cm–1; 1H NMR (CDCl3) δ 1.43 (t, 3H), 2.65 (t, 2H), 3.78 (s, 3H), 3.84 (t, 2H), 3.88 (s, 3H), 4.46 (q, 2H), 6.20 (s, 1H), 6.67 (s, 1H), 6.78 (s, 1H), 7.31 - 7.52 (m, 4H) ppm; MS, m/z (%):473 (M+ + 2, 3.5), 471 (M+, 10.1), 293 (100.0), 91 (18.9). 20c IR (KBr) ν 1708 (C=O) cm–1; 1H NMR (CDCl3) δ 2.66 (t, 2H), 3.79 (s, 3H), 3.84 (t, 2H), 3.88 (s, 3H), 3.96 (s, 3H), 6.23 (s, 1H), 6.69 (s, 1H), 6.81 (s, 1H), 7.31 - 7.48 (m, 5H) ppm; 13C NMR (CDCl3) δ 27.00, 45.79, 52.86, 55.83, 56.45, 85.82, 108.41, 110.66, 121.58, 125.01, 130.22, 131.90, 136.85, 138.88, 142.83, 147.58, 149.67, 150.70, 158.01, 160.64 ppm; MS, m/z (%): 423 (M+, 8.9), 293 (100.0), 91 (21.4). 20d IR (KBr) ν 1705 (C=O) cm–1; 1H NMR (CDCl3) δ 2.43 (s, 3H), 2.66 (t, 2H), 3.79 (s, 3H), 3.84 (t, 2H), 3.88 (s, 3H), 3.96 (s, 3H), 6.23 (s, 1H), 6.69 (s, 1H), 6.81 (s, 1H), 7.31 - 7.48 (m, 4H) ppm; 13C NMR (CDCl3) δ 21.15, 26.92, 45.78, 52.86, 55.82, 56.44, 85.68, 108.47, 110.51, 122.54, 125.36, 130.10, 131.79, 136.83, 138.78, 142.83, 147.39, 149.86, 150.61, 157.92, 160.51 ppm; MS, m/z (%): 437 (M+, 9.3), 293 (100.0), 91 (20.3). 20e IR (KBr) ν 1704 (C=O) cm–1; 1H NMR (CDCl3) δ 2.62 (t, 2H), 3.75 (s, 3H), 3.84 (t, 2H), 3.88 (s, 3H), 3.95 (s, 3H), 6.21 (s, 1H), 6.75 (s, 1H), 7.01 (s, 1H), 7.25 - 7.49 (m, 4H) ppm; MS, m/z (%): 459 (M+ + 2, 4.1), 457 (M+, 10.2), 293 (100.0), 91 (20.9). 20f 1H NMR (CDCl3) δ 2.90 (t, 2H), 3.86 (s, 3H), 3.98 (s, 3H), 4.09 (t, 2H), 6.62 (s, 1H), 7.07 - 8.11 (m, 11H) ppm; 13C NMR (CDCl3) δ 26.16, 47.94, 56.01, 56.16, 86.68, 107.02, 111.55, 116.32, 123.02, 125.85, 127.32, 127.64, 128.66, 129.68, 141.00, 141.42, 142.82, 144.47, 148.48, 151.12, 161.81, 168.40 ppm; MS, m/z (%): 486 (M+, 8.2), 324 (100.0). 20g 1H NMR (CDCl3) δ 2.35 (s, 3H), 2.89 (t, 2H), 3.88 (s, 3H), 4.00 (s, 3H), 4.08 (t, 2H), 6.57 (s, 1H), 7.07-8.07 (m, 10H) ppm; 13C NMR (CDCl3) δ 21.43, 26.16, 47.84, 55.96, 56.15, 86.65, 107.06, 111.44, 116.32, 123.06, 125.84, 127.30, 127.59, 128.86, 129.68, 140.95, 141.41, 142.82, 144.38, 148.34, 151.06, 161.80, 168.39 ppm; MS, m/z (%): 500 (M+, 6.7), 324 (100.0). 20h 1H NMR (CDCl3) δ 2.91 (t, 2H), 3.86 (s, 3H), 3.88 (s, 3H), 4.01 (t, 2H), 6.59 (s, 1H), 7.07 - 8.10 (m, 10H) ppm; MS, m/z (%): 522 (M+ + 2, 3.9), 520 (M+, 9.6), 324 (100.0). 20i 1H NMR (DMSO-d6) δ 2.82 (t, 2H), 3.75 (s, 3H), 3.90 (s, 3H), 4.12 (t, 2H), 6.75 (s, 1H), 7.12 - 8.07 (m, 9H) ppm; 13C NMR (DMSO-d6) δ 25.54, 47.01, 55.51, 55.86, 108.17, 111.12, 111.64, 111.82, 112.78, 116.30, 122.70, 125.61, 129.04, 130.01, 139.98, 144.92, 146.08, 147.80, 150.67, 151.12, 161.21, 161.54 ppm; MS, m/z (%): 492 (M+, 7.9), 324 (100.0), 127 (22.1), 73 (25.0). 20j 1H NMR (DMSO-d6) δ 2.82 (t, 2H), 3.75 (s, 3H), 3.90 (s, 3H), 4.13 (t, 2H), 6.68 (s, 1H), 6.76-8.08 (m, 9H) ppm; 13C NMR (DMSO-d6) δ 25.54, 46.92, 55.51, 55.86, 108.19, 111.12, 111.63, 111.84, 112.78, 116.19, 122.70, 125.67, 129.05, 130.01, 140.01, 144.96, 146.07, 147.80, 150.68, 151.06, 157.46, 161.10 ppm; MS, m/z (%): 476 (M+, 10.6), 324 (100.0). 24a IR (KBr) ν 1647 (C=O) cm–1; 1H NMR (DMSO-d6) δ 2.40 (s, 3H), 2.48 (s, 3H), 2.71 (t, 2H), 3.79 (s, 3H), 3.92 (t, 2H), 3.98 (s, 3H), 6.24 (s, 1H), 6.82 (s, 1H), 6.88 (s, 1H), 7.42 - 7.93 (m, 4H) ppm; MS, m/z (%): 421 (M+, 10.4), 293 (100.0), 91 (19.1). 24b IR (KBr) ν 1649 (C=O) cm–1; 1H NMR (DMSO-d6) δ 2.42 (s, 3H), 2.68 (t, 2H), 3.78 (s, 3H), 3.90 (t, 2H), 3.95 (s, 3H), 6.24 (s, 1H), 6.83 (s, 1H), 6.88 (s, 1H), 7.48 - 7.91 (m, 4H) ppm; MS, m/z (%): 443 (M+ + 2, 5.1), 441(M+, 12.8), 293 (100.0), 91 (22.9).  H. M. Hassaneen et al. / Natural Science 3 (2011) 651-660 Copyright © 2011 SciRes. OPEN ACCESS 657657 24c IR (KBr) ν 1616 (C=O) cm–1; 1H NMR (CDCl3) δ 2.47 (s, 3H), 2.68 (t, 2H), 3.81 (s, 3H), 3.87 (t, 2H), 3.90 (s, 3H), 6.32 (s, 1H), 6.71 (s, 1H), 6.84 (s, 1H), 7.36-8.30 (m, 9H) ppm; 13C NMR (CDCl3) δ 21.26, 26.95, 46.00, 55.92, 56.53, 86.65, 108.62, 110.62, 122.67, 125.29, 126.01, 128.25, 130.25, 130.26, 131.98, 133.14, 135.55, 137.11, 138.89, 147.46, 150.72, 151.28, 158.22, 184.25 ppm; MS, m/z (%): 483 (M+, 6.7), 293 (100.0), 91 (16.8), 105 (66.8). 24d IR (KBr) ν 1616 (C=O) cm–1; 1H NMR (CDCl3) δ 2.67 (t, 2H), 3.79 (s, 3H), 3.87 (t, 2H), 3.91 (s, 3H), 6.34 (s, 1H), 6.70 (s, 1H), 6.84 (s, 1H), 7.35 - 8.32 (m, 9H) ppm; MS, m/z (%): 505 (M+ + 2, 3.9), 503(M+, 9.5), 293 (100.0), 91 (17.2), 105 (67.8). 24e IR (KBr) ν 1672 (C=O), 3385 (NH) cm–1; 1H NMR (CDCl3) δ 2.68 (t, 2H), 3.81 (s, 3H), 3.83 (t, 2H), 3.91 (s, 3H), 6.29 (s, 1H), 6.63 - 8.70 (m, 12H), 11.75 (s, 1H) ppm; MS, m/z (%): 484 (M+, 7.6), 293 (100.0). 24f IR (KBr) ν 1670 (C=O), 3386 (NH) cm–1; 1H NMR (CDCl3) δ 2.47 (s, 3H), 2.68 (t, 2H), 3.80 (s, 3H), 3.85 (t, 2H), 3.89 (s, 3H), 6.22 (s, 1H), 6.66-8.65 (m, 11H), 11.73 (s, 1H) ppm; MS, m/z (%): 498 (M+, 9.5), 293 (100.0). 24g IR (KBr) ν 1670 (C=O), 3388 (NH) cm–1; 1H NMR (CDCl3) δ 2.66 (t, 2H), 3.81 (s, 3H), 3.83 (t, 2H), 3.89 (s, 3H), 6.32 (s, 1H), 6.66 - 8.66 (m, 11H), 11.74 (s, 1H) ppm; MS, m/z (%): 520 (M+ + 2, 2.7), 518 (M+, 8.3), 293 (100.0). Figure 3. Synthesis of pyrazoles 13. other hand, H (5) is linked to the carbon attached to ni- trogen atom and thus it’s deshielded to appear in the region δ 7.5 - 8.5 ppm [26-28]. The 1H NMR spectra of isolated reaction products revealed, in each case, a singlet signal at δ 8.5 ppm which indicates the presence of the pyrazole H (5) rather than H (4) in the structure of the isolated products. The proposed mechanism leading to the formation of the latter product suggested that the studied reaction starts with regioselective 1, 3-dipolar cycloaddition of nitrilimines 10 to C=C of the enaminones 7 to afford the cycloadducts 11 which gave the pyrazole derivatives 13 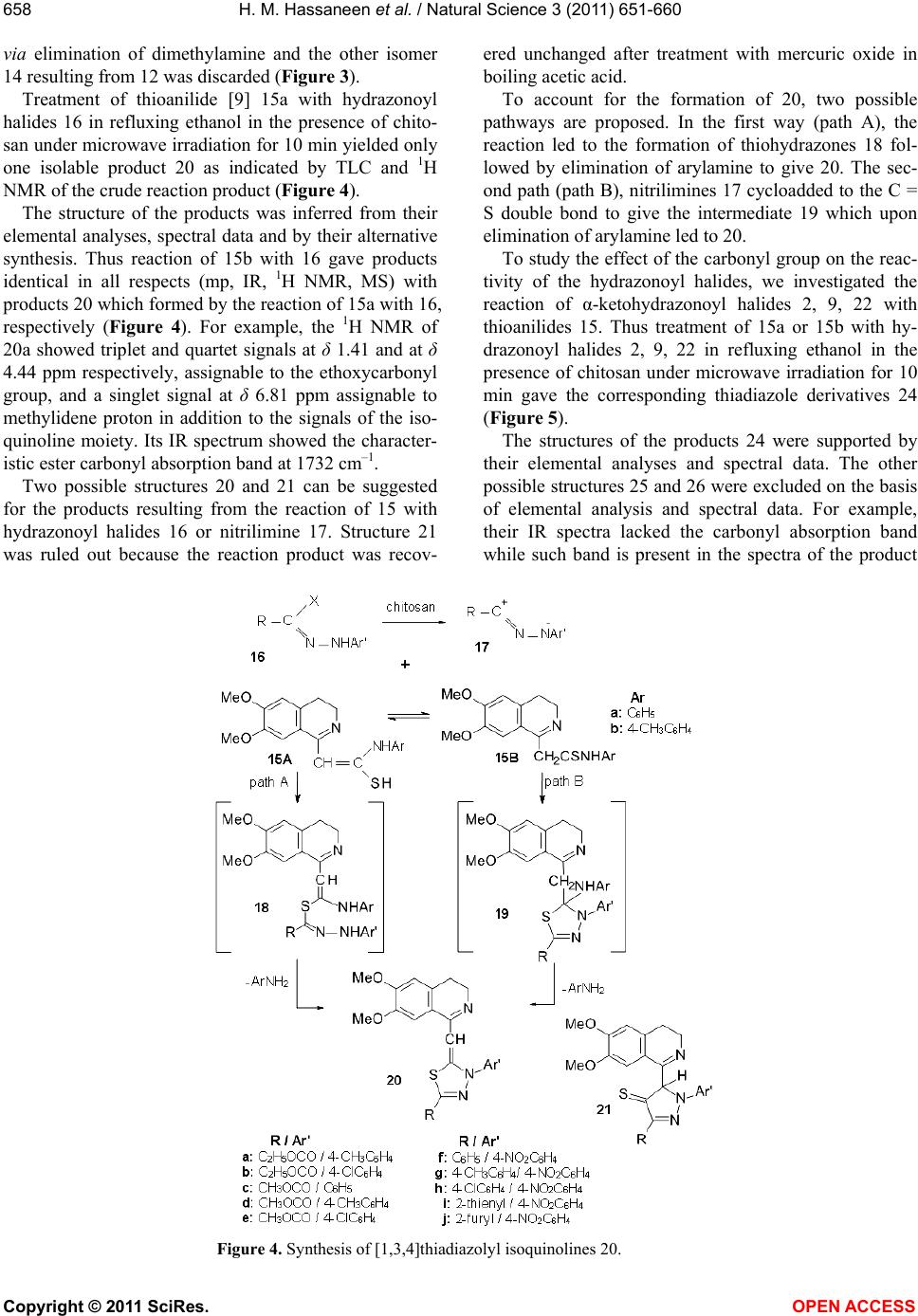 H. M. Hassaneen et al. / Natural Science 3 (2011) 651-660 Copyright © 2011 SciRes. OPEN ACCESS 658 via elimination of dimethylamine and the other isomer 14 resulting from 12 was discarded (Figure 3). Treatment of thioanilide [9] 15a with hydrazonoyl halides 16 in refluxing ethanol in the presence of chito- san under microwave irradiation for 10 min yielded only one isolable product 20 as indicated by TLC and 1H NMR of the crude reaction product (Figure 4). The structure of the products was inferred from their elemental analyses, spectral data and by their alternative synthesis. Thus reaction of 15b with 16 gave products identical in all respects (mp, IR, 1H NMR, MS) with products 20 which formed by the reaction of 15a with 16, respectively (Figure 4). For example, the 1H NMR of 20a showed triplet and quartet signals at δ 1.41 and at δ 4.44 ppm respectively, assignable to the ethoxycarbonyl group, and a singlet signal at δ 6.81 ppm assignable to methylidene proton in addition to the signals of the iso- quinoline moiety. Its IR spectrum showed the character- istic ester carbonyl absorption band at 1732 cm–1. Two possible structures 20 and 21 can be suggested for the products resulting from the reaction of 15 with hydrazonoyl halides 16 or nitrilimine 17. Structure 21 was ruled out because the reaction product was recov- ered unchanged after treatment with mercuric oxide in boiling acetic acid. To account for the formation of 20, two possible pathways are proposed. In the first way (path A), the reaction led to the formation of thiohydrazones 18 fol- lowed by elimination of arylamine to give 20. The sec- ond path (path B), nitrilimines 17 cycloadded to the C = S double bond to give the intermediate 19 which upon elimination of arylamine led to 20. To study the effect of the carbonyl group on the reac- tivity of the hydrazonoyl halides, we investigated the reaction of α-ketohydrazonoyl halides 2, 9, 22 with thioanilides 15. Thus treatment of 15a or 15b with hy- drazonoyl halides 2, 9, 22 in refluxing ethanol in the presence of chitosan under microwave irradiation for 10 min gave the corresponding thiadiazole derivatives 24 (Figure 5). The structures of the products 24 were supported by their elemental analyses and spectral data. The other possible structures 25 and 26 were excluded on the basis of elemental analysis and spectral data. For example, their IR spectra lacked the carbonyl absorption band while such band is present in the spectra of the product Figure 4. Synthesis of [1,3,4]thiadiazolyl isoquinolines 20. 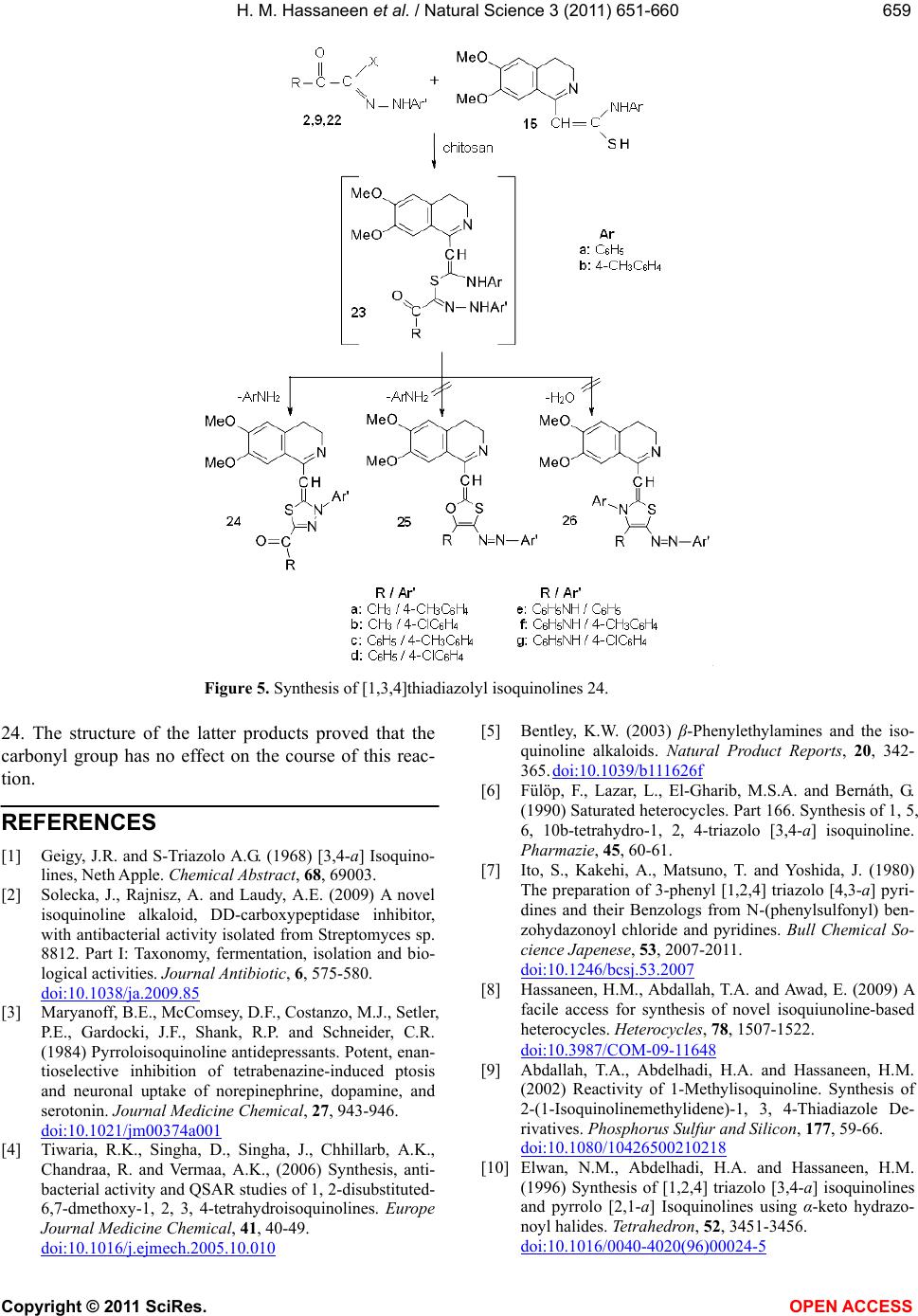 H. M. Hassaneen et al. / Natural Science 3 (2011) 651-660 Copyright © 2011 SciRes. OPEN ACCESS 659659 Figure 5. Synthesis of [1,3,4]thiadiazolyl isoquinolines 24. 24. The structure of the latter products proved that the carbonyl group has no effect on the course of this reac- tion. REFERENCES [1] Geigy, J.R. and S-Triazolo A.G. (1968) [3,4-a] Isoquino- lines, Neth Apple. Chemical Abstract, 68, 69003. [2] Solecka, J., Rajnisz, A. and Laudy, A.E. (2009) A novel isoquinoline alkaloid, DD-carboxypeptidase inhibitor, with antibacterial activity isolated from Streptomyces sp. 8812. Part I: Taxonomy, fermentation, isolation and bio- logical activities. Journal Antibiotic, 6, 575-580. doi:10.1038/ja.2009.85 [3] Maryanoff, B.E., McComsey, D.F., Costanzo, M.J., Setler, P.E., Gardocki, J.F., Shank, R.P. and Schneider, C.R. (1984) Pyrroloisoquinoline antidepressants. Potent, enan- tioselective inhibition of tetrabenazine-induced ptosis and neuronal uptake of norepinephrine, dopamine, and serotonin. Journal Medicine Chemical, 27, 943-946. doi:10.1021/jm00374a001 [4] Tiwaria, R.K., Singha, D., Singha, J., Chhillarb, A.K., Chandraa, R. and Ver m aa, A.K., (2006) Synthesis, anti- bacterial activity and QSAR studies of 1, 2-disubstituted- 6,7-dmethoxy-1, 2, 3, 4-tetrahydroisoquinolines. Europe Journal Medicine Chemical, 41, 40-49. doi:10.1016/j.ejmech.2005.10.010 [5] Bentley, K.W. (2003) β-Phenylethylamines and the iso- quinoline alkaloids. Natural Product Reports, 20, 342- 365. doi:10.1039/b111626f [6] Fülöp, F., Lazar, L., El-Gharib, M.S.A. and Bernáth, G. (1990) Saturated heterocycles. Part 166. Synthesis of 1, 5, 6, 10b-tetrahydro-1, 2, 4-triazolo [3,4-a] isoquinoline. Pharmazie, 45, 60-61. [7] Ito, S., Kakehi, A., Matsuno, T. and Yoshida, J. (1980) The preparation of 3-phenyl [1,2,4] triazolo [4,3-a] pyri- dines and their Benzologs from N-(phenylsulfonyl) ben- zohydazonoyl chloride and pyridines. Bull Chemical So- cience Japenese, 53, 2007-2011. doi:10.1246/bcsj.53.2007 [8] Hassaneen, H.M., Abdallah, T.A. and Awad, E. (2009) A facile access for synthesis of novel isoquiunoline-based heterocycles. Heterocycles, 78, 1507-1522. doi:10.3987/COM-09-11648 [9] Abdallah, T.A., Abdelhadi, H.A. and Hassaneen, H.M. (2002) Reactivity of 1-Methylisoquinoline. Synthesis of 2-(1-Isoquinolinemethylidene)-1, 3, 4-Thiadiazole De- rivatives. Phosphorus Sulfur and Silicon, 177, 59-66. doi:10.1080/10426500210218 [10] Elwan, N.M., Abdelhadi, H.A. and Hassaneen, H.M. (1996) Synthesis of [1,2,4] triazolo [3,4-a] isoquinolines and pyrrolo [2,1-a] Isoquinolines using α-keto hydrazo- noyl halides. Tetrahedron, 52, 3451-3456. doi:10.1016/0040-4020(96)00024-5  H. M. Hassaneen et al. / Natural Science 3 (2011) 651-660 Copyright © 2011 SciRes. OPEN ACCESS 660 [11] Abdallah, T.A., Hassaneen, H.M. and Abdelhadi, H.A. (2009) Synthesis of tetra- and penta- heterocyclic com- pounds incorporated isoquinoline moiety, Heterocycles, 78, 373-378. doi:10.3987/COM-08-11481 [12] Awad, E.M., Elwan, N.M., Hassaneen, H.M., Linden A. and Heimgartner, H. (2002) New routes to fused isoqui- noline, Helvetica Chimica Acta, 85, 320-331. doi:10.1002/1522-2675(200201)85:1<320::AID-HLCA3 20>3.0.CO;2-X [13] Al-matar, H.M., Khalil, K.D., Meier, H. and Elnagdi, M.H. (2008) Chitosan as heterogeneous catalyst in Mi- chael additions: The reaction of cinnamonitriles with ac- tive methyls, active methylenes and phenols, Arkivoc Xvi, 288-301. [14] Guibal, E. (2005) Heterogeneous catalysis on chito- san-based materials: a review, Progress in Polymer Sci- ence, 30, 71-109. doi:10.1016/j.progpolymsci.2004.12.001 [15] Bollini, M., Gonzalez, M. and Bruno, A. (2009) Micro- wave-assisted rapid and efficient synthesis of C-alkyl imidazoisoquinolinone derivatives, Tetrahedron Letters, 50, 1507-1509. doi:10.1016/j.tetlet.2009.01.083 [16] Andrade, C.K.Z., Barreto, A.S. and Silva, W.A. (2008) Microwave assisted solvent-, support- and catalyst-free synthesis of enaminones, Arkivoc Xii, 226-232. [17] Lidstrom, P.J., Tierney, J., Wathey, B. and Westman, J. (2001) Microwave assisted organic synthesis—a review, Tetrahedron, 57, 9225-9283. doi:10.1016/S0040-4020(01)00906-1 [18] Bortolini, O., D’Agostino, M., De Nino, A., Maiuolo, L., Nardi, M. and Sindona, G. (2008) Solvent-free, micro- wave assisted 1,3-cycloaddition of nitrones with vinyl nucleobases for the synthesis of N,O-nucleosides, Tetra- hedron, 64, 8078-8081. doi:10.1016/j.tet.2008.06.074 [19] El Ashry, E.H. and Kassem, A.A. (2006) Account of microwave irradiation for accelerating organic reactions, Arkivoc Xii, 1-16. [20] Nair, M.D. and Metha, S.R. (1967) Long range coupling in heterocyclic compounds, Indian Journal Chemical, 12B, 5. [21] Battersby, A.R., Openshaw, H.T. and Wood, H.C.S. (1953) The synthesis of emetine and related compounds. Part II. The synthesis of (±)-rubremetinium bromide, Journal Chemical Socience, 2463-2470. [22] Gomha, S.M. and Riyadh, S.M. (2009) Synthesis of tria- zolo [4,3-b] [1,2,4,5] tetrazines and triazolo [3,4-b] [1,3,4] thiadiazines using chitosan as ecofriendly catalyst under microwave irradiation, Arkivoc Xii, 58-68. [23] Hassaneen, H.M., Hassaneen, H.M.E. and Mohammed, Y.Sh. (2011) Synthesis, Reactions and Antibacterial Ac- tivity of 3-Acetyl [1,2,4] triazolo [3,4-a] isoquinoline Derivatives using Chitosan as Heterogeneous Catalyst under Microwave Irradiation, Verlag der Zeitschrift für Naturforschung, 66b, 299-310. [24] Dawood, K. M. (2005) Synthesis of Spiro-pyrazole-3, 3’- thiopyrano [2,3-b] pyridines and Azolo [a] pyrido [2’,3’:5,6] thiopyrano [3,4-d] pyrimidines as New Ring Systems with Antifungal and Antibacterial Activities, Journal Heterocyclic Chemical, 42, 221-225. doi:10.1002/jhet.5570420207 [25] Farag, A.M., Mayhoubb, A.S., Barakatb, S.E. and Bayo- mi, A.H. (2008) Regioselective synthesis and antitumor screening of some novel N-phenylpyrazole derivatives. Bioorganic and Medicinal Chemistry, 16, 881-889. doi:10.1016/j.bmc.2007.10.015 [26] Komarova, E.S., Makarov, V.A., Alekseeva, G.V. and Granik, V.G. (2006) Synthesis of derivatives of a new heterocyclic system pyrazolo [3,4-b] pyrido [1’,2’:1,2] imidazo [4,5-d] pyridine, Russian Chemical Bull Interna- tional Edition, 55, 735-740. doi:10.1007/s11172-006-0322-z [27] He, F.Q., Liu, X.H., Wang, B.L. and Li, Z.M. (2008) Synthesis and biological activities of novel bis-heterocy- clic pyrrodiazole derivatives, Heteroatom Chemical, 19, 21-27. doi:10.1002/hc.20369 [28] Amer, F.A., Hammouda, M., El-Ahl, A.S. and Abdelwa- hab, B.F. (2007) Synthesis of Important New Pyrrolo [3,4-c] pyrazoles and Pyrazolyl-Pyrrolines from Hetero- cyclic β-Ketonitriles, Journal Chinese Chemical So- cience, 54, 1543-1552.
|