 American Journal of Anal yt ical Chemistry, 2011, 2, 447-455 doi:10.4236/ajac.2011.24054 Published Online August 2011 (http://www.SciRP.org/journal/ajac) Copyright © 2011 SciRes. AJAC Determination of Clopidogrel Carboxylic Acid in Human Plasma by LC-MS/MS Mohamed El-Husseiny El-Sadek1, Samia Mahmoud Moustafa2, Hussien Omar Kadi3, Abdul Moneim Ali Al-Hakami2 1Faculty of Pharmacy, Zagazig University, Zagazig, Egypt 2Faculty of Pharmacy, Suez Canal University, Suez, Egypt 3Faculty of Medicine, Sana’a University, Sana’a, Yemen E-mail: alhakami2@gmail.com Received April 2, 2011; revised May 12, 2011; accepted May 19, 2011 Abstract Background: Clopidogrel, is a thienopyridine derivative labeled for use to prevent thrombosis after coro- nary artery stenting. Pharmacokinetics of clopidogrel is studied indirectly by quantification of carboxylic acid which is a major metabolite of Clopidogrel. Objective: The aim of this work is to develop and validate a rapid, simple and sensitive LC/MS/MS assay method for the determination of Clopidogrel carboxylic acid in human plasma using Clopidogrel-D4-carboxylic acid as internal standard. Methods: Analytes was ex- tracted from 200 μl of plasma by a simple liquid-liquid extraction using diethyl ether – n-hexane (80:20, v/v). The chromatographic separations were achieved on a C18 column using Methanol, de-ionized water and formic acid as a mobile phase at flow rate of 0.5 ml/minute. Analysis was monitored by multiple reactions monitoring mode based on m/z transition of 308.10→113 for Clopidogrel carboxylic acid and 312.10→129 for internal standard. Result: The method had a total run time of about 4 minutes. The lower limit of quanti- fication (LLOQ) was 25 ng/ml showing good linearity over the working range of 25 – 3000 ng/ml (r ≥ 0.999). The intra- and inter day accuracies were 90% - 98% and 92.138% - 96.889% respectively (deviation within acceptable range ≤ 10%).Conclusion: It was shown that this method is suitable for pharmacokinetic study following oral administration of Clopidogrel and can be successfully applied to the therapeutic drug moni- toring of Clopidogrel in Clopidogrel-treated patients. Keywords: Clopidogrel, Clopidogrel Carboxylic Acid, Liquid Chromatography, Tandem Mass Spectrometry 1. Introduction Clopidogrel hydrogen sulfate (Figure 1), methyl (+)-(S)- α-(o-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridin-5(4 H)-acetate hydrogen sulfate, is a novel thienopyridine derivative that irreversibly blocks adenosine diphosphate (ADP) and is important in platelet aggregation, the cross-linking of platelets by fibrin. Clopidogrel is chem- ically related to Ticlopidine with superior side effects profile and dosing requirements [1-3]. The empirical formula of Clopidogrel bisulfate is C16H16ClNO2S.H2SO4 and its molecular weight is 419.9 [4]. The molecule of clopidogrel contains an asymmetrical carbon at C-7 leading to the existence of two enanti- omers (R and S). Studies showed that the active com- pound clopidogrel is the S-enantiomer [5,6]. Clopidogrel free base was unstable due to a labile proton in the chiral center and was susceptible to racemization and hydroly- sis of methyl ester group [6,7]. The drug is rapidly but incompletely absorbed after oral administration and extensively metabolized to an active metabolite. The parent drug or its active metabo- lite remains undetectable in plasma. The major circulat- ing compound, however, is an inactive carboxylic de- rivative, which is used to document the pharmacokinetic profile of clopidogrel [8]. The pharmacokinetic parameters of clopidogrel have been characterized by this inactive carboxylic acid me- tabolite in human plasma. An inactive carboxylic deriva- tive represents 85% of circulating metabolites in human plasma [9]. The elimination half life of this compound is  M. E.-H. EL-SADEK ET AL. 448 H3COOC H N SCl H2SO4 Figure 1. Chemical structure of clopidogrel bisulfate. 8 hours [10-12]. The platelet inhibition with clopidogrel lasts the lifespan of the platelet (7-10 days) [12-14]. Clopidogrel is inactive in vitro and hepatic biotrans- formation via the cytochrome P450 pathway, is essential for its in vivo antiplatelet activity [15]. The active me- tabolite, 5-thiol compound, is formed by the oxidation of clopidogrel to 2-oxoclopidogrel and subsequent hydroly- sis. The active metabolite is highly labile and remains undetected in plasma [6,16]. Clopidogrel and its metabolite bind reversibly to hu- man plasma proteins (94% - 98%) and are excreted in both urine and feces with an elimination half- life of eight hours [17]. Clopidogrel is a potent, non-competitive inhibitor of adenosine diphosphate (ADP) induced platelet aggrega- tion, irreversibly inhibiting the binding of ADP to its platelet membrane receptors [18]. The inhibition is spe- cific and does not significantly affect cyclooxygenase or arachidonic acid metabolism [19,20]. Clopidogrel is the drug of choice to prevent thrombosis after coronary ar- tery stenting [21,22]. Quantification of carboxylic acid metabolite would be an indirect approach for studying the pharmacokinetics of clopidogrel [12,23-25]. The present study report a simple and sensitive liquid chromatography coupled to tandem mass spectrometric method (LC-MS/MS) for determination of Clopidogrel carboxylic acid in human plasma using Clopidogrel-d4- -carboxylic acid as the internal standard. The developed method was successfully applied to a pharmacokinetic study after oral administration of Clopidogrel (75 mg) to healthy volunteers. 2. Materials 2.1. Reagents and Chemicals Clopidogrel carboxylic acid and Clopidogrel-d4-carbo- xylic acid were obtained from TRC. Methanol and For- mic acid, HPLC grade and n-hexane were purchased from Merck, Germany. Diethyl ether was purchased from VWR, EC. De-ionized water was purchased from Milli. 2.2. Instrumentation and LC/MS Conditions HPLC1100 Agilant from Agilant, Germany consisting of agilant 1100 binary pump, agilant 1100 autosampler and agilant1100 degasser. The chromatographic separations were achieved using a symmetryC18 column (3.5µm 4.6x75 mm; Waters, USA). The mobile phase consisted of Methanol, de-ionized water and formic acid (75%:25%: 0.1%). The flow rate of the mobile phase was set at 0.5 ml/ min. the column oven temperature was 30˚C. The HPLC system was coupled to an API 4000 MS/ MS triple-quadrupole system detector equipped with a turbo ion spray ionization (ESI) source ( Applied Bio- systems MDS, SCIEX, Canada). The turbo ion spray ionization source was operated in a positive mode. The ion spray voltage was adjusted to 5500 V. the mass spec- trometer was operated at a unit resolution for both Q1 and Q3 in multiple reaction monitoring (MRM) mode. The transition of precursor to product ion was monitored at 308.10→113 for Clopidogrel carboxylic acid and 312.10→129 for internal standard. The method had a total run time 4 minutes. 3. Methodology 3.1. Standard and Stock Solutions Preparation Clopidogrel carboxylic acid stock solution 1 mg/ml prepared in methanol; Clopidogrel carboxylic acid working standard solu- tion 100 μg/ml prepared in methanol; Clopidogrel-d4-carboxylic acid Stock Standard Solu- tion 100 µg/ml prepared in methanol; Clopidogrel-d4-carboxylic acid working solution 1000 ng/ml prepared in de-ionized water; 3.2. Calibration Curve and Quality Controls Spiking Preparation In Human Plasma The calibration standards of Clopidogrel carboxylic acid 25, 50, 100, 200, 500, 750, 1000, 2000 and 3000 ng/ml, and 75, 1500 and 2250 ng/ml quality controls were pre- pared by diluting the clopidogrel carboxylic acid solution (100 µg/ml) with drug-free human plasma. 200 µl ali- quots were transferred to 10 ml glass tubes and stored at –20oC. Calibration standards of Clopidogrel carboxylic acid were extracted from all 9 concentration levels and assayed. 3.3. Sample Extraction The internal standard solution (100 µl of Clopidogrel- -d4-carboxylic acid 1000 ng/ml in de-ionized water) was added to each human plasma sample in glass tube, vortex for 30 seconds. Then 30 µl of concentrated formic acid were added, vortex for 30 seconds. Six ml of (20% n- Copyright © 2011 SciRes. AJAC  M. E.-H. EL-SADEK ET AL.449 -hexane:80% diethyl ether) were added, vortex for about 60 seconds followed by centrifugation for 5 minutes at 4000 r.p.m. The upper organic layer were decanted to a clean 10 ml glass tubes and evaporated in water bath at 400C under gentle stream of nitrogen gas. The residue was reconstituted with 200 µl of mobile phase and 25 µl was injected into LC/MS/MS 4. Standardization and Calculation The method's linearity for Clopidogrel carboxylic acid determination in human plasma was confirmed for a range of concentrations between (25 – 3000) ng/ml for Clopidogrel carboxylic acid respectively. Calibration curves were determined by a weighted quadratic regres- sion equation (Y = a2X2 + a1X + a0) were used for Clo- pidogrel carboxylic acid. The best-fit peak area ratios vs. concentrations were used to back-calculate the concen- trations, where y represents the (peak area of Clopidogrel carboxylic acid /peak area of internal standard) and a weighting of 1/x (a0, a1, a2, are the polynomial coeffi- cients of quadratic equation). Chromatograms processing, data generation and concentrations back calculations were all performed by the Analyst software (Applied Biosystems, MDS, SCIEX, Canada). Summary of de- scriptive statistics was done by Excel spreadsheets. 4.1. Method Validation Method validation was conducted in accordance with the ICH and USFDA guidelines and satisfies the require- ments of applicable statutes and regulations[26,27]. Ac- cordingly, the method validation was evaluated in terms of: 4.1.1. Specificity, Selectivity and Matrix Effect The specificity of the method was determined by screen- ing six different batches of human blank plasma and the injection of six LLOQ samples prepared using six dif- ferent batches of human plasma. Specificity of the me- thod was verified by the absence of any co-eluted peaks of endogenous plasma components at the retention times of the drug or the internal standard. Five out of six sam- ples of LLOQ should meet the LLOQ acceptance criteria (not more than 20% deviation from the theoretical val- ues). Specificity was conducted by analyzing 6 blank, zero and 6 LLOQ samples from six different plasma batches. 4.1.2. Linearity For the determination of linearity, standard calibration curves of 9 points (non-zero standards) in addition to the blank and standard zero samples were prepared. On each day of the validation course, a calibration curve was prepared and its goodness of fit was calculated by least square linear regression equation, excluding the results of the blank and standard zero sample from the regres- sion function. The concentrations of calibration standards were back calculated applying the obtained regression equation. At least 75% of non-zero standards, should meet the following acceptance criteria. Not more than 20% deviation at LLOQ. Not more than 15% deviation for standards above the LLOQ. 4.1.3. Accuracy and Precision Accuracy and precision evaluation was conducted over the first three days of the validation time course. At each day, QC samples were calculated employing the regres- sion of the calibration curve prepared at the same day. The accuracy and precision deviation values should be within 20% for the LLOQ and 15% for the QC's above the LLOQ. 4.1.3.1. Intra-day Accuracy and Precision The intra-day accuracy and precision of the assay was measured by analyzing four QC samples at each concen- tration level LLOQ, low QC, medium QC and high QC samples (25, 75, 1500 and 2250 ng/ml) Clopidogrel car- boxylic acid on three consecutive days with six replicate sample each day, where their concentrations were back calculated. The deviation of the mean from the nominal value serves as the measure of accuracy. Acceptance Criteria: ±20% for QCs at LLOQ and ±15% for QCs at low, mid and high concentrations. Ac- cepted ration is 67% of each level and 67% for the total. 4.1.3.2. Inter-day Accuracy and Precision The inter day accuracy and precision was investigated at each concentration level (25, 75, 1500 and 2250) ng/ml for Clopdogrel carboxylic acid over three days. Analysis was carried out using eighteen QC samples at each con- centration level. Acceptance criteria: ±20% for QCs at LLOQ and ±15% for QCs at low, mid and high concentrations. Ac- cepted ratio is 67% of each level and 67% for the total. 4.1.3.3. Absolute Analytical Recovery The detector response obtained from the injection of the extracted QC samples was compared to the detector re- sponse of an equivalent pure authentic standard solution that was prepared to contain a drug and internal standard concentration assuming 100% recovery and reconstituted in dry extracted blank samples from six different batches. The absolute recoveries were calculated for Clopidogrel carboxylic acid and internal standards by comparing the relevant peak areas of the extracted QC samples with the Copyright © 2011 SciRes. AJAC  M. E.-H. EL-SADEK ET AL. Copyright © 2011 SciRes. AJAC 450 peak areas of an equivalent un-extracted pure authentic standard solution reconstitute in the dry extracted blank samples. The absolute analytical recovery was calculated for Clopidogrel carboxylic acid at three concentration levels (75, 1500 and 2250) ng/ml, while for the internal standard, absolute analytical recovery was calculated at the nominal concentration of 500 ng/ml for Clopido- grel-d4-carboxylic acid Acceptance criteria: Recovery close to 100% are de- sirable, the extent of absolute analytical recovery of an analyte and/or the internal standard may be as low as 50% to 60% but not more than 110%; if the recovery is precise, accurate, and reproducible. 4.1.4. Sensiti vi ty The lowest standard concentration in the calibration curve is considered as the lower limit of quantification (LLOQ) that meets the following acceptance criteria: LLOQ response is five times the response of the blank sample. The sensitivity was tested by preparing and analyzing six QC samples at the LLOQ with a complete set of cali- bration curve on each day of the three days of validation. 4.1.5. Stability 4.1.5.1. Stability in Biological Plasma Samples The stability of Clopidogrel carboxylic acid and the in- ternal standards (Clopidogrel-d4-carboxylic acid) was studied using six QC samples for each time interval ses- sion, at both the low and the high concentration levels (75 and 2250) ng/ml Clopidogrel carboxylic acid. 1) Short-term stability Sufficient number of QC samples at each concentra- tion level were allocated to carry out the short-term sta- bility. Six low and the high concentration levels were prepared and frozen at –20oC for more than 12 hrs then thawed at room temperature (RT) and left on the bench top at room temperature for 6 hours and then analyzed. Stability was calculated by comparing the tested QC samples with theoretical nominal concentrations. Acceptance criteria: % CV is within ±15% for QCs at low and high concentrations. Accepted ratio is 67% of each level and 67% for the total, stability ±15%. 2) Maximum number of injections per run The maximum number of injections per run is vali- dated by injecting a standard calibration curve, and QC's at three levels (low, mid, high) in duplicate or more with blank samples spaced between the QC's. QC samples are distributed as the unknown samples in routine analysis. The maximum number of injections per run was stud- ied for 104 and it was passed. 4.1.5.2. Stock Solution Stability Clopidogrel carboxylic acid and Clopidogrel-d4-carbox- ylic acid stock solution stability (prepared in methanol) were evaluated for 6 hours at room temperature, and for 23 days at –20oC. Clopidogrel carboxylic acid and Colpidogre-d4-carb- oxylic acid working solution stability (prepared in de-io- nized water) were evaluated for 6 hours at room tem- perature, and 10 days at –20oC. Stability was calculated by comparing the pertinent responses obtained from the test stock solutions(s) with the responses of freshly prepared ones. 4.1.6. Matrix Dilution Integrity Samples of concentration higher than ULOQ should be diluted with a complementary volume of the same bio- logical matrix. Thus, the resultant dilute sample would be processed and the obtained area ratio can be easily fitted to the regression equation of the calibration curve. The dilution should bring down the concentration to fall within the linear range. Acceptance criteria: % CV and accuracy should be within ±15% for QC dilution and the accepted ratio is 67% per level. 5. Result 5.1. Mass Spectrometry and Chromatography The MRM chromatograms of Clopidogrel carboxylic acid and Clopidogrel-d4-carboxylic acid obtained by ex- traction of human plasma are shown in (Figures 2-5). The retention times for Clopidogrel and internal standard was 1.82. No endogenous or extraneous peaks interfered with the assy. The lower limit of quantification of Clo- pidogrel carboxylic acid was 25 ng/ml. the calibration curve was linear over a concentration from 25 – 3000 ng/ml. In this study, matrix matched standards were used in constructing the calibration curve, therefore, any po- ssible indirect interference caused by the endogenous components was minimized. 5.2. Method Validation Specificity of the method was verified by the absence of any co-eluted peaks of endogenous plasma component at the retention times of the drug or the internal standard. All samples passed the acceptance criteria. The chroma- togram for a blank plasma sample indicating that the method is specific, and the results of the six LLOQ shows that there is no matrix effect on LLOQ (Figure 2). The method is linear over the range (25 – 3000) ng/ml for Clopidogrel carboxylic acid, (r ≥ 0.999), where at the LLOQ accuracy obtained was 94% (less than 20% devia- tion) and 96.76% - 103% for standard points higher than 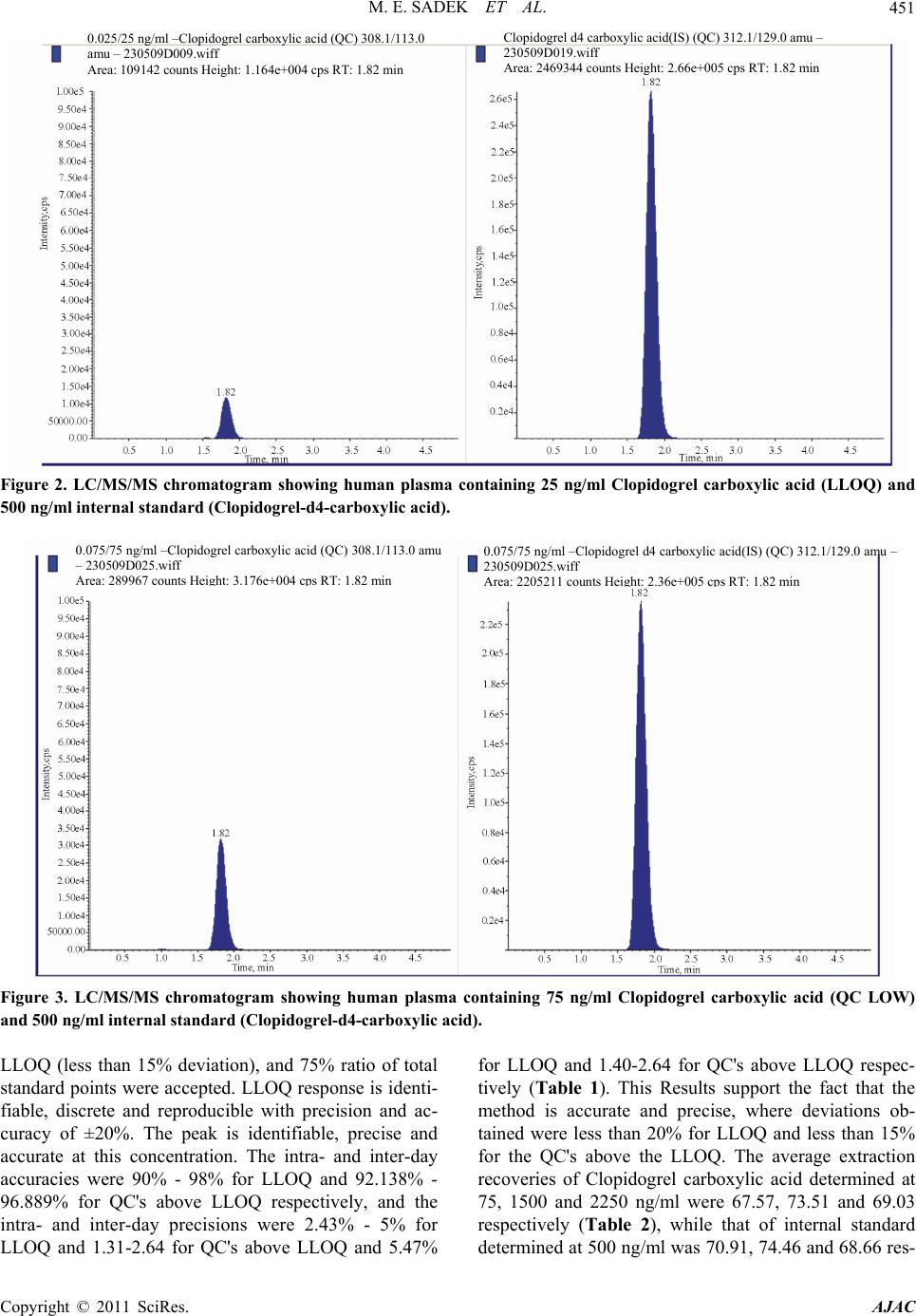 M. E. SADEK ET AL.451 Clopidogrel d4 carboxylic acid(IS) (QC) 312.1/129.0 amu – 230509D019.wiff Area: 2469344 counts Height: 2.66e+005 cps RT: 1.82 min 0.025/25 ng/ml –Clopidogrel carboxylic acid (QC) 308.1/113.0 amu – 230509D009.wiff Area: 109142 counts Height: 1.164e+004 cps RT: 1.82 min Figure 2. LC/MS/MS chromatogram showing human plasma containing 25 ng/ml Clopidogrel carboxylic acid (LLOQ) and 500 ng/ml internal standard (Clopidogrel-d4-carboxylic acid). 0.075/75 ng/ml –Clopidogrel carboxylic acid (QC) 308.1/113.0 amu – 230509D025.wiff Area: 289967 counts Height: 3.176e + 0.075/75 ng/ml –Clopidogrel d4 carboxylic acid(IS) (QC) 312.1/129.0 amu – 230509D025.wiff Ar 22 211 nt H i ht 2 + RT 1 2 min Figure 3. LC/MS/MS chromatogram showing human plasma containing 75 ng/ml Clopidogrel carboxylic acid (QC LOW) and 500 ng/ml internal standard (Clopidogrel-d4-carboxylic acid). LLOQ (less than 15% deviation), and 75% ratio of total standard points were accepted. LLOQ response is identi- fiable, discrete and reproducible with precision and ac- curacy of ±20%. The peak is identifiable, precise and accurate at this concentration. The intra- and inter-day accuracies were 90% - 98% for LLOQ and 92.138% - 96.889% for QC's above LLOQ respectively, and the intra- and inter-day precisions were 2.43% - 5% for LLOQ and 1.31-2.64 for QC's above LLOQ and 5.47% for LLOQ and 1.40-2.64 for QC's above LLOQ respec- tively (Table 1). This Results support the fact that the method is accurate and precise, where deviations ob- tained were less than 20% for LLOQ and less than 15% for the QC's above the LLOQ. The average extraction recoveries of Clopidogrel carboxylic acid determined at 75, 1500 and 2250 ng/ml were 67.57, 73.51 and 69.03 respectively (Table 2), while that of internal standard determined at 500 ng/ml was 70.91, 74.46 and 68.66 res- Copyright © 2011 SciRes. AJAC  M. E. SADEK ET AL. 452 Clopidogrel d4 carboxylic acid(IS) (QC) 312.1/129.0 amu – 230509D031.wiff Area: 2329774 counts Height: 2.48e+005 cps RT: 1.82 min 3.000/1500 ng/ml –Clopidogrel carboxylic acid (QC) 308.1/113.0 amu – 230509D031.wiff Area: 5234674 counts Height: 5.397e+005 cps RT: 1.82 min Figure 4. LC/MS/MS chromatogram showing human plasma sample containing 1500 ng/ml Clopidogrel carboxylic acid (QC Medium) and 500 ng/ml internal standard (Clopidogrel-d4-carboxylic acid) . 4.500/2250 ng/ml –Clopidogrel carboxylic acid (QC) 308.1/113.0 amu – 230509D037.wiff Area: 6294723 counts Height: 6.374e+005 cps RT: 1.82 min 4.500/2250 ng/ml –Clopidogrel carboxylic acid (QC) 308.1/113.0 amu – 230509D037.wiff Area: 6294723 counts Height: 6.374e+005 cps RT: 1.82 min Figure 5. LC/MS/MS chromatogram showing human plasma containing 2250 ng/ml Clopidogrel carboxylic acid (QC High) and 500 ng/ml internal standard (Clopidogrel-d4-carboxylic acid). pectively with high degree of precision, accuracy and reproducibility. Clopidogrel carboxylic acid is stable up to 6 hours at room temperature as supported by the % CV obtained (2.12%) for the low concentration (75 ng/ml) and (1.35%) for the high concentration (2250 ng/ml) for all the QC samples. The method has shown that the stock solution for Clopidogrel carboxylic acid and Clopidogrel d4-carboxylic acid in methanol is stable for 6 hours at room temperature and up to 23 days at –20oC, while that for the serial solution in de-ionized water is 6 hours at room temperature and up to 10 days at –20oC. Clopido- grel carboxylic acid was stable with absolute percentages Copyright © 2011 SciRes. AJAC 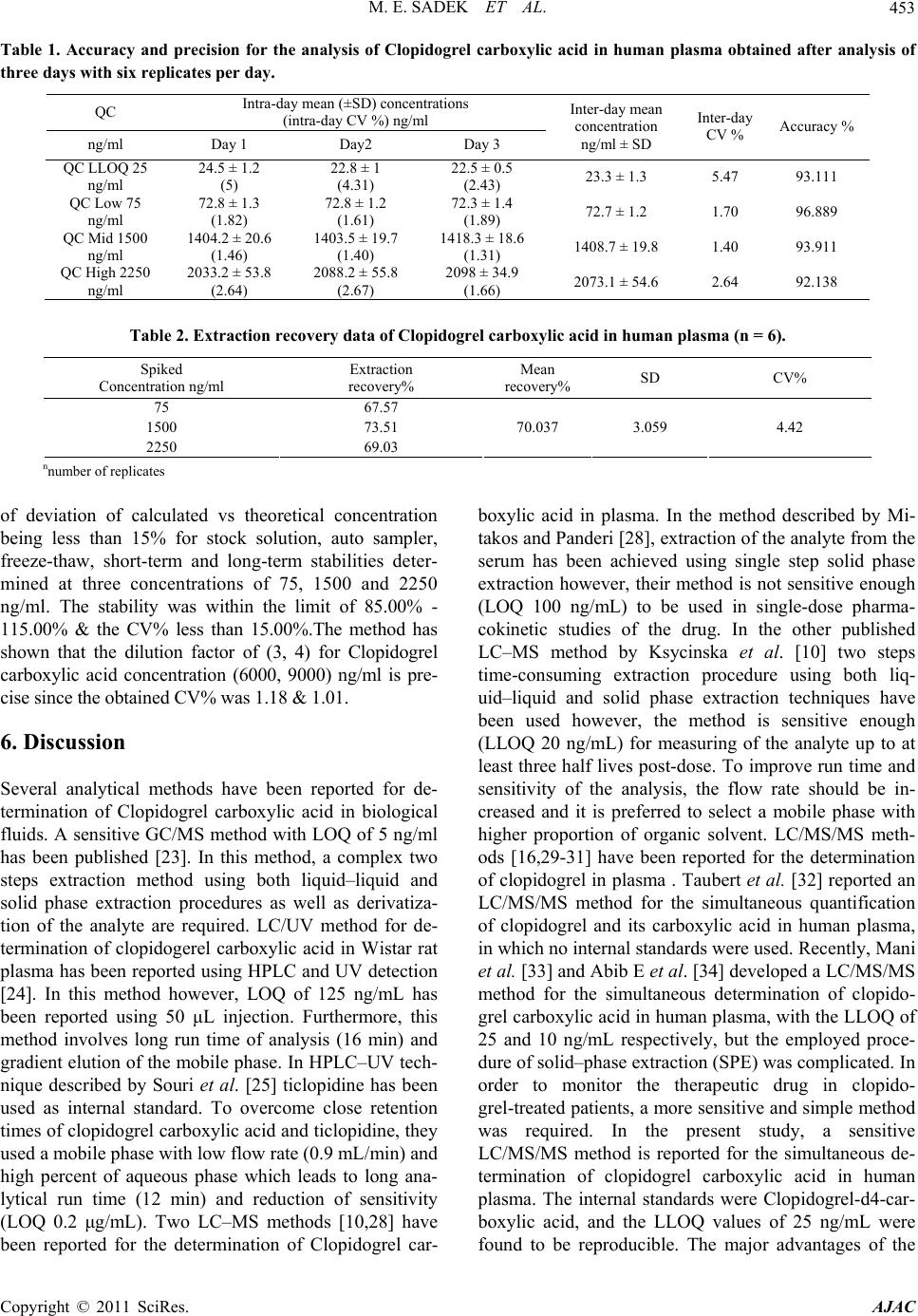 M. E. SADEK ET AL.453 Table 1. Accuracy and precision for the analysis of Clopidogrel carboxylic acid in human plasma obtained after analysis of three days with six replicates per day. QC Intra-day mean (±SD) concentrations (intra-day CV %) ng/ml ng/ml Day 1 Day2 Day 3 Inter-day mean concentration ng/ml ± SD Inter-day CV % Accuracy % QC LLOQ 25 ng/ml 24.5 ± 1.2 (5) 22.8 ± 1 (4.31) 22.5 ± 0.5 (2.43) 23.3 ± 1.3 5.47 93.111 QC Low 75 ng/ml 72.8 ± 1.3 (1.82) 72.8 ± 1.2 (1.61) 72.3 ± 1.4 (1.89) 72.7 ± 1.2 1.70 96.889 QC Mid 1500 ng/ml 1404.2 ± 20.6 (1.46) 1403.5 ± 19.7 (1.40) 1418.3 ± 18.6 (1.31) 1408.7 ± 19.8 1.40 93.911 QC High 2250 ng/ml 2033.2 ± 53.8 (2.64) 2088.2 ± 55.8 (2.67) 2098 ± 34.9 (1.66) 2073.1 ± 54.6 2.64 92.138 Table 2. Extraction recovery data of Clopidogrel carboxylic acid in human plasma (n = 6). Spiked Concentration ng/ml Extraction recovery% Mean recovery% SD CV% 75 67.57 1500 73.51 2250 69.03 70.037 3.059 4.42 nnumber of replicates of deviation of calculated vs theoretical concentration being less than 15% for stock solution, auto sampler, freeze-thaw, short-term and long-term stabilities deter- mined at three concentrations of 75, 1500 and 2250 ng/ml. The stability was within the limit of 85.00% - 115.00% & the CV% less than 15.00%.The method has shown that the dilution factor of (3, 4) for Clopidogrel carboxylic acid concentration (6000, 9000) ng/ml is pre- cise since the obtained CV% was 1.18 & 1.01. 6. Discussion Several analytical methods have been reported for de- termination of Clopidogrel carboxylic acid in biological fluids. A sensitive GC/MS method with LOQ of 5 ng/ml has been published [23]. In this method, a complex two steps extraction method using both liquid–liquid and solid phase extraction procedures as well as derivatiza- tion of the analyte are required. LC/UV method for de- termination of clopidogerel carboxylic acid in Wistar rat plasma has been reported using HPLC and UV detection [24]. In this method however, LOQ of 125 ng/mL has been reported using 50 μL injection. Furthermore, this method involves long run time of analysis (16 min) and gradient elution of the mobile phase. In HPLC–UV tech- nique described by Souri et al. [25] ticlopidine has been used as internal standard. To overcome close retention times of clopidogrel carboxylic acid and ticlopidine, they used a mobile phase with low flow rate (0.9 mL/min) and high percent of aqueous phase which leads to long ana- lytical run time (12 min) and reduction of sensitivity (LOQ 0.2 μg/mL). Two LC–MS methods [10,28] have been reported for the determination of Clopidogrel car- boxylic acid in plasma. In the method described by Mi- takos and Panderi [28], extraction of the analyte from the serum has been achieved using single step solid phase extraction however, their method is not sensitive enough (LOQ 100 ng/mL) to be used in single-dose pharma- cokinetic studies of the drug. In the other published LC–MS method by Ksycinska et al. [10] two steps time-consuming extraction procedure using both liq- uid–liquid and solid phase extraction techniques have been used however, the method is sensitive enough (LLOQ 20 ng/mL) for measuring of the analyte up to at least three half lives post-dose. To improve run time and sensitivity of the analysis, the flow rate should be in- creased and it is preferred to select a mobile phase with higher proportion of organic solvent. LC/MS/MS meth- ods [16,29-31] have been reported for the determination of clopidogrel in plasma . Taubert et al. [32] reported an LC/MS/MS method for the simultaneous quantification of clopidogrel and its carboxylic acid in human plasma, in which no internal standards were used. Recently, Mani et al. [33] and Abib E et al. [34] developed a LC/MS/MS method for the simultaneous determination of clopido- grel carboxylic acid in human plasma, with the LLOQ of 25 and 10 ng/mL respectively, but the employed proce- dure of solid–phase extraction (SPE) was complicated. In order to monitor the therapeutic drug in clopido- grel-treated patients, a more sensitive and simple method was required. In the present study, a sensitive LC/MS/MS method is reported for the simultaneous de- termination of clopidogrel carboxylic acid in human plasma. The internal standards were Clopidogrel-d4-car- boxylic acid, and the LLOQ values of 25 ng/mL were found to be reproducible. The major advantages of the Copyright © 2011 SciRes. AJAC  M. E.-H. EL-SADEK ET AL. 454 method are selectivity and sensitivity (LLOQ 25 ng/ml) reduction in run time of analysis (4.0 min) which allows determination of the analyte more precisely. Representa- tive chromatograms are shown in Figures 2-5. All sam- ples were found to show no interference at the retention times of the analytes. 7. Conclusions The developed method showed a linear dynamic range of 25-3000 ng/ml, with good intra-and inter-day accuracy and precision. It was shown that this method is suitable for pharmacokinetic study following oral administration of Clopidogrel to male volunteers under fasting condi- tions. The method has been successfully applied to the therapeutic drug monitoring of clopidogrel in clopido- grel-treated patients. The LLOQ of the assay was suffi- cient to characterize the absorption kinetics of Clopido- grel in human. 8. References [1] D. C. Mills, R. Puri, C. J. Hu, C. Minniti, G. Grana, M. D. Freedman, R. F. Colman and R. W. Colman, “Clopido- grel Inhibits the Binding of ADP Analogues to the Re- ceptor Mediating Inhibition of Platelet Adenylate Cy- clase,” Arteriosclerosis Thrombosis, Vol. 12, No. 4, 1992, pp. 430-436. doi:10.1161/01.ATV.12.4.430 [2] P. W. Majerus and D. M. Tollefsen, “Blood Coagulation and Anticoagulant, Thrombolytic, and Antiplatelet Drugs,” In: L. L. Brunton, Ed., The Pharmacological Basis of Therapeutics, 11th Edition, The McGraw-Hill Companies, New York, 2006, p. 1483. [3] PLAVIXTM, 2011. www.RXlist.com [4] Safety, 2011. http://www.fda.gov/medwatch/safety/2007/May_PI/Plavi x_PI.pdf [5] P. Savi, J. Combalbert, C. Gaich, M. C. Rouchon, J. P. Maffrand, Y. Berger and J. M. Herbert, “The Antiaggre- gating Activity of Clopidogrel Is Due to a Metabolic Ac- tivation by the Hepatic Cytochrome P450-1A,” Thromb Haemostasis, Vol. 72, 1994, pp. 313-317. [6] J. M. Pereillo, M. Maftouh, A. Andrieu, M. F. Uzabiaga, O. Fedeli, P. Savi, M. Pascal, J. M. Herbert, J. P. Maf- frand and C. Picard, “Structure and Stereochemistry of the Active Metabolite of Clopidogrel,” Drug Metabolism and Disposition, Vol. 30, No. 11, 2002, pp. 1288-1295. doi:10.1124/dmd.30.11.1288 [7] Y. Gomez, E. Adams and J. Hoogmartens, “Analysis of Purity in 19 Drug Product Tablets Containing Clopido- grel: 18 Copies Versus Theoriginal Brand,” Journal of Pharmaceutical and Biomedical Analysis, Vol. 34, No. 2, 2004, pp. 341-348. [8] J. M. Herbert, D. Frehel, E. Vallee, G. Kieffer, D. Gouy, Y. Berger, G. Defreyn and J. P. Maffrand, “Clopidogrel, a Novel Antiplateletand Antithrombotic Agent,” Cardio- vascular Drugs, Vol. 11, No. 2, 1993, pp. 180-198. [9] T. R. Rao, P. R. Usha, M. U. Naidu, J. A. Gogtay and M. Meena, “Bioequivalence and Tolerability Study of Two Brands of Clopidogrel Tablets, Using Inhibition of Plate- let Aggregation and Pharmacodynamic Measures,” Cur- rent Therapeutic Research, Vol. 64, No. 9, 2003, pp. 685- 696. doi:10.1016/j.curtheres.2003.09.014 [10] H. Ksycinska, P. Rudzki and M. Bukowska-Kiliszek, “Determination of Clopidogrel Metabolite (SR26334) in Human Plasma by LC–MS,” Journal of Pharmaceutical and Biomedical Analysis, Vol. 41, No. 2, 2006, pp. 533- 539. doi:10.1016/j.jpba.2005.11.035 [11] P. C. A. Kam and C. M. Nethery, “The Thienopyridine Derivatives (Platelet Adenosine Diphosphate Receptor Antagonists), Pharmacology and Clinical Developments,” Anaesthesia, Vol. 58, No. 1, 2008, pp. 28-35. doi:10.1046/j.1365-2044.2003.02960.x [12] J. McEwen, G. Strauch, P. Perles, G. Fowter, T. More- land, J. P. Dickinson, R. Meontels, J. Mosser and J. Nec- cian, “Clopidogrel Bioavailability Is Unaffected by Food or Antacids,” The Journal of Clinical Pharmacology, Vol. 36, No. 9, 1996, p. 856. [13] G. Vilahur, B. G. Choi, M. U. Zafar, J. F. Viles-Gonzalez, D. A. Vorchheimer, V. Fuster and J. J. Badimon, “Nor- malization of Platelet Reactivity in Clopidogrel-Treated Subjects,” Journal of Thrombosis and Haemostasis, Vol. 5, No. 1, 2007, pp. 82-90. doi:10.1111/j.1538-7836.2006.02245.x [14] R. H. Hongo, J. Ley, S. E. Dick and R. R. Yee, “The Effect of Clopidogrel in Combination with Aspirin When Given before Coronary Artery Bypass Grafting,” Journal of the American College of Cardiology, Vol. 40, No. 2, 2002, pp. 231-237. doi:10.1016/S0735-1097(02)01954-X [15] K. A. Kim, P. W. Park and J. Y. Park, “Effect of CYP- 3A5*3 Genotype on the Pharmacokinetics and Antiplate- let Effect of Clopidogrel in Healthy Subjects,” European Journal of Clinical Pharmacology, Vol. 64, No. 6, 2008, pp. 589-597. doi:10.1007/s00228-008-0471-0 [16] R. V. S. Nirogi, V. N. Kandikere, M. Shukla, K. Mudi- gonda, S. Maurya and R. Boosi, “Quantification of Clo- pidogrel in Human Plasma by Sensitive Liquid Chroma- tography/Tandem Mass Spectrometry,” Mass Spectrome- try, Vol. 20, No. 11, 2006, pp. 1695-1700. [17] R. A. Kulkarni, “Clopidogrel in Cardiovascular Disor- ders,” Journal of Postgraduate Medicine, Vol. 46, No. 4, 2000, pp. 312-313. [18] G. Escolar and M. Heras, “Clopidogrel: A Selective In- hibitor of Platelet ADP Receptors,” Drugs Today, Vol. 36, No. 4, 2000, p. 187. [19] A. A. Weber, S. Reimann and K. Schrör, “Specific Inhi- bition of ADP Induced Platelet Aggregation by Clopido- grel in Vitro,” British Journal of Pharmacology, Vol. 126, No. 2, 1999, pp. 415-420. doi:10.1038/sj.bjp.0702276 [20] K. Schrör. “The Basic Pharmacology of Ticlopidine and Clopidogrel,” Platelets, Vol. 4, No. 5, 1993, pp. 252-261. doi:10.3109/09537109309013225 [21] P. A. Gurbel, C. M. O’Connor, C. C. Cummings and V. L. Copyright © 2011 SciRes. AJAC  M. E.-H. EL-SADEK ET AL. Copyright © 2011 SciRes. AJAC 455 Serebruany, “Clopidogrel: The Future Choice for Pre- venting Platelet Activationduring Coronary Stenting?” Pharmacological Research, Vol. 40, No. 2, 1999, pp. 107-111. doi:10.1006/phrs.1999.0478 [22] CAPRIE Steering Committee, “A Randomized Blinded Trial of Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE),” Lancet, Vol. 348, No. 9038, 1996, pp. 1329-1339. doi:10.1016/S0140-6736(96)09457-3 [23] P. Lagorce, Y. Perez, J. Ortiz, J. Necciari and F. Bressolle, “Assay Method for the Carboxylic Acid Metabolite of Clopidogrel in Human Plasma by Gas Chromatogra- phy–Mass Spectrometry,” Journal of Chromatography B: Biomedical Sciences, Vol. 720, No. 1-2, 1998, pp. 107- 117. doi:10.1016/S0378-4347(98)00452-6 [24] S. S. Singh, K. Sharma, D. Barot, P. R. Mohan and V. B. Lohray, “Estimation of Carboxylic Acid Metabolite of Clopidogrel in Wistar Rat Plasma by HPLC and Its Ap- plication to a Pharmacokinetic Study,” Journal of Chro- matography B. Analytical Technologies in the Biomedical and Life Sciences, Vol. 821, No. 2, 2005, pp. 173-180. doi:10.1016/j.jchromb.2005.05.013 [25] E. Souri, H. Jalalizadeh, A. Kebriaee-Zadeh, M. Shekar- chi and A. Dalvandi, “Validated HPLC Method for De- termination of Carboxylic Acid Metabolite of Clopido- grel in Human Plasma and Its Application to a Pharma- cokinetic Study,” Biomedical Chromatography, Vol. 20, 2006, pp. 1309-1314. doi:10.1002/bmc.697 [26] ICH Steering Committee, “Validation of Analytical Pro- cedures: Methodology,” 1996. http://www.pharmweb.net/pomirroor/pw9/ifpma/ichl.htm l [27] U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), “Guidance for Industry: Bioanalytical Method Validation,” May 2001. [28] A. Mitakos and I. Panderi, “Determination of the Car- boxylic Acid Metabolite of Clopidogrel in Human Plasma by Liquid Chromatography–Electrospray Ionization Mass Spectrometry,” Analytica Chimica Acta, Vol. 505, No. 1, 2004, pp. 107-114. doi:10.1016/S0003-2670(03)00019-9 [29] A. Robinson, J. Hillis, C. Neal and A. C. Leary, “The Validation of a Bioanalytical Method for the Determina- tion of Clopidogrel in Human Plasma,” Journal of Chr- omatography B, Vol. 848, No. 2, 2007, pp. 344-354. doi:10.1016/j.jchromb.2006.10.076 [30] A. Lainesse, Y. Ozalp, H. Wong and R. S. Alpan, “Bio- equivalence Study of Clopidogrel Bisulfate Film-Coated Tablets,” Arzneimittelforshung, Vol. 54, No. 9, 2004, pp. 600-604. [31] B. S. Shin and S. D. Yoo “Determination of Clopidogrel in Human Plasma by Liquid Chromatography/Tandem Mass Spectrometry: Application to a Clinical Pharma- cokinetic Study,” Biomedical Chromatography, Vol. 21, No. 9, 2007, pp. 883-889. doi:10.1002/bmc.850 [32] D. Taubert, A. Kastrati, S. Harlfinger, O. Gorchakova, A. Lazar, N. Beckerath, A. Schomong and E.Schomig, “Pharmacokinetics of Clopidogrel after Administration of a High Loading Dose,” Thromb Haemostasis, Vol. 92, 2004, pp. 311-316. [33] H. Mani, S. W. Toennes, B. Linnemann, D. A. Urbanek, J. Schwonberg, G. F. Kauert and E. Lindhoff-Last, “Deter- mination of Clopidogrel Main Metabolite in Plasma: A Useful Tool for Monitoring Therapy?” Therapeutic Drug Monitoring, Vol. 30, No. 1, 2008, pp. 84-89. doi:10.1097/FTD.0b013e31815c13fd [34] E. Abib, L. F. Duarte, M. L. P. Vanunci, D. A. Oliveira de, S. Antonelli, et al., “Comparative Biological Avail- ability of Clopidogrel Formulation in Healthy Volunteers After a Single Dose Administration,” Journal of Bio- equivalence & Bioavailability, Vol. 2, No. 2, 2010, pp. 45-49.
|