 American Journal of Anal yt ical Chemistry, 2011, 2, 411-421 doi:10.4236/ajac.2011.24050 Published Online August 2011 (http://www.SciRP.org/journal/ajac) Copyright © 2011 SciRes. AJAC An Economical Method for Preparative Purification of Five Alkaloids from Coptis chinensis Franch by High-Speed Counter-Current Chromatography Using Singled Prepared Solvent System by GC Lianhong Yin, Lina Xu, Xiaona Wang, Binan Lu, Yingnan Li, Mingming Hu, Yuetao Liu, Jinyong Peng* College of Pharmacy, Dalian Medical University, Dalian, China E-mail: *yinlianhong1015@163.com Received August 8, 2010; revised February 22, 2011; accepted May 23, 2011 Abstract Coptis chinensis Franch, a widely used Traditional Chinese Medicine, shows various kinds of bioactivity. The major active components of the herb are considered to be alkaloids. Thus, preparative separation of these alkaloids is critical important for further pharmacology and mechanism studies. In the paper, five alka- loids from C. chinensis were purified by HSCCC using the solvent system composed of chloroform-metha- nol-water (2:1:1, v/v/v) single prepared. The content of each solvent in solvent system were determined by gas chromatography (GC), then according the ratios of solvents in each phase to prepare the mobile and sta- tionary phase respectively. And a comparative study was carried out between together preparation and single preparation of the solvent system. The purities and recoveries of all the products were over 98.5% and 92%. However, 134 mL chloroform, 336 mL methanol and 452 mL water were saved when the two phase were singled by GC. Our research showed an economical method for separating alkaloids from C. chinensis by HSCCC using the solvent system single prepared by GC. Keywords: Alkaloid, Coptis chinensis Franch, High Speed Counter-Current Chromaotgraphy, Gas Chromatography, Solvent System 1. Introduction Coptis chinensis Franch (Huanglian in Chinese), a fa- mous traditional Chinese medicine in China, has been used for centuries to treat many kinds of disorders, such as dysentery, cholera, hypertension, leukemia, inflamma- tion and lung disease [1-3]. The major bioactive compo- nents of the herb are considered to be alkaloids including palmatine, berberine, epiberberine, jatrorrhizine and cop- tisine [4]. Pharmacological studies and clinical practice have demonstrated that these alkaloids have antibacterial, anti-inflammatory, anticancer and anti-HIV effects [5-7]. Thus, preparative separation of the alkaloids from the C. chinensis is critical important for further pharmacology and mechanism studies. Some methods such as silica gel, polyamide, preparative RPLC and high-speed counter- current chromatogramphy (HSCCC) have been reported previously. But the conventional column chromatogra- phy is tedious and requiring multiple chromatography steps, preparative RPLC have a smaller load ranges [8,9]. In addition, the adsorptions on stationary phase material and the solvents consume in both of two methods are serious. Thus, HSCCC as a good effective, high recovery and big load sample method is expected and used frequently to separate the alkaloids in recent years. HSCCC, a unique continuous liquid-liquid partition chromatographic technique [10-12], allows directly in- troduction of crude samples into the column without pre-preparation, and yields a highly efficient separation in several hours, when sample load ranges from milli- grams to kilograms [13,14]. It also can purify some compounds with an excellent sample recovery, which are having similar polarities [15,16]. As an efficient liquid chromatography preparative technique, HSCCC has a great potential for separation and purification of various 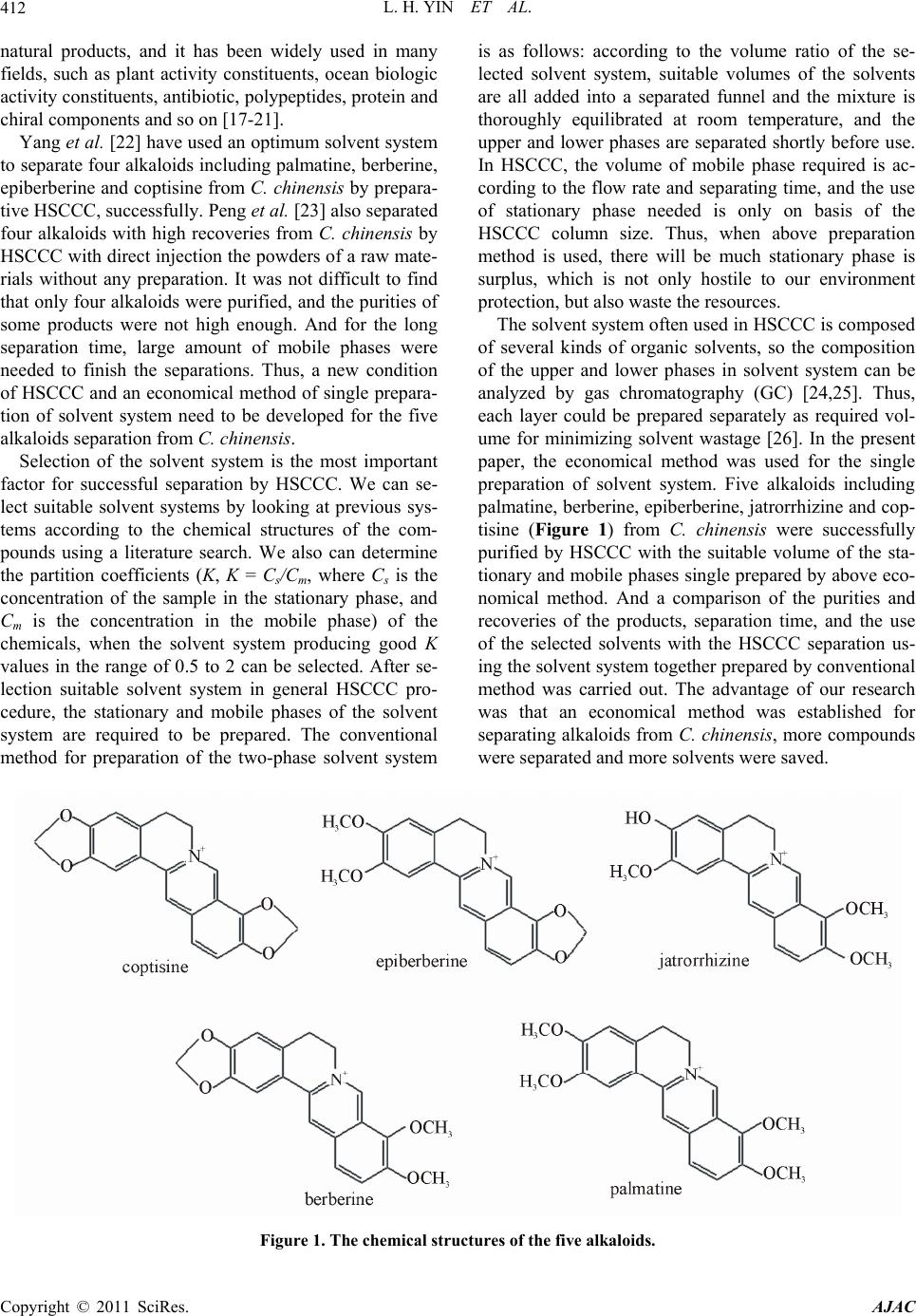 L. H. YIN ET AL. 412 natural products, and it has been widely used in many fields, such as plant activity constituents, ocean biologic activity constituents, antibiotic, polypeptides, protein and chiral components and so on [17-21]. Yang et al. [22] have used an optimum solvent system to separate four alkaloids including palmatine, berberine, epiberberine and coptisine from C. chinensis by prepara- tive HSCCC, successfully. Peng et al. [23] also separated four alkaloids with high recoveries from C. chinensis by HSCCC with direct injection the powders of a raw mate- rials without any preparation. It was not difficult to find that only four alkaloids were purified, and the purities of some products were not high enough. And for the long separation time, large amount of mobile phases were needed to finish the separations. Thus, a new condition of HSCCC and an economical method of single prepara- tion of solvent system need to be developed for the five alkaloids separation from C. chinensis. Selection of the solvent system is the most important factor for successful separation by HSCCC. We can se- lect suitable solvent systems by looking at previous sys- tems according to the chemical structures of the com- pounds using a literature search. We also can determine the partition coefficients (K, K = Cs/Cm, where Cs is the concentration of the sample in the stationary phase, and Cm is the concentration in the mobile phase) of the chemicals, when the solvent system producing good K values in the range of 0.5 to 2 can be selected. After se- lection suitable solvent system in general HSCCC pro- cedure, the stationary and mobile phases of the solvent system are required to be prepared. The conventional method for preparation of the two-phase solvent system is as follows: according to the volume ratio of the se- lected solvent system, suitable volumes of the solvents are all added into a separated funnel and the mixture is thoroughly equilibrated at room temperature, and the upper and lower phases are separated shortly before use. In HSCCC, the volume of mobile phase required is ac- cording to the flow rate and separating time, and the use of stationary phase needed is only on basis of the HSCCC column size. Thus, when above preparation method is used, there will be much stationary phase is surplus, which is not only hostile to our environment protection, but also waste the resources. The solvent system often used in HSCCC is composed of several kinds of organic solvents, so the composition of the upper and lower phases in solvent system can be analyzed by gas chromatography (GC) [24,25]. Thus, each layer could be prepared separately as required vol- ume for minimizing solvent wastage [26]. In the present paper, the economical method was used for the single preparation of solvent system. Five alkaloids including palmatine, berberine, epiberberine, jatrorrhizine and cop- tisine (Figure 1) from C. chinensis were successfully purified by HSCCC with the suitable volume of the sta- tionary and mobile phases single prepared by above eco- nomical method. And a comparison of the purities and recoveries of the products, separation time, and the use of the selected solvents with the HSCCC separation us- ing the solvent system together prepared by conventional method was carried out. The advantage of our research was that an economical method was established for separating alkaloids from C. chinensis, more compounds were separated and more solvents were saved. Figure 1. The chemical structures of the five alkaloids. Copyright © 2011 SciRes. AJAC  L. H. YIN ET AL.413 2. Experimental 2.1. Plant Materials and Chemicals C. chinensis was purchased from a local drug store (Da- lian, China) and authenticated by Dr. Yunpeng Diao (Dalian Medical University, Dalian, China). The stan- dards including palmatine, berberine, epiberberine, cop- tisine and jatrorrhizine were all purchased from the Chi- nese National Institute of Control of Pharmaceutical and Biological Products, Beijing, China. Methanol used for HPLC was chromatographic grade (TEDIA, USA). Phosphoric acid and all other organic solvents used for sample preparation or HSCCC separa- tion were analytical grade and purchased from ShenLian Chemical Factory (Shenyang, China). Reverse osmosis Milli-Q water (18 MΩ) (Millipore, USA) was used for all solutions and dilutions. 2.2. Apparatus The HSCCC instrument used in this study was a TBE-300A high-speed counter-current chromatograph (Shanghai Tauto Biotech Co., Ltd., Shanghai, China) with three multilayer coil separation columns connected in series (I.D. of the tubing = 1.6 mm, total volume = 260 mL) and a 20 mL sample loop. The revolution radius or the distance between the holder axis and central axis of the centrifuge (R) was 5 cm, and the β values of the mul- tilayer coil varied from 0.5 at the internal terminal to 0.8 at the external terminal (β = r/R, where r is the distance from the coil to the holder shaft). The revolution speed of the instrument could be regulated with a speed controller in the range between 0 and 999 rpm. The HSCCC system was equipped with a model TBP-50A constant-flow pump (Shanghai Tauto Biological Company, China), a UV-Vis detector (Model UV-8823B, Beijing, China), and a model N2000 workstation (Zhejiang University, Hangzhou, China). The experimental temperature was adjusted by HX 1050 constant temperature circulating implement (Beijing Boyikang Lab Implement, Beijing, China). The HPLC analyses were performed on an Agilent 1200 system (Agilent Technologies, Waldbronn, Ger- many), equipped with 1322A online vacuum degasser, G1311A quaternary pump, G1329A autosampler, G1314B UV detector and Chemstation software (Agilent techno- logies). The solvent analysis was carried out with GC-14C (SHIMADZU, Japan) equipped with FID detector and a capillary column (30 m × 0.32 mm, i.d. 0.25 μm film thickness). 2.3. Preparation of the Crude Extracts and Sample Solution The pulverized plant material of 800 g was extracted three times with 70% aqueous ethanol (solvent: sample ratio = 10:1, v/w) under reflux and 4 h for each. The fil- trate was collected and evaporated under reduced pres- sure at 60˚C, and the residue was obtained. Then, the residue was re-dissolved in water, which was adjusted to pH 2.0 by hydrogen chloride and stayed for one night. After that, the solution was filtered and the pH value was adjusted 10.0 with NaOH solution. Subsequently, the solution was extracted by chloroform, and the under- layer was separated and evaporated under reduced pres- sure at 40˚C to dryness. Deep yellow power was pro- duced, which was lyophilized and stored in a refrigerator for further HSCCC isolation. The sample solution was prepared by dissolving 300 mg crude extract in 20 mL of the solvent mixture con- sisting of equal volumes of stationary and mobile phases single or together prepared of the solvent system com- posed of chloroform-methanol-water (2:1:1, v/v/v). 2.4. The Single Preparation of two-Phase Solvent System used in HSCCC In this study, we applied GC to single prepare the sta- tionary and mobile phases of the solvent system used in HSCCC. The stationary and mobile phases of the se- lected solvent system were first prepared together by the traditional method. Then the contents of chloroform and methanol in the two phases were analyzed by GC using n-butanol as the internal standard and acetone as the dis- solvent. In the paper, different internal standards, capillary columns and temperature programs were investigated to optimize GC chromatographic conditions. Finally, a good separation for peaks of chloroform, methanol and n-butanol was achieved when the separation carried out on the following conditions: GC equipped with FID de- tector and a capillary column (30 m × 0.32 mm, i.d. 0.25 μm film thickness) was used; the column temperature was held at 30˚C for 10 min, and then programmed to 120˚C at 10˚C/min. The injector and detector tempera- tures were set at 150˚C and 180˚C, respectively, and in- jections were made in the split mode with a split ratio 1: 20. Nitrogen was used as the carrier gas. The contents of chloroform and methanol in the stationary and mobile phases were determined individually, and suitable vol- umes of each solvent were calculated for single prepara- tion of the stationary and mobile phases. First, the correlations between the volumes and the weights of the stationary and mobile phases of the se- Copyright © 2011 SciRes. AJAC  L. H. YIN ET AL. 414 lected solvent system were investigated, and we found that excellent calibration curves were existed. The linear ranges (mL), regression equations (Y = aX + b) and R2 of each phase were obtained compared with their weights. For stationary phase, in the range of 8.7 ~ 400 mL, Y = 0.9262X + 0.1727 (R2 = 0.9999); for mobile phase, in the range of 11.4 ~ 1500 mL, Y = 1.3612X + 0.9451 (R2 = 0.9999), in which Y is the weight of stationary phase or mobile phase and X means the volume of each phase. In GC analysis, the standard solution containing chlo- roform, methanol and internal standard at the concentra- tions of 0.02942, 0.01582 and 0.04040 g/mL, respec- tively, was prepared, which was used for subsequent determination. Then, 20 mL stationary and mobile phases were measured with the weights of 18.6967 and 28.1691 g, respectively, and dissolved in acetone to prepare sam- ple solutions. The internal standard was also added to afford the concentration of 0.04040 g/mL. All the solu- tions mentioned above were analyzed by GC, and the peak areas were recorded. The concentrations (g/mL) of chloroform and methanol in each phase were calculated according to the following formulae (1) and (2) [27]. Thus, the concentrations of chloroform and methanol were 0.03535 g/mL and 0.41203 g/mL in the stationary phase, and 1.28125 g/mL and 0.09390 g/mL in the mo- bile phase, respectively. So, according to the formula (3), the concentrations of water in the two phases were 0.48746 g/ mL and 0.03331 g/mL, respectively. SS a R C f C (1) '' X Xa SS A Cf C (2) where, a is the correction factor; S is the peak area of internal standard; is the peak area of standard; S is the concentration of internal standard; C C is the concentration of standard; X is the peak area of ana- lyte in test sample; X is the concentration of analyte in test sample; C ' S and are the peak area and con- centration of internal standard in the test sample. ' S C WTC CCCC M (3) where, W is the concentration of water in one phase; Tis the density of one phase (g/ml), C and C C C Care the concentrations of chloroform and methanol in one phase, respectively. For the known density of each solvent, we can switch the concentration of each solvent (g/mL) X to volume ratio (mL/mL) X. In stationary phase, the volume ra- tios of chloroform, methanol and water were 0.02403, 0.52090 and 0.48746 mL/mL; and in mobile phase, the volume ratios were 0.87100, 0.11870 and 0.03331 mL/mL, respectively C T Then, according to the formula (4), we can calculate the volumes of chloroform, methanol and water in the stationary and mobile phases. X VVTX (4) where, is the total volume of the stationary and mo- bile phases needed in HSCCC; X V is the volume of solvent needed to prepare the stationary or mobile phase. V 2.5. The Rraditional together Preparation of two-phase Solvent System used in HSCCC The HSCCC experiments were performed with a two- phase solvent system composed of chloroform-metha- nol-water (2:1:1, v/v/v). Two methods for preparation of the stationary and mobile phases were carried out. One, the stationary and mobile phases were prepared together by the conventional method. After thoroughly equili- brating, the two phases of the solvent mixtures in a sepa- rating funnel at room temperatures were separated shortly before use. The other, the stationary and mobile phases were single prepared, according to the results of GC analysis, namely 1102 mL chloroform, 150 mL methanol and 42 mL water were added together to pre- pare the mobile phase, and 6 mL chloroform, 135 mL methanol and 127 mL water were mixed to prepare the stationary phase. 2.6. HSCCC Separation Procedure In each separation, the multiplayer coil column was first entirely filled with the upper phase as the stationary phase. Then the mobile phase was pumped into the ‘head’-end of the HSCCC coil column at a suitable flow rate of 1.5 mL/min, while the HSCCC apparatus was rotated at a speed of 800 rpm. After a clear mobile phase was eluted from the tail outlet and the two phases had established a hydrodynamic equilibrium throughout the column, the sample solution was injected through the injection valve. The effluent from the outlet of the col- umn was continuously monitored at 280 nm and each peak fraction was collected according to the elution pro- file. After the separation was completed, the centrifuge was stopped and retention of the stationary phase was measured by collecting the column contents by forcing them out of the column with pressurized gas. 2.7. HPLC Analysis for Purity Determination In the present paper, the crude sample and each HSCCC peak fraction were all analyzed by HPLC. The analysis was carried out on an Agilent Eclipse Plus C18 (150 mm × 4.6 mm, i.d., 5 μm), and the mobile phase composed of methanol (A)-3% phosphoric acid (B) (pH 2.0, adjusted Copyright © 2011 SciRes. AJAC  L. H. YIN ET AL.415 by triethylamine) with a gradient mode (0 ~ 15 min, 25%A→30%A; 25 ~ 38 min, 30%A; 38 ~ 60 min, 30%A→35%A). The flow rate was controlled at 0.8 mL/min, and the detection wavelength was selected at 270 nm. 2.8. Chemical Structure Identification and Confirmation Identification of the HSCCC peak fractions was carried out by MS (API 3200 mass spectrometer, Applied Bio- systems, USA), UV spectra (U-3010 UV, Hitachi, Japan), and the standards. 3. Results and Discussion 3.1. Optimization Suitable HPLC Analytical Conditions A sensitive method for determination of the alkaloids in crude extract of C. chinensis is a prerequisite to the HSCCC separation. In recent years, many methods have been developed to detect the alkaloids in C. chinensis, such as capillary electrophoresis (CE), LC-MS and LC-MS-MS [28-34]. Among them, CE analysis is high sensitive and excellent, but the pre-condition and back- ground solution are complex, and the technique can not be controlled easily. LC-MS and LC-MS-MS technique are fast, sensitive and accurate, but the apparatus is too expensive and not all research departments can afford it. By now, there are many HPLC analytical methods have been reported to determine alkaloids in C. chinensis [35,36]. However, we can easily find that some flaws were existed in reported papers including long separation time, not good separation, complex mobile phase and the limited number of detected compounds. In the present paper, different chromatographic column packed with different materials purchased from different companies, mobile phases composed of acetonitrile-water and metha- nol-water with some modifiers including phosphoric buffer, acetic acid, formic acid, phosphoric acid, formic acid adjusted by ammonia or by triethylamine with dif- ferent pH values, and different gradient elution modes were all investigated. Good separation was achieved when the process was carried out on an Agilent Eclipse Plus C18 (150 mm × 4.6 mm, i.d., 5 μm), and the mobile phase composed of methanol (A)-3% phosphoric acid (B) (pH 2.0, adjusted by triethylamine) with a gradient elu- tion mode (0 ~ 15 min, 25%A→30%A; 25 ~ 38 min, 30%A; 38 ~ 60 min, 30%A→35%A). The detection wavelength was selected at 270 nm and the flow rate was controlled at 0.8 mL/min. On the optimized conditions, the beautiful chromatograms of crude sample and mixed standards are shown in Figure 2(a) and Figure 2(b), in which the peaks 1, 2, 3, 4 and 5 correspond to coptisine, epiberberine, jatrorrhizine, berberine and palmatine, re- spectively. The advantage of the method was that the mobile phase was simple and no salt or buffer was needed. The separated time was mediate, and all the al- kaloids were separated fully. 3.2. Optimization of HSCCC Conditions According to HPLC analysis shown in Figure 2(a), there are five major components in the crude extract of C. chinensis. Many papers have been reported to separate alkaloids from this plant by HSCCC [22,23]. The solvent system composed of chloroform–methanol–water is con- sidered to be one of the classical solvent systems in al- kaloids purification, but the ratios between the solvents were not invariable. The crude extracts of Sophora fla- vescens Ait. were separated and purified by high-speed counter-current chromatography (HSCCC) with a two- phase solvent system composed of chloroform-metha- nol-2.3 × 10−2 M NaH2PO4 (27.5:20:12.5, v/v/v) [37], and the optimum solvent systems CHCl3-MeOH-0.3 M/0.2 M HCl (4:1.5:2, v/v/v) were used to separate lap- paconitine, ranaconitine, N-deacetyllappaconitine and N-deacetylranaconitine from Aconitum sinomontanum Nakai [38]. So, in our research, the solvent systems com- posed of chloroform-methanol-water at the volume ratios of 4:3:2 (v/v/v) and 2:1:1 (v/v/v) and chloroform-etha- nol-water at the volume ratios of 4:3:2 (v/v/v) and 2:1:1 (v/v/v) were further optimized. The stationary and mo- bile phases of those four kinds of solvent systems were all prepared by together and single preparation methods, and the K-values of five compounds were determined. The results are listed in Table 1. It was obvious that the K-values of the compounds epiberberine, coptisine and jatrorrhizine, and the compounds palmatine and berber- ine had no difference when the solvent system composed of chloroform-ethanol-water (2:1:1, v/v/v) was used as the two-phase solvent system in spite of the methods of preparation. When chloroform-ethanol-water (4:3:2, v/v/v) or chloroform-methanol-water (4:3:2, v/v/v) were used as the separation systems, the targets could not be well separated and the purities of the compounds were not satisfactory. Thus, they were not suitable for HSCCC separation of the five alkaloids from the crude extract of C. chinensis. The other solvent system tested in present study was chloroform-methanol-water (2:1:1, v/v/v), and suitable K-values were produced not only in condition of together preparation of the stationary and mobile phases, but also in condition of single preparation method. Thus, the solvent system composed of chloroform-methanol- water (2:1:1, v/v/v) was selected to purify the targets Copyright © 2011 SciRes. AJAC 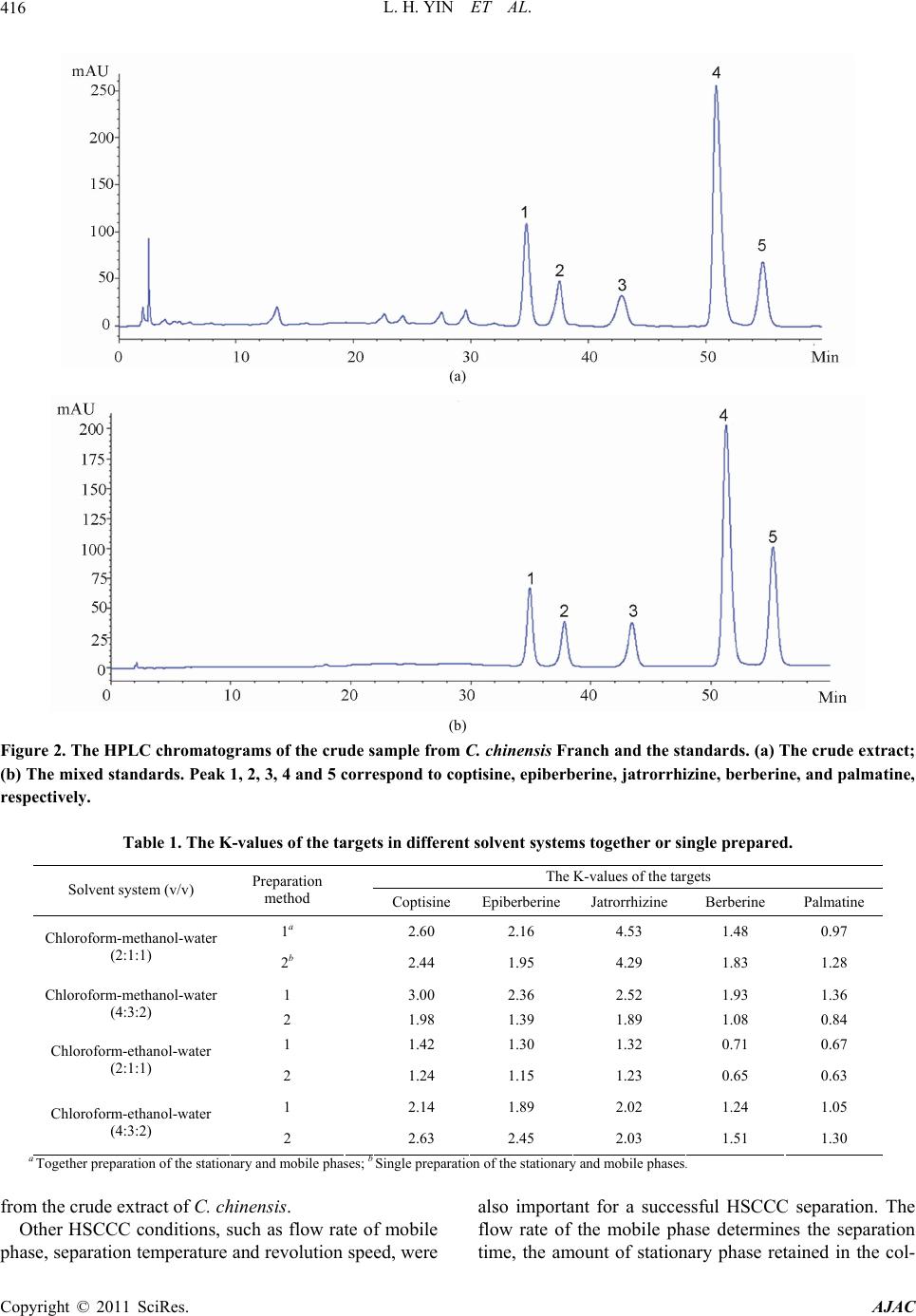 L. H. YIN ET AL. Copyright © 2011 SciRes. AJAC 416 (a) (b) Figure 2. The HPLC chromatograms of the crude sample from C. chinensis Franch and the standards. (a) The crude extract; (b) The mixed standards. Peak 1, 2, 3, 4 and 5 correspond to coptisine, epiberberine, jatrorrhizine, berberine, and palmatine, respectively. Table 1. The K-values of the targets in different solvent systems together or single prepared. The K-values of the targets Solvent system (v/v) Preparation method Coptisine EpiberberineJatrorrhizine Berberine Palmatine 1a 2.60 2.16 4.53 1.48 0.97 Chloroform-methanol-water (2:1:1) 2b 2.44 1.95 4.29 1.83 1.28 1 3.00 2.36 2.52 1.93 1.36 Chloroform-methanol-water (4:3:2) 2 1.98 1.39 1.89 1.08 0.84 1 1.42 1.30 1.32 0.71 0.67 Chloroform-ethanol-water (2:1:1) 2 1.24 1.15 1.23 0.65 0.63 1 2.14 1.89 2.02 1.24 1.05 Chloroform-ethanol-water (4:3:2) 2 2.63 2.45 2.03 1.51 1.30 a Together preparation of the stationary and mobile phases; b Single preparation of the stationary and mobile phases. from the crude extract of C. chinensis. Other HSCCC conditions, such as flow rate of mobile phase, separation temperature and revolution speed, were also important for a successful HSCCC separation. The flow rate of the mobile phase determines the separation time, the amount of stationary phase retained in the col-  L. H. YIN ET AL.417 2 umn, and therefore the peak resolution. The different temperatures may cause different retentions of the sta- tionary phases, and the speed may produce the volume of the stationary phase retained leading to lower peak reso- lution or excessive sample band broadening by violent pulsation of the column. So, different flow rates of the mobile phase, different temperatures and different revo- lution speeds were investigated. Finally, we found that the flow rate of mobile phase set at 1.5 mL/min, the temperature sated at 30˚C, and the apparatus rotated at 800 rpm were the best conditions for this HSCCC separation. Under the optimized conditions, the crude extract was successfully separated by HSCCC using the solvent sys- tem composed of chloroform-methanol-water (2:1:1, v/v/v). Figure 3(a) shows the chromatogram of the crude extract purified by HSCCC when the stationary and mo- bile phases of the solvent system were single prepared, and Figure 3(b) shows the chromatogram of the crude extract isolated by HSCCC when the stationary and mo- bile phases of the solvent system were together prepared. Five fractions (, , , and ) were collected aⅠⅡⅢⅣ Ⅴc- cording to the profiles shown in Figure 3(a) and Figure 3(b), respectively. 3.3. Purity and Recovery Retermination The obtained fractions from HSCCC were all analyzed by HPLC compared with the standards for purity deter- mination. The outcomes of the contents of the five alka- loids in crude sample, the purity, and recovery of the obtained targets are listed in Table 2. It was obvious that, in the separation performed by HSCCC using single prepared stationary and mobile phases, the purities and recoveries of the five alkaloids were over 98% and 92%, respectively. In the process performed by HSCCC using together prepared stationary and mobile phases, the puri- ties and recoveries of the products had no differences compared with single preparation method. Namely, the purities and recoveries of the obtained compounds were good, no matter which preparation method of solvent system were used. 3.4. Chemical Structure Identification The obtained fractions, , , and were identⅠⅡⅢⅣ Ⅴi- fied by MS, UV and the standards. The retention times of the separated compounds were the same compared with the standards. The MS and UV spectra data are agree- ment with those in the literature [4]. Thus, the obtained fractions , , , and were identified to be paⅠⅡⅢⅣⅤl- matine, berberine, epiberberine, coptisine and jatrorrhiz- ine, respectively. 3.5. Comparison Study of Single and Together Preparation of the Stationary and Mobile Phases Through the HSCCC chromatograms and HPLC analysis, we can easily find that there was no difference between the stationary and mobile phases of the selected solvent system prepared together and single. In HSCCC separa- tion using single prepared stationary and mobile phases, the retention of the stationary phase was 70%, and the separation time was 800 min and total of 1265 mL of the mobile phase was required, which was calculated as the following formula (5), and 260 mL stationary phase was required only on basis of the HSCCC column size. 1tmp VVV (5) where Vtmp means the total volume of the mobile phase required in HSCCC separation; V1 is the volume of the stationary phase eluted out from the column for hydro- dynamic equilibrium, and V2 is the use of the mobile phase to elute the targets, which was calculated accord- ing to the formula (6), where Tis separation time and is the flow rate of the mobile phase. 2 M VT F (6) The compositions of the mobile phase and stationary phase were analyzed by GC to calculate the solvent con- sume. According to the volume of each phase used, the solvent used in the procedure was obtained. Thus, total of 1108 mL chloroform, 285 mL methanol and 169 mL water were needed in HSCCC procedure with single preparation method. In the separation procedure using together prepared stationary and mobile phases, the retention of the sta- tionary phase was 77%, and the separation time was 820 min, and total of 1290 mL mobile phase was needed, which was also calculated with the formulae (5) and (6). 260 mL stationary phase was also required. The solvent system composed of chloroform-methanol-water (2:1:1, v/v/v) was accurately prepared to obtain the required mobile and stationary phases. There were three calibra- tion curves established to describe the correlations be- tween the use of the mobile phase with chloroform, methanol and water volumes. The linearity of the solvent volume (X, mL) and the mobile phase volume (Y, mL) was investigated in the range of 15 - 1500 mL. Y = 1.0372X + 1.6264 (R2= 0.9999, for chloroform); Y = 2.0743X + 1.6264 (R2 = 0.9999, for methanol); Y = 2.0743X + 1.6264 (R2 = 0.9999, for water). Thus, 1242 mL chloroform, 621 mL methanol and 621 mL water were required to prepare the solvent system according to the calibration curves, when the stationary and mobile phases were prepared together. Thus, 134 mL chloroform, 336 mL methanol and 452 Copyright © 2011 SciRes. AJAC  L. H. YIN ET AL. 418 (a) (b) Figure 3. The HSCCC chromatograms of the crude extract from C. chinensis Franch. HSCCC conditions: Two-phase solvent system: Chloroform-methanol-water (2:1:1, v/v/v); flow rate: 1.5 mL/min; revolution speed: 800 rpm; detection wavelength: 280 nm; sample size: 300 mg; separation temperature: 30˚C. Ⅰ, , , and ⅡⅢⅣ Ⅴare the collected fractions. (a) the station- ary and mobile phases are single prepared by GC; (b) the stationary and mobile phases are together prepared by the conven- tional method. Copyright © 2011 SciRes. AJAC  L. H. YIN ET AL. Copyright © 2011 SciRes. AJAC 419 Table 2. The detailed information for separating the five alkaloids from C. chinensis Franch by HSCCC using different sol- vent system preparation methods. Together preparation method Single preparation method Compound Contenta Amountb Recoveryc Purity Amount Recovery Purity Palmatine 7.42% 20.8 mg 92.07% 98.53% 21.3 mg 95.13% 99.42% Berberine 13.00% 38.1 mg 96.00% 98.27% 37.4 mg 95.51% 99.60% Epiberberine 5.70% 16.3 mg 94.05% 98.67% 16.4 mg 94.71% 98.75% Coptisine 8.84% 25.4 mg 95.12% 99.31% 25.1 mg 93.81% 99.12% Jatrorrhizine 1.31% 3.7 mg 93.98% 99.82% 3.7 mg 93.50% 99.31% aThe content of the component in the crude extract of C. chinensis Franch; bThe amount of the component separated by HSCCC; c22 Re cov(%)100% 11 PW ery PW . Where P1 is the content of the compound in crude sample; W1 is the amount of the crude sample for HSCCC isolation; P2 is the purity of the obtained compound; W2 is the amount of the obtained compound from HSCCC. P1 and P2 were cal- culated by external standard method of HPLC. Table 3. The details of the HSCCC separation with different preparative methods and the expenditures of the three solvents. Preparation methoda Retention of the stationary phase Separation time Stationary phase requiredb Mobile phase requiredc Required of the two phases Chloroform MethanolWater Mobile phase () 1m V1242 mL 621 mL 621 mL Together preparation 77% 820 min 260 mL 1290 mL Stationary phased () 1 V0 mL 0 mL 0 mL Total volume () 11tms VV V 1 1242 mL 621 mL 621 mL Mobile phase () 2m V1102 mL 150 mL 42 mL Single prepa- ration 75% 800 min 260 mL 1265 mL )Stationary phase (2 V6 mL 135 mL 127 mL Total volume () 22tms VV V 2 1108 mL 285 mL 169 mL Saved reagent () 12St t VVV 134 mL 336 mL 452 mL aThe Preparation method means the method to prepare the stationary and mobile phases in HSCCC process; bThe use of the stationary phase in HSCCC is the column volume of the apparatus; cThe use of the mobile phase in HSCCC process is calculated according to the flow rate of the mobile phase and the separation time; dThe stationary phase and mobile phase are prepared together. mL water were saved when the stationary and mobile phases were single prepared in HSCCC purification. All the details are shown in Table 3. 4. Conclusions In this paper, five alkaloids from C. chinensis were suc- cessfully separated by HSCCC using the solvent system composed of chloroform-methanol-water (2:1:1, v/v/v), of which the stationary and mobile phases were singled prepared by GC. The results indicated that the method for the alkaloids separation is applied, and the single preparation technique of the two phases by GC in HSCCC was efficient and solvent saving. And an eco- nomical method was established for separating alkaloids from C. chinensis. The single preparation of solvent sys- tem by GC might be widely used in long time HSCCC separation and purification. 5. Acknowledgements This research was partially supported by the Key Labo- ratory of Liaoning University (No. 2008S072), Liaoning, China. 6. References [1] H. H. Yu, K. J. Kim, J. D. Cha, H. K. Kim, Y. E. Lee, N. Y. Choi and Y. O. You, “Antimicrobial Activity of Ber- berine Alone and in Combination with Ampicillin or Ox- acillin against Methicillin-Resistant Staphylococcus Aureus,” Journal of Medicial Food, Vol. 8, No. 4, 2005, pp. 454-461. doi:10.1089/jmf.2005.8.454 [2] H. A. Jung., N. Y. Yoon, H. J. Bae, B. S. Min and J. S. Choi, “Ingibitory Activities of the Alkaloids from Copti- dis Rhizoma against Aldose Reductase,” Archives of Pharmacal Research, Vol. 31, No. 11, 2008, pp. 1405- 1412. doi:10.1007/s12272-001-2124-z [3] J. Liu, C. He, K. Zhou, J. Wang and J. X. Kang, “Coptis Extracts Enhance the Anticancer Effect of Estrogen Re- ceptor Antagonists on Human Breast Cancer Cells,” Bio- chemical and Biophysical Research Communications, Vol. 378, No. 2, 2009, pp. 174-178. doi:10.1016/j.bbrc.2008.10.169 [4] J. H. Chen, F. M. Wang, J. Liu, F. S. C. Lee, X. R. Wang and H. G. Yang, “Analysis of Alkaloids in Coptis  L. H. YIN ET AL. 420 Chinensis Franch by Accelerated Solvent Extraction Combined with Ultra Performance Liquid Chroma- tographic Analysis with Photodiode Array and Tandem Mass Spectrometry Detections,” Analytia Chimuca Acta, Vol. 613, No. 2, 2008, pp. 184-195. doi:10.1016/j.aca.2008.02.060 [5] H. L. Yang, S. S. Yu and Y. H. Pei, “Studies on Chemical Constituents from leaves of Lysidice Brevicalyx,” Zhong- guo Zhong Yao Za Zhi, Vol. 33, No. 22, 2008, pp. 2633- 2635. [6] C. L. Kuo, C. W. Chi and T. Y. Liu, “The Anti-Infam- matory Potential of Berberine in Vitro and in Vivo,” Cancer Letter, Vol. 203, No. 2, 2004, pp. 127-137. doi:10.1016/j.canlet.2003.09.002 [7] L. Q. Tang, W. Wei, L. M. Chen and S. Liu, “Effects of Berberine on Diabetes Induced by Alloxan and a High- Fat/High-Cholesterol Diet in Rats,” Journal of Ethno- pharmacology, Vol. 108, No. 1, 2006, pp. 109-115. doi:10.1016/j.jep.2006.04.019 [8] L. P. Qu, G. R. Fan, J. Y. Peng and H. M. Mi, “Isolation of Six Isoflavones from Semen Sojae Praeparatum by Preparative HPLC,” Fitoterapia, Vol. 78, No. 3, 2007, pp. 200-204. doi:10.1016/j.fitote.2006.11.002 [9] Y. Q. Yin, Z. B. Shen and L. Y. Kong, “Studies on Chemical Constituents from Ipomoea Batatas,” Lishizhen Medicine and Materia Medica Research, Vol. 19, No. 11, 2008, pp. 1501-1503. [10] Y. Ito and R. L. Bowman, “Counter-Current Chromatog- raphy: Liquid-Liquid Partition Chromatography without Solid Support,” Science, Vol. 167, No. 3916, 1970, pp. 281-283. doi:10.1126/science.167.3916.281 [11] Y. Ito, J. L. Sandlin and W. G. Bowers, “High-Speed Preparative Counter Current Chromatography with a Coil Planet Centrifuge,” Journal of Chromatography, Vol. 244, 1982, pp. 247-258. doi:10.1016/S0021-9673(00)85688-5 [12] Y. Ito, “Counter Current Chromatography: Theory and Practice,” Marcel Dekker, New York, 1988, pp. 79-442. [13] Y. Ma, Y. Ito and A. Foucault, “Resolution of Gram Quantities of Racemates by High-Speed Counter-Current Chromatography,” Journal of Chromatography A, Vol. 704, No. 1, 1995, pp. 75-81. doi:10.1016/0021-9673(95)00148-G [14] L. J. Chen, Q. Zhang, G. L. Yang, L. Y. Fan, J. Tang, I. Garrard, S. N. Ignatova, D. Fisher and I. A. Sutherland. “Rapid Purification and Scale-up of Honokiol and Mag- nolol Using High-Capacity High-Speed Counter-Current Chromatography,” Journal of Chromatography A, Vol. 1142, No. 2, 2007, pp. 115-122. doi:10.1016/j.chroma.2006.09.098 [15] D. S. Abrams and J. M. Prausnitz, “Statistical Thermo- dynamics of Liquid Mixtures: A New Expression for the Excess Gibbs Free Energy of Partly or Completely Mis- cible Systems,” AIChE Journal, Vol. 21, 1975, pp. 116- 128. doi:10.1002/aic.690210115 [16] S. Y. Shi, K. L. Huang, Y. P. Zhang and S. Liu, “Prepara- tive Isolation and Purification of Two Flavonoid Gly- cosides from Taraxacum Mongolicum by High-Speed Counter-Current Chromatography,” Separation and puri- fication technology, Vol. 60, No. 1, 2008, pp. 81-85. doi:10.1016/j.seppur.2007.07.047 [17] J. Y. Peng, G. R. Fan, L. P. Qu, Z. Xin and Y. T. Wu, “Application of Preparative High-Speed Counter-Current Chromatography for Isolation and Separation of Schizan- drin and Gomisin A from Schisandra Chinensis,” Journal of Chromatography A, Vol. 1082, No. 2, 2005, pp. 203- 207. doi:10.1016/j.chroma.2005.05.091 [18] H. B. Li, F. Chen, T. Y. Zhang, F. Q. Yang and G. Q. Xu, “Preparative Isolation and Purification of Lutein from the Microalga Chlorella vulgaris by High-Speed Counter- Current Chromatography,” Journal of Chromatography A, Vol. 905, No. 1-2, 2001, pp. 151-155. doi:10.1016/S0021-9673(00)00987-0 [19] C. Seger, K. Eberhart, S. Sturm, H. Strasser and H. Stuppner, “Apolar Chromatography on Sephadex LH-20 Combined with High-Speed Counter-Current Chroma- tography: High Yield Strategy for Structurally Closely Related an Analyses-Destruxin Derivatives from Metarhi- zium anisopliae as a Case Study,” Journal of Chroma- tography A, Vol. 1117, No. 1, 2006, pp. 67-73. doi:10.1016/j.chroma.2006.03.055 [20] W. B. Zhi, Q. Y. Deng, J. N. Song, M. Gu and F. Ouyang, “One-Step Purification of Alphamylase from the Cultiva- tion Supernatant of Recombinant Bacillus Subtilis by High-Speed Counter-Current Chromatography with Aqueous Polymer Two-Phase Systems,” Journal of Chromatography A, Vol. 1070, No. 1-2, 2005, pp. 215- 219. doi:10.1016/j.chroma.2005.02.065 [21] E. Pérez, M. J. Santos and C. Minguillón, “Application of Cellulose and Amylose Arylcarbamates as Chiral Selec- tors in Counter-Current Chromatography,” Journal of Chromatography A, Vol. 1107, No. 1-2, 2006, pp. 165- 174. doi:10.1016/j.chroma.2005.12.061 [22] F. Q. Yang, T. Y. Zhang, R. Zhang and Y. Ito, “Applica- tion of Analytical and Preparative High-Speed Counter- Current Chromatography for Separation of Alkaloids from Coptis chinensis Franch,” Journal of Chromatog- raphy A, Vol. 829, No.1-2, 1998, pp. 137-141. doi:10.1016/S0021-9673(98)00776-6 [23] J. Y. Peng, X. Han, Y. W. Xu, Y. Qi, L. N. Xu and Q. W. Xu, “New Approach of Application High Speed Coun- tercurrent Chromatography Coupled with Direct Injection the Powders of a Raw Materials without Any Preparation, for Isolation and Separation of Four Alkaloids with High Recoveries from Coptis chinensis Franch,” Journal of Liquid Chromatography & Related Technologies, Vol. 30, No. 17-20, 2007, pp. 2929-2940. doi:10.1080/10826070701588984 [24] P. Jacobs, W. Dewé, A. Flament, M. Gibella and A. Cec- cato, “A New Validation Approach Applied to the GC Determination of Impurities in Organic Solvents,” Jour- nal of Pharmaceutical and Biomedical Analysis, Vol. 40,No. 2, 2006, pp. 294-304. doi:10.1016/j.jpba.2005.06.036 [25] F. J. Arrebola, M. J. González-Rodríguez, A. Garrido Frenich, A. Marín-Juan and J. L. Martínez Vidal, “De- termination of Halogenated Solvents Content in Olive Oil Copyright © 2011 SciRes. AJAC  L. H. YIN ET AL. Copyright © 2011 SciRes. AJAC 421 by Two Completely Automated Headspace Techniques Coupled to Gas Chromatography-Mass Spectrometry,” Analytica Chimica Acta, Vol. 552, No. 1-2, 2005, pp. 60- 66. doi:10.1016/j.aca.2005.07.054 [26] I. J. Garrard, L. Janaway and D. Fisher, “Minimising Solvent Usage in High Speed, High Loading, and High Resolution Isocratic Dynamic Extraction,” Journal of Liquid Chromatography & Related Technologies, Vol. 30, No. 1-4, 2007, pp. 151-163. doi:10.1080/10826070601077252 [27] W. Y. Liu, “Pharmaceutical Analysis,” People’s Medical Publishing House, 2006, pp. 75-80. [28] Y. M. Liu and S. J. Shen, “Determination of Coptisine, Berberine and Palmatine in Traditional Chinese Medici- nal Preparations by Capillary Electrophoresis,” Journal of Chromatography A, Vol. 639, No. 2, 1993, pp. 323-328. doi:10.1016/0021-9673(93)80269-E [29] S. W. Sun and H. M. Tseng, “Improved Detection of Coptidis Alkaloids by Field-Amplified Sample Stacking in Capillary Electrophoresis,” Journal of Pharmaceutical and Biomedical Analysis, Vol. 36, No. 1, 2004, pp. 43-48. doi:10.1016/j.jpba.2004.04.017 [30] S. Yu, X. Y. Pang, Y. X. Deng, L. Liu, Y. Liang, X. D. Liu, L. Xie, G. J. Wang and X. T. Wang, “A Sensitive and Specific Liquid Chromatography Mass Spectrometry Method for Simultaneous Determination of Berberine, Palmatine, Coptisine, Epiberberine and Jatrorrhizine from Coptidis rhizoma in Rat Plasma,” International Journal of Mass Spectrometry, Vol. 268, No. 1, 2007, pp. 30-37. doi:10.1016/j.ijms.2007.08.003 [31] S. W. Sun and H. M. Tseng, “Sensitivity Improvement on Detection of Coptidis Alkaloids by Sweeping in Capillary Electrophoresis,” Journal of Pharmaceutical and Bio- medical Analysis, Vol. 37, No. 1, 2005, pp. 39-45. doi:10.1016/j.jpba.2004.09.042 [32] W. C. Chuang, D. S. Young, L. K. Liu and S. J. Sheu, “Liquid Chromatographic-Electrospray Mass Spectro- metric Analysis of Coptidis rhizoma,” Journal of Chro- matography A, Vol. 755, No. 1, 1996, pp. 19-26. doi:10.1016/S0021-9673(96)00591-2 [33] Y. T. Deng, Q. F. Liao, S. H. Li, K. H. Bi, B. Y. Pan and Z. Y. Xie, Simultaneous Determination of Berberine, Palmatine and Jatrorrhizine by Liquid Chromatogra- phy-Tandem Mass Spectrometry in Rat Plasma and Its Application in a Pharmacokinetic Study after Oral Ad- ministration of Coptis-Evodia Herb Couple,” Journal of Chromatography B, Vol. 863, No. 2, 2008, pp. 195-205. doi:10.1016/j.jchromb.2007.12.028 [34] H. L. Li, W. D. Zhang, R. H. Liu, C. Zhang, T. Han, X. W. Wang, X. L. Wang, J. B. Zhu and C. L. Chen, “Si- multaneous Determination of Four Active Alkaloids from a Traditional Chinese Medicine Corydalis saxicola Bun- ting. (Yanhuanglian) in Plasma and Urine Samples by LC-MS-MS,” Journal of Chromatography B, Vol. 831, No. 1-2, 2006, pp. 140-146. doi:10.1016/j.jchromb.2005.11.049 [35] H. S. Lee, Y. E. Eom and D. O. Eom, “Narrowbore High Performance Liquid Chromatography of Berberine and Palmatine in Crude Drugs and Pharmaceuticals with Ion-Pair Extraction Using Cobalt Thiocyanate Reagent,” Journal of Pharmaceutical and Biomedical Analysis, Vol. 21, No. 1, 1999, pp. 59-63. doi:10.1016/S0731-7085(99)00113-2 [36] Y. X. Luo, D. X. Wen, S. J. Jiang, X. L. Zeng, J. N. Jiang, Y. C. Xu and P. D. Xie, “Studies on HPLC Fingerprint of Herba Corydalis Saxicolae,” Chinese Journal of Phar- macutical Analysis, Vol. 27, No. 11, 2007, pp. 1749- 1751. [37] J. Y. Ling, G. Y. Zhang, Z. J. Cui and C. K. Zhang, “Su- percritical Fluid Extraction of Quinolizidine Alkaloids from Sophora Flavescens Ait. and Purification by High-Speed Counter-Current Chromatography,” Journal of Chromatography A, Vol. 1145, No. 1-2, 2007, pp. 123-127. doi:10.1016/j.chroma.2007.01.080 [38] F. Y. Yang and Y. Ito, “Preparative Separation of Lap- paconitine, Ranaconitine, N-deacetyllappaconitine and N-deacetylranaconitine from Crude Alkaloids of Sample Aconitum Sinomontanum Nakai by High-Speed Counter- Current Chromatography,” Journal of Chromatography A, Vol. 943, No. 2, 2002, pp. 219-225. doi:10.1016/S0021-9673(01)01464-9
|