 Materials Sciences and Applicatio n, 2011, 2, 777-785 doi:10.4236/msa.2011.27107 Published Online July 2011 (http://www.SciRP.org/journal/msa) Copyright © 2011 SciRes. MSA Synthesis of Amine-Type Adsorbents with Emulsion Graft Polymerization of 4-Hydroxybutyl Acrylate Glycidylether Hongjuan Ma1, Kazuaki Morita2, Hiroyuki Hoshina1, Noriaki Seko1 1Quantum Beam Science Directorate, Japan Atomic Energy Agency, Takasaki, Japan; 2Graduate School of Engineering, Gunma University, Kiryu, Japan Email: ma.hongjuan@jaea.go.jp Received February 16th, 2011; revised February 21st, 2011; accepted May 21st, 2011. ABSTRACT Radiation induced graft polymerization on polymeric matrix followed by functionalization is widely accepted for the prepa ration of metal adsorben ts. In this paper, a pre-irradiation method was used for emulsion graft polymerization of 4-hydroxybutyl acrylate glycid ylether (4-HB) onto polyethylene/polypropylene (PE/PP) nonwoven fabric. The degree of grafting (Dg) which can be calculated by weight increment was determined as a function of reaction time, irradiation dose, and monomer concentration. After 30 kGy irradiation, with 4-HB concentration of 5%, surfactant Span 20 of 0.5% at 40˚C for 2 h, the trunk polymer was made grafted at a Dg of 135%. 4-HB-grafted PE/PP nonwoven fabric was modified by ethylenediamine (EDA) in isopropyl alcohol (IPA) as a solvent at 60˚C. With a Dg of 135%, the amine group dens ity of the adsorb ent is 2.8 mmol/g. The adsorption test was carried out by batch experiment in several metal ion solutions, and the removal ra tio from the EDA modified adsorbent of the metal ions is in the order of Cu 2+ > Pb2+ > Zn2+ > Ni2+ > Li+. Compared with glycidyl methacrylate (GMA) which is a typical functional monomer for graft po- lymerization, 4-HB-grafted adsorbent exhibited not only better mechanical property but also higher adsorption capac- ity of Cu2+ and Pb2+. Keywords: Graft Polymerization, Pre-irradiation, 4-hydroxybutyl Acrylate Glycidylether (4-HB), Glycidyl methacrylate (GMA), Amine-Type Adso rbent 1. Introduction Aquatic pollution by heavy metals has attracted world- wide attention for many decades. Several heavy metals such as cadmium, chromium, copper, lead, mercury, nickel, zinc, etc. are included on the U.S. Environmental Protection Agency’s (USEPA) list of priority pollutants [1]. Copper is an essential micronutrient, vital for the body in small amounts. However, humans can present symptoms from temporary stomach and intestinal disor- ders to kidney or liver damage at high amount over 1.3 mg·dm–3 [2]. Lead intoxication has been a problem throughout history [3,4], adverse health effects of lead are well documented: it may cause severe damage to the kidney, nervous system, reproductive system, liver and brain and causes sickness or death [4,5]. The permissible limits for lead in drinking water given by USEPA are 0.015 mg·dm–3 [6] and for wastewaters is 0.1 mg·dm–3 given by both USEPA and Bureau of Indian Standards (BIS) [7,8]. The potential sources of Cu2+ ions are, es- sentially, pulp, paper and wood preservative-employing mills, industrial waste streams of metal cleaning and plating baths, fertilizer industry, et al. [9]. Lead has been used for many years in products of everyday life (paint, household plumbing, water pipes and et al. [10-12]. Hence, both Cu2+ and Pb2+ ions are ubiquitous and fre- quently found toxic metals in surface water. And it be- comes mandatory for the removal of lead from drinking and wastewaters. Therefore, several processes have been used and developed over years to remove such metals from industrial wastewater: biological process [13-15], ion exchange [16], membrane filtration [17,18], adsorp- tion or electrochemical recovery [19,20] et al. For the adsorption task, both inorganic [21,22] and organic espe- cially polymeric materials [23] have been explored suc- cessfully for years. Many techniques have been sug- gested in the literature for the synthesis of polymeric 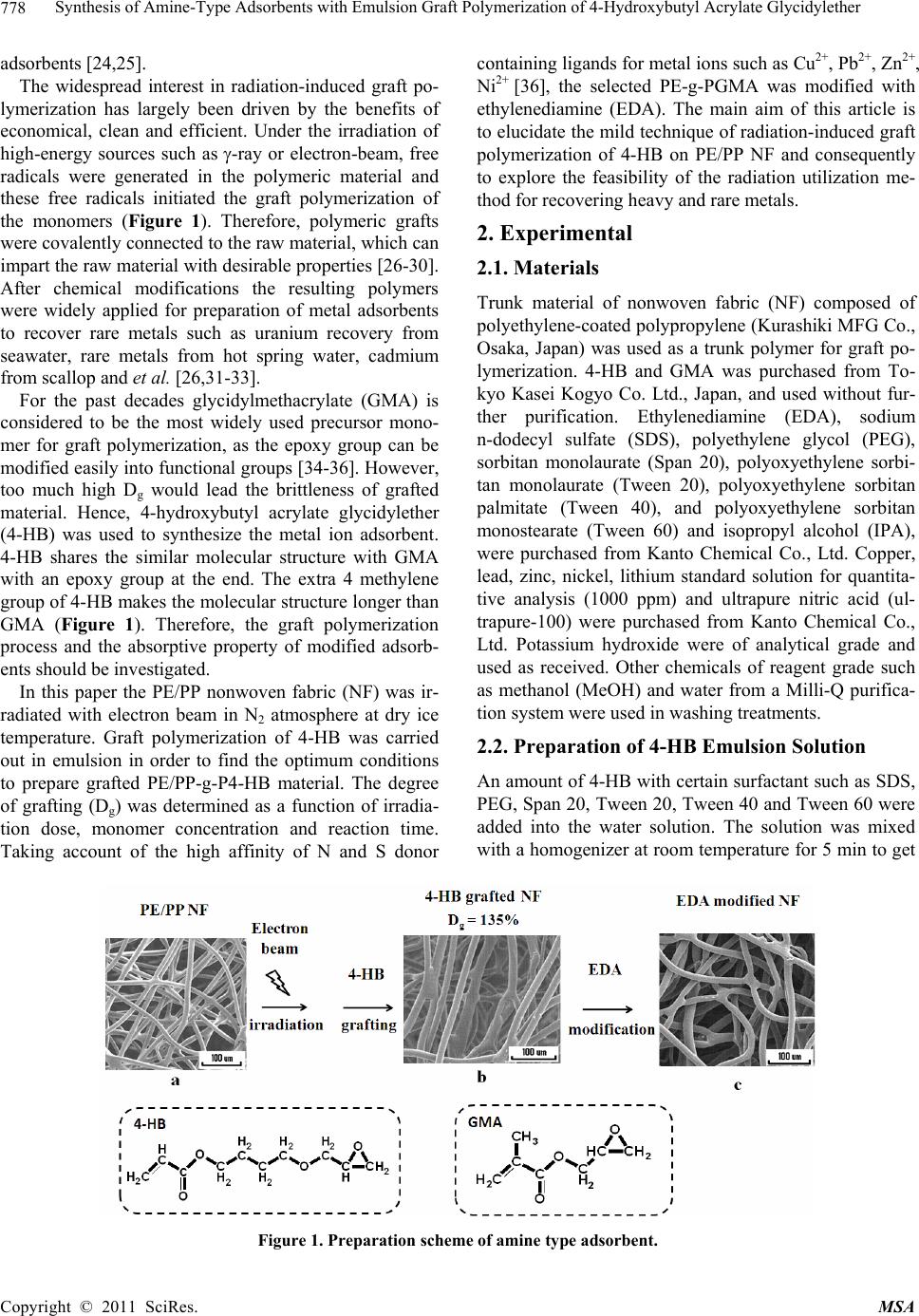 Synthesis of Amine-Type Adsorbents with Emulsion Graft Polymerization of 4-Hydroxybutyl Acrylate Glycidylether 778 adsorbents [24,25]. The widespread interest in radiation-induced graft po- lymerization has largely been driven by the benefits of economical, clean and efficient. Under the irradiation of high-energy sources such as -ray or electron-beam, free radicals were generated in the polymeric material and these free radicals initiated the graft polymerization of the monomers (Figure 1). Therefore, polymeric grafts were covalently connected to the raw material, which can impart the raw material with desirable properties [26-30]. After chemical modifications the resulting polymers were widely applied for preparation of metal adsorbents to recover rare metals such as uranium recovery from seawater, rare metals from hot spring water, cadmium from scallop and et al. [26,31-33]. For the past decades glycidylmethacrylate (GMA) is considered to be the most widely used precursor mono- mer for graft polymerization, as the epoxy group can be modified easily into functional groups [34-36]. However, too much high Dg would lead the brittleness of grafted material. Hence, 4-hydroxybutyl acrylate glycidylether (4-HB) was used to synthesize the metal ion adsorbent. 4-HB shares the similar molecular structure with GMA with an epoxy group at the end. The extra 4 methylene group of 4-HB makes the molecular structure longer than GMA (Figure 1). Therefore, the graft polymerization process and the absorptive property of modified adsorb- ents should be investigated. In this paper the PE/PP nonwoven fabric (NF) was ir- radiated with electron beam in N2 atmosphere at dry ice temperature. Graft polymerization of 4-HB was carried out in emulsion in order to find the optimum conditions to prepare grafted PE/PP-g-P4-HB material. The degree of grafting (Dg) was determined as a function of irradia- tion dose, monomer concentration and reaction time. Taking account of the high affinity of N and S donor containing ligands for metal ions such as Cu2+, Pb2+, Zn2+, Ni2+ [36], the selected PE-g-PGMA was modified with ethylenediamine (EDA). The main aim of this article is to elucidate the mild technique of radiation-induced graft polymerization of 4-HB on PE/PP NF and consequently to explore the feasibility of the radiation utilization me- thod for recovering heavy and rare metals. 2. Experimental 2.1. Materials Trunk material of nonwoven fabric (NF) composed of polyethylene-coated polypropylene (Kurashiki MFG Co., Osaka, Japan) was used as a trunk polymer for graft po- lymerization. 4-HB and GMA was purchased from To- kyo Kasei Kogyo Co. Ltd., Japan, and used without fur- ther purification. Ethylenediamine (EDA), sodium n-dodecyl sulfate (SDS), polyethylene glycol (PEG), sorbitan monolaurate (Span 20), polyoxyethylene sorbi- tan monolaurate (Tween 20), polyoxyethylene sorbitan palmitate (Tween 40), and polyoxyethylene sorbitan monostearate (Tween 60) and isopropyl alcohol (IPA), were purchased from Kanto Chemical Co., Ltd. Copper, lead, zinc, nickel, lithium standard solution for quantita- tive analysis (1000 ppm) and ultrapure nitric acid (ul- trapure-100) were purchased from Kanto Chemical Co., Ltd. Potassium hydroxide were of analytical grade and used as received. Other chemicals of reagent grade such as methanol (MeOH) and water from a Milli-Q purifica- tion system were used in washing treatments. 2.2. Preparation of 4-HB Emulsion Solution An amount of 4-HB with certain surfactant such as SDS, PEG, Span 20, Tween 20, Tween 40 and Tween 60 were added into the water solution. The solution was mixed with a homogenizer at room temperature for 5 min to get Figure 1. Preparation scheme of amine type adsorbent. Copyright © 2011 SciRes. MSA  Synthesis of Amine-Type Adsorbents with Emulsion Graft Polymerization of 4-Hydroxybutyl Acrylate Glycidylether 779 a homogeneous emulsion state. The micelle size of 4-HB/Span 20 was evaluated by fiber optics dynamic light scattering spectrophotometer (FDLS-3000, Otsuka Electronics, Co. Ltd., Japan). 2.3. Graft Polymerization and Chemical Modification The NF in long sheet was cut into 3 cm × 7 cm square pieces which were then packed into polyethylene bags. After the replacement of the air in the polyethylene bag by nitrogen gas, the NF pieces were cooled at dry-ice temperature and exposed to an electron beam irradiation. The irradiated NF pieces were transferred to a glass am- poule and the air inside was evacuated to remove O2. Then, an aqueous emulsion containing 4-HB and Span 20 was drawn into the glass ampoule via suction. Graft po- lymerization of 4-HB was carried out by keeping the glass ampoule in a water bath at 40˚C. After the graft polymerization, the grafted NF pieces were washed three times with methanol and dried. After drying under the reduced pressure, the amount of 4-HB grafted onto NF was evaluated by the degree of grafting (Dg). Dg was defined as follows: Dg (%) = (Wi – W0) / W0 × 100, where, W0 is the initial weight of NF and Wi weight of NF after graft polymerization. 4-HB-grafted NF was aminated by 70% EDA in IPA at 60˚C to introduce the adsorption unit [37]. After 3 h reaction, the 4-HB-grafted NF was taken out from the solution, washed with distilled water. After drying under reduced pressure, the density of amine group of the ad- sorbent was estimated by: Amine group density (mmol/g-adsorbent) (%) = (Zi – Z0) / Zi / M × 100, where, Z0 and Zi are the weights of 4-HB-grafted NF be- fore and after chemical modification and M the molecu- lar weight of the EDA. 2.4. Metal Adsorption Metal adsorption of the amine type adsorbent fabrics was performed by batch adsorption. 0.02 g EDA modified NF was soaked in 45 ml of metal ions of 10 ppm. The re- moval ratio of metal ions was evaluated as follows: Removal Ratio (%) = (C0 – Ci) / C0 × 100, where, C0 and Ci are the concentrations of the metal ions in solution before and after adsorption. 2.5. Analysis The graft polymerization of 4-HB-grafted NF was dem- onstrated by Fourier transform infrared (FT-IR) in a transmittance mode on a PerkinElmer Spectrum One FT-IR. The NF before and after graft polymerization was observed with a scanning electron microscope (SEM, Hitachi SEMDX Type N) after sputtered with gold. The concentration of the remaining metal ion was measured by optical emission spectrometer (Optima 4,300 DV). 3. Results and Discussion 3.1. Emulsion Stability In order to get suitable graft polymerization condition the stability of 4-HB emulsion solution prepared by several different surfactants was investigated to optimize the grafting condition. 5 wt% of 4-HB was added into 1 wt% certain surfactant water solution. The resulting emulsions of 4-HB with surfactant were in a milky state with dif- ferent size of micelle after high speed magnetic stir by a homogenizer. For a stable emulsion not only the milky state but also the size of micelle should keep constant all along and their stabilities of homogeneous emulations states were summarized in Table 1. In the case of SDS and PEG the emulsion could not maintain a milky state and separated into layers within half an hour. Milky state prepared with Tween’s maintained for 6 h and then gradually changed into separated layers (Figure 2(d)). The most stable emulsion of 5 wt% 4-HB in water was obtained with the addition of Span 20, in which the milky state can keep for at least 24 h (Figure 2). In order to optimize the state of 4-HB/Span 20 emulsion, the con- centration of Span 20 was changed and the result was shown in Figure 3. Obviously, the size of micelle in- creased from 200 to 600 nm when the concentration of Span 20 was reduced from 1.0 to 0.1 wt%. Therefore, the optimal emulsion of 4-HB was obtained when the con- centration of Span 20 was set at 0.5 wt%. 3.2. Graft Polymerization The NF was irradiated at five different irradiation doses: 10, 20, 30, 40 and 50 kGy to investigate the effect of irra- diation dose on Dg. The emulsion graft polymerization was carried out at 40˚C in the aqueous emulsion of 5% 4-HB and 0.5% Span 20. Figure 4 depicts the Dg of 4-HB onto the NF as a function of irradiation dose after 1 h. The results indicated that the Dg of 4-HB increased linearly with the increase of the absorbed dose below 40 kGy. Grafting saturation with a Dg of 180% was achieved at Table 1. Stability of 4-HB emulsion prepared by different surfactants. Surfactant Stability of milky state SDS <0.5 h PEG <0.5 h Span 20 24 h Tween 20 6 h Tween 40 6 h Tween 60 6 h Copyright © 2011 SciRes. MSA  Synthesis of Amine-Type Adsorbents with Emulsion Graft Polymerization of 4-Hydroxybutyl Acrylate Glycidylether 780 Figure 2. Milky state of the emulsion prepared with Span 20 (a), keep for 48 h (b), Tween 20 (c), and keep for 24 h (d). 050100 150 200 250 200 400 600 800 0.1% 0.3% 0.5% 1.0% Micelle Diameter (nm) Time (min) Figure 3. Micelle diameter at various concentrations of Span 20, (■) 0.1%, (○) 0.3%, (∆) 0.5% and (▼) 1.0%. 0 1020304050 0 50 100 150 200 Dg (%) 1 h 5 % 4HB 0.5 % Span 20 40 0C Dose (kGy) Figure 4. Effect of pre-irradiation dose on Dg, with 5% 4-HB, 0.5% Span-20 at 40˚C after 1 h grafting. 50 kGy. It is well known that pre-irradiation induced graft copolymerization relies heavily on the amount of trapped radicals generated in the trunk polymer. The li- nearly increased Dg is due to the amount of efficient trapped radicals with the dose increment, which is ac- companied by an increment in the initiation, followed by predominance in propagation and suppression in termi- nation [38]. However, as already reported in literature, once the rate of graft polymerization is too high to ex- ceed the diffusion rate of monomer into the fabric, the simultaneous cross-linking in the fabric suppresses the diffusion of 4-HB into polyethylene [39]. Hence, pre- irradiation dose below 50 kGy is considered to be favor- able for 4-HB graft polymerization onto PE/PP NF. Figure 5 shows the effect of 4-HB concentration on Dg in the case of 50 kGy pre-irradiation at 40˚C after fixed time interval of 0.5, 1, 2, 3 and 4 h. Set grafting time of 1 h for example, a concentration of 2.5% 4-HB yielded a Dg of 60%. At the concentration of 5% the Dg reached 175% which is enough for precursor for applica- tion to metal ion adsorbent [39]. Then the increase of concentration led a slower increment and gave a platform value of 290% at the concentration of 15%. The further increase of 4-HB concentration would result in a ho- mo-polymerization, which turned out to be uneconomical and unnecessary. Evidence from the above suggests a concentration of 5% 4-HB would be appropriate for this graft polymerization. Figure 6 shows the time conversion of Dg at 40˚C in the aqueous emulsion of 5% 4-HB at pre-irradiation dose of 30 and 50 kGy, respectively. At pre-irradiation dose of 50 kGy, Dg increased drastically with grafting time and reached 130% after 30 min, and gradually leveled off after 3 h with a Dg of 280%. As the diffusion rate of the monomer to the internal radicals in the polymer substrate was impeded by graft polymerization and homo-poly- merization, and eventually resulted in a certain value of Dg. At pre-irradiation dose of 30 kGy, Dg increased stea- dily with grafting time and reached 130% after 2 h. As a Dg of 130% is enough for precursor for application to metal ion adsorbent, it is more economical and easier to control the Dg at the dose of 30 kGy rather than 50 kGy. 051015 0 100 200 300 400 500 600 50 kGy 0.5% S pan 20 40 0C Dg (%) Monom er Concentration (% ) 0.5h 1h 2h 3h 4h Figure 5. Effect of monomer concentration on Dg, after 50 kGy irradiation with 0.5% Span20 at 40 oC, (■) 0.5 h, (○) 1 h, (∆) 2 h, (▼) 3h and (□) 4 h. Copyright © 2011 SciRes. MSA  Synthesis of Amine-type Adsorbents with Emulsion Graft Polymerization of 4-hydroxybutyl Acrylate Glycidylether Copyright © 2011 SciRes. MSA 781 ameter (Figure 1(a)). After graft polymerization, the diameter of the fiber was doubled to 20 µm at the Dg of 130% (Figure 1(b)). In our previous study, the soft trunk NF became hard after grafted with GMA and then turned 01234 0 50 100 150 200 250 300 350 5% 4HB 0.5% Span 20 40 0C 50 kGy N2 dry ice temperature 30 kGy Dg (%) Time (h) to brittle at a high Dg of 100%. However the 4-HB grafted-type material can keep soft even at a high Dg of 280%. The tensile curves are displayed in Figure 8, in which the three curves followed the similar propensity but with some discrepancy. From tensile strength and breaking elongation of single NF it is clearly that the GMA grafted NF can easily be broken under tension compared with 4-HB grafted one. When compare the molecular structure of 4-HB with GMA depicted in Fig- ure 1 the reason can be presumed as the 4 methylene groups of 4-HB. This subtle distinction makes the mo- lecular structure of monomer flexible, further makes the side chain of polymer longer and flexible during the process of graft polymerization. Accordingly, not only the physical properties such as topography and mechani- cal properties of the grafted material but also their capa- bility were improved, which will be elaborated below. Figure 6. Time course of 4-HB grafting on NF, (■) 50 kGy and (●) 30 kGy irradiation dose. The graft polymerization of NF with 4-HB was dem- onstrated by FT-IR. An example of a spectrum is given in Figure 7 together with a spectrum of the trunk poly- mer. After the NF was grafted with 4-HB, the strong ab- sorption at about 1726 cm–1 (C=O stretching) and 1251 cm–1 (-C-O- stretching) were observed. Besides, 847 cm–1 represented the characteristic vibrations of epoxy groups. Meanwhile, the characteristic vibrations of the carbonyl group increased with the increase of Dg. These results indicate that the introduction of poly-4-HB onto PE/PP fabric is clearly produced. 3.3. Modification of PE-g-P4-HB All above results show that the optimum conditions to prepare 4-HB-grafted NF have been achieved by using relatively low irradiation dose at 30 kGy, low monomer concentration of 5%. And then amine-type adsorbents were synthesized by reaction of EDA with 4-HB grafted NF. Figure 7c shows the infrared spectra of the grafted NF with EDA group contents. As compared to the spec- trum of the unmodified copolymers (Figure 7(b)), the The SEM images of the surface sections of trunk NF and grafted material are shown in Figure 1. The surface section image of the fabric revealed the fiber morphology, crisscrossed by a network of fibers with 10 µm in di- 0 20 40 60 80 100 0 20 40 60 80 100 1600 1200800 0 20 40 60 80 100 4HB grafted Dg = 138% EDA modified 4HB grafted Dg = 138% PE/PP T % T % T % a 847 cm-1 1251 cm-1 1726 cm-1 b W aven um b ers (cm -1) c Figure 7. FT-IR spectra of NF, 4-HB-grafted NF and modified 4-HB-grafted NF with EDA.  Synthesis of Amine-Type Adsorbents with Emulsion Graft Polymerization of 4-Hydroxybutyl Acrylate Glycidylether 782 0246810 0 1 2 3 4 5 6 7 GMA 124% 4-HB 124% trunk PE/PP Tension (MPa) Length (m m ) Figure 8. The typical tensile curves of NF, 4-HB-grafted NF and GMA-grafted NF. characteristic vibrations of epoxy groups at 847 cm–1 disappeared because of the ring opening reaction. The density of amine group in EDA modified NF with dif- ferent Dg were shown in Figure 9. In the case of material with low Dg the density of amine group almost linearly depended on the Dg, which also indicated that amine group could easily be introduced into grafted poly-4-HB. After modification, a Dg of 100% 4-HB-grafted NF yielded 2.5 mmol-epoxy groups in 1 g grafted material. The density of amine group preserved increasing but the ratio slowed down distinctly and finally reached a pla- teau value of 3.0 mmol/g at the Dg of 290%. In the SEM image shown in Figure 1 the modified material seemed similar with the unmodified NF, which means no trail of destruction happened during the modification process. 3.4. Absorptive Property of Modified Adsorbents The grafted NF with a Dg of 135% was treated by EDA at the optimized condition of modification, and the den- sity of amine group was 2.8 mmol/g. Metal adsorption of the resulting adsorbent NF was performed by batch ad- sorption of 10 ppm metal ions. The absorptive behavior of the material depended on the pH value of the solution (Figure 10). Generally, the removal ratio of the metal ions increased with the increment of pH value and reached a platform of 95% for Cu2+, 82% for Pb2+ and 73% for Zn2+ respectively at pH 5 after 5 h adsorption. The capability of this EDA modified NF was also studied in Ni2+ and Li+ solutions. However, the removal ratio was very low. After 24 h, removal ratio of Ni2+ at pH 5 was only 23% (Figure 11). In the case of Li+, EDA modified NF was almost impotent (Figures 10, 11). Hence, the removal ratio of the EDA modified adsorbent is in the order of Cu2+ > Pb2+ > Zn2+ > Ni2+ > Li+. When compare the capability of 4-HB-grafted material with GMA-grafted one, certain Dg was designed before 050100 150 200 250 300 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 70% EDA in IPA 60 oC 2h Amine group density (mmol/g) Dg (%) Figure 9. The density of amine groups in EDA-type ad- sorbents after 2 h modification at 60˚C. 23456 0 20 40 60 80 100 Removal ratio (%) Pb2+ Cu2+ Ni2+ Zn2+ Li+ pH Figure 10. Removal ratio of metal ions at different pH val- ues, (■) Pb2+, (○) Cu2+, (∆) Ni2+, (▼) Zn2+ and (♦) Li+. 012345 202 0 20 40 60 80 100 4 Time (h) Removal ratio (%) Pb2+ Cu2+ Ni2+ Zn2+ Li+ pH 5, 10 ppm Figure 11. Removal ratio of metal ions with initial concen- tration of 10 ppm at pH 5, (■) Pb2+, (○) Cu2+, (∆) Ni2+, (▼) Zn2+ and (◄) Li+. hand in order to control the density of amine groups. Af- ter treated by EDA the 4-HB-grafted NF with a Dg of Copyright © 2011 SciRes. MSA 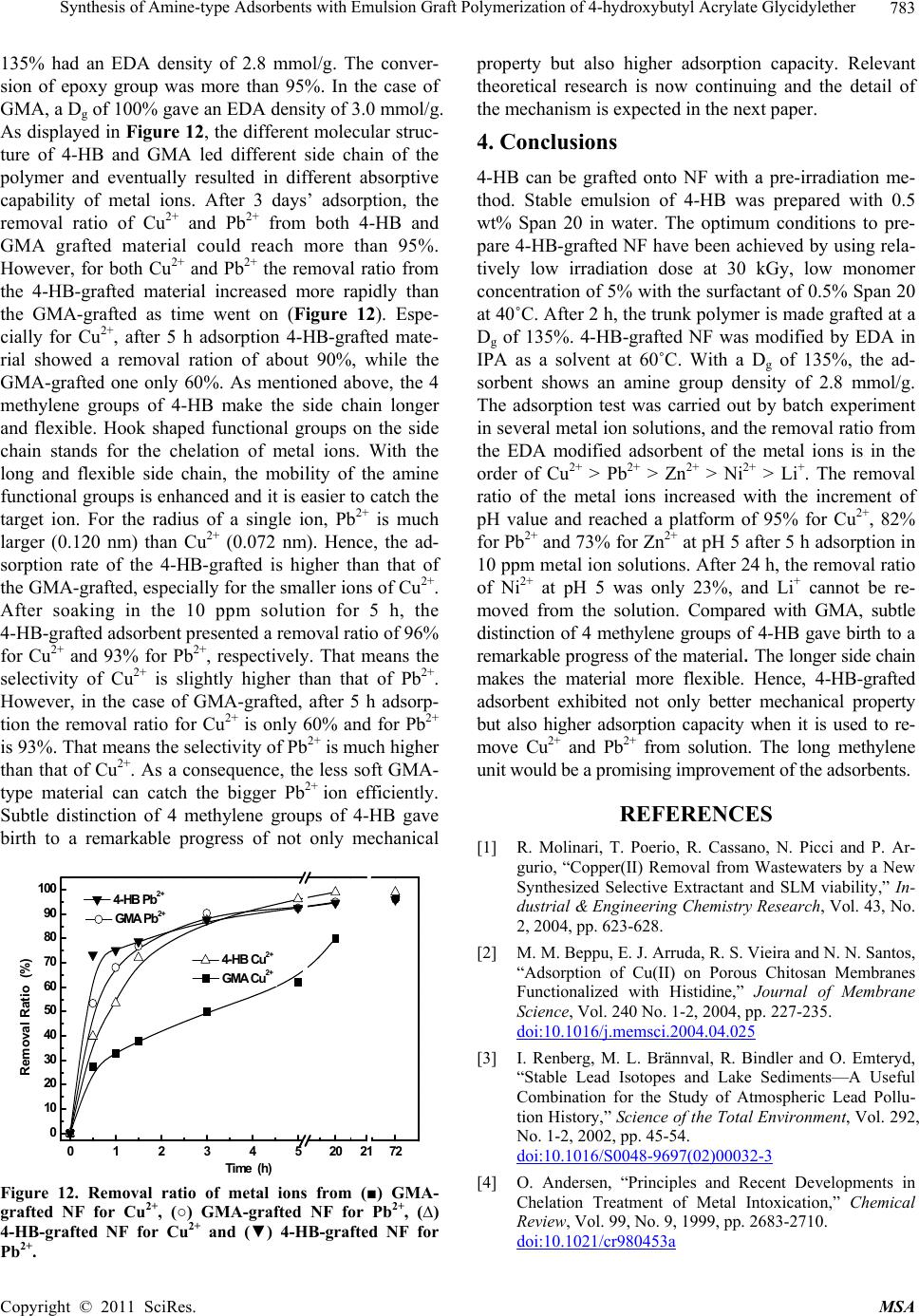 Synthesis of Amine-type Adsorbents with Emulsion Graft Polymerization of 4-hydroxybutyl Acrylate Glycidylether783 135% had an EDA density of 2.8 mmol/g. The conver- sion of epoxy group was more than 95%. In the case of GMA, a Dg of 100% gave an EDA density of 3.0 mmol/g. As displayed in Figure 12, the different molecular struc- ture of 4-HB and GMA led different side chain of the polymer and eventually resulted in different absorptive capability of metal ions. After 3 days’ adsorption, the removal ratio of Cu2+ and Pb2+ from both 4-HB and GMA grafted material could reach more than 95%. However, for both Cu2+ and Pb2+ the removal ratio from the 4-HB-grafted material increased more rapidly than the GMA-grafted as time went on (Figure 12). Espe- cially for Cu2+, after 5 h adsorption 4-HB-grafted mate- rial showed a removal ration of about 90%, while the GMA-grafted one only 60%. As mentioned above, the 4 methylene groups of 4-HB make the side chain longer and flexible. Hook shaped functional groups on the side chain stands for the chelation of metal ions. With the long and flexible side chain, the mobility of the amine functional groups is enhanced and it is easier to catch the target ion. For the radius of a single ion, Pb2+ is much larger (0.120 nm) than Cu2+ (0.072 nm). Hence, the ad- sorption rate of the 4-HB-grafted is higher than that of the GMA-grafted, especially for the smaller ions of Cu2+. After soaking in the 10 ppm solution for 5 h, the 4-HB-grafted adsorbent presented a removal ratio of 96% for Cu2+ and 93% for Pb2+, respectively. That means the selectivity of Cu2+ is slightly higher than that of Pb2+. However, in the case of GMA-grafted, after 5 h adsorp- tion the removal ratio for Cu2+ is only 60% and for Pb2+ is 93%. That means the selectivity of Pb2+ is much higher than that of Cu2+. As a consequence, the less soft GMA- type material can catch the bigger Pb2+ ion efficiently. Subtle distinction of 4 methylene groups of 4-HB gave birth to a remarkable progress of not only mechanical 0123452021 0 10 20 30 40 50 60 70 80 90 100 72 4-HB Pb2+ GMA Pb2+ 4-HB Cu2+ Removal Ratio (%) Tim e (h) GMA Cu2+ Figure 12. Removal ratio of metal ions from (■) GMA- grafted NF for Cu2+, (○) GMA-grafted NF for Pb2+, (∆) 4-HB-grafted NF for Cu2+ and (▼) 4-HB-grafted NF for Pb2+. property but also higher adsorption capacity. Relevant theoretical research is now continuing and the detail of the mechanism is expected in the next paper. 4. Conclusions 4-HB can be grafted onto NF with a pre-irradiation me- thod. Stable emulsion of 4-HB was prepared with 0.5 wt% Span 20 in water. The optimum conditions to pre- pare 4-HB-grafted NF have been achieved by using rela- tively low irradiation dose at 30 kGy, low monomer concentration of 5% with the surfactant of 0.5% Span 20 at 40˚C. After 2 h, the trunk polymer is made grafted at a Dg of 135%. 4-HB-grafted NF was modified by EDA in IPA as a solvent at 60˚C. With a Dg of 135%, the ad- sorbent shows an amine group density of 2.8 mmol/g. The adsorption test was carried out by batch experiment in several metal ion solutions, and the removal ratio from the EDA modified adsorbent of the metal ions is in the order of Cu2+ > Pb2+ > Zn2+ > Ni2+ > Li+. The removal ratio of the metal ions increased with the increment of pH value and reached a platform of 95% for Cu2+, 82% for Pb2+ and 73% for Zn2+ at pH 5 after 5 h adsorption in 10 ppm metal ion solutions. After 24 h, the removal ratio of Ni2+ at pH 5 was only 23%, and Li+ cannot be re- moved from the solution. Compared with GMA, subtle distinction of 4 methylene groups of 4-HB gave birth to a remarkable progress of the material. The longer side chain makes the material more flexible. Hence, 4-HB-grafted adsorbent exhibited not only better mechanical property but also higher adsorption capacity when it is used to re- move Cu2+ and Pb2+ from solution. The long methylene unit would be a promising improvement of the adsorbents. REFERENCES [1] R. Molinari, T. Poerio, R. Cassano, N. Picci and P. Ar- gurio, “Copper(II) Removal from Wastewaters by a New Synthesized Selective Extractant and SLM viability,” In- dustrial & Engineering Chemistry Research, Vol. 43, No. 2, 2004, pp. 623-628. [2] M. M. Beppu, E. J. Arruda, R. S. Vieira and N. N. Santos, “Adsorption of Cu(II) on Porous Chitosan Membranes Functionalized with Histidine,” Journal of Membrane Science, Vol. 240 No. 1-2, 2004, pp. 227-235. doi:10.1016/j.memsci.2004.04.025 [3] I. Renberg, M. L. Brännval, R. Bindler and O. Emteryd, “Stable Lead Isotopes and Lake Sediments—A Useful Combination for the Study of Atmospheric Lead Pollu- tion History,” Science of the Total Environment, Vol. 292, No. 1-2, 2002, pp. 45-54. doi:10.1016/S0048-9697(02)00032-3 [4] O. Andersen, “Principles and Recent Developments in Chelation Treatment of Metal Intoxication,” Chemical Review, Vol. 99, No. 9, 1999, pp. 2683-2710. doi:10.1021/cr980453a Copyright © 2011 SciRes. MSA  Synthesis of Amine-Type Adsorbents with Emulsion Graft Polymerization of 4-Hydroxybutyl Acrylate Glycidylether 784 [5] S. Manahan, “Environmental Chemistry,” Brooks/Colei, California, USA, 1984. [6] US Environmental Protection Agency (USEPA), “Guide- lines for Drinking Water,” March 2001. [7] K. V. S. G. Muralikrishna, “Chemical Analysis of Water and Soil—A Laboratory Manual, Environmental Protec- tion Society,” National Institute of Ecology and Envi- ronment, Kakinada, India, 1997. [8] IS: 10500, Drinking Water Standards, Bureau of Indian Standards (BIS), 1995. [9] R. Gündŏgan, B. Acemiŏglu and M. H. Alma, “Copper (II) Adsorption from Aqueous Solution by Herbaceous Peat,” Journal of Colloid and Interface Science, Vol. 269, No. 2, 2004, pp. 303-309. doi:10.1016/S0021-9797(03)00762-8 [10] T. A. Garcia and L. Corredor, “Biochemical Changes in the Kidneys after Perinatal Intoxication with Lead and/or Cadmium and Their Antagonistic Effects When Coad- ministered,” Ecotoxicology Environmental Safety, Vol. 57, No. 2, 2004, pp. 184-189. doi:10.1016/S0147-6513(03)00063-0 [11] J. Goel, K. Kadirvelu, C. Rajagopal, and V. K. Garg, “Removal of Lead(II) from Aqueous Solution by Adsorp- tion on Carbon Aerogel Using a Response Surface Meth- odological Approach,” Industrial & Engineering Chemis- try Research, Vol. 44, No. 7, 2005, pp. 1987-1994. doi:10.1021/ie0490684 [12] T. Tokimoto, N. Kawasaki, T. Nakamura, J. Akutagawa and S. Tanada, “Removal of Lead Ions in Drinking Water by Coffee Grounds as Vegetable Biomass,” Journal of Colloid and Interface Science, Vol. 281, No. 1, 2005, pp. 56-61. doi:10.1016/j.jcis.2004.08.083 [13] C. Huang, Y. C. Chung and M.R. Liou, “Adsorption of Cu(II) and Ni(II) by Pelletized Biopolymer,” Journal of Hazardous Materials, Vol. 45, No. 2-3, 1996, pp. 265- 277. doi:10.1016/0304-3894(95)00096-8 [14] G. Issabayeva, M. K. Aroua and N. M. Nik Sulaiman, “Removal of Lead from Aqueous Solutions on Palm Shell Activated Carbon,” Bioresource Technology, Vol. 97, No. 18, 2006, pp. 2350-2355. doi:10.1016/j.biortech.2005.10.023 [15] K. Chandra Sekhar, C. T. Kamala, N.S. Chary, A. R. K. Sastry, T. Nageswara Rao and M. Vairamani, “Removal of Lead from Aqueous Solutions Using an Immobilized Biomaterial Derived from a Plant Biomass,” Journal of Hazardous Materials, Vol. 108, No. 1-2, 2004, pp. 111- 117. doi:10.1016/j.jhazmat.2004.01.013 [16] A. Janin, J. F. Blais, G. Mercier and P. Drogui, “Selective Recovery of Cr and Cu in Leachate from Chromated Cop- per Arsenate Treated Wood Using Chelating and Acidic Ion Exchange Resins,” Journal of Hazardous Materials, Vol. 169, No. 1-3, 2009, pp. 1099-1105. doi:10.1016/j.jhazmat.2009.04.066 [17] M. M. Beppu, E. J. Arruda, R. S. Vieira and N. N. Santos, “Adsorption of Cu(II) on Porous Chitosan Membranes Functionalized with Histidine,” Journal of Membrane Science, Vol. 240, No. 1-2, 2004, pp. 227-235. [18] A. Saeed, M. Iqbal and M. W. Akhtar, “Removal and Recovery of Lead(II) from Single and Multimetal (Cd, Cu, Ni, Zn) Solutions by Crop Milling Waste (Black Gram Husk),” Journal of Hazardous Materials, Vol. 117, No. 1, 2005, pp. 65-73. doi:10.1016/j.jhazmat.2004.09.008 [19] R. S. Yeh, Y. Y. Wang and C. C. Wan, “Removal of Cu-EDTA Compounds Via Electrochemical Process With Coagulation,” Water Research, Vol. 29, No. 2, 1995, pp. 597-599. doi:10.1016/0043-1354(94)00169-8 [20] H. Cheng, “Cu(II) Removal from Lithium Bromide Re- frigerant by Chemical Precipitation and Electrocoagula- tion,” Separation and Purification Technology, Vol. 52, No. 1, 2006, pp. 191-195. doi:10.1016/j.seppur.2006.03.021 [21] M. Q. Jiang, X. Y. Jin, X. Q. Lu and Z. L. Chen, “Ad- sorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto Natural Kaolinite Clay,” Desalination, Vol. 252, No. 1-3, 2010, pp. 33-39. doi:10.1016/j.desal.2009.11.005 [22] J. Oliva, J. De Pablo, J. L. Cortina, J. Cama and C. Ayora, “The Use of Patite II to Remove Divalent Metal Ions Zinc(II), Lead(II), Manganese(II) and Iron(II) from Water in Passive Treatment Systems: Column Experiments,” Journal of Hazardous Materials, Vol. 184, No. 1-3, 2010, pp. 364-374. doi:10.1016/j.jhazmat.2010.08.045 [23] Y. Xu and D. Zhao, “Removal of Lead from Contami- nated Soils Using Poly(Amidoamine) Dendrimers,” In- dustrial & Engineering Chemistry Research, Vol. 45 No. 5, 2006, pp. 1758-1765. [24] H. Chen and A. Wang, “Adsorption Characteristics of Cu(II) from Aqueous Solution onto Poly(Acrylamide)/ Attapulgite Composite,” Journal of Hazardous Materials, Vol. 165, No. 1-3, 2009, pp. 223-231. doi:10.1016/j.jhazmat.2008.09.097 [25] A. Nagendran, A. Vijayalakshmi, D. Lawrence Arocki- asamy, K. H. Shobana and D. Mohan, “Toxic Metal Ion Separation by Cellulose Acetate/Sulfonated Poly(Ether Imide) Blend Membranes: Effect of Polymer Composi- tion and Additive Original Research Article,” Journal of Hazardous Materials, Vol. 155, No. 3, 2008, pp. 477-485. doi:10.1016/j.jhazmat.2007.11.088 [26] K. Saito, S. Yamada, S. Furusaki, T. Sugo and J. Oka- moto, “Characteristics of Uranium Adsorption by Ami- doxime Membrane Synthesized by Radiation-Induced Graft Polymerization,” Journal of Membrane Science, Vol. 34, No. 1, 1987, pp. 307-315. doi:10.1016/S0376-7388(00)83171-3 [27] M. M. Nasef, H. Saidi and H. Mohd Nor, “Radiation- Induced Graft Copolymerization for Preparation of Cation Exchange Membranes: A Review,” Malaysia Nuclear Science Journal, Vol. 17, 1999, pp. 27-43. [28] T. Kawai, K. Sugita, K. Saito and T. Sugo, “Extension and Shrinkage of Polymer Brush Grafted onto Porous Membrane Induced by Protein Binding,” Macromolecules, Vol. 33, No. 4, 2000, 1306-1309. doi:10.1021/ma9819642 [29] F. Cardona, G. A. George, D. J. T. Hill, F. Rasoul and J. Maeji, “Copolymers Obtained by the Radiation-Induced Copyright © 2011 SciRes. MSA  Synthesis of Amine-type Adsorbents with Emulsion Graft Polymerization of 4-hydroxybutyl Acrylate Glycidylether Copyright © 2011 SciRes. MSA 785 Grafting of Styrene onto Poly(Tetrafluoroethylene-Co- Perfluoropropylvinyl Ether) Substrates. 1. Preparation and Structural Investigation,” Macromolecules, Vol. 35, No. 2, 2002, pp. 355-364. doi:10.1021/ma0022295 [30] M. Tamada, N. Seko and F.Yoshii, “Application of Ra- diation-Graft Materials for Metal Adsorbent and Cross- linked Natural Polymer for Healthcare Products,” Radia- tion Physics and Chemistry, Vol. 71, No. 1-2, 2004, pp. 223-227. doi:10.1016/j.radphyschem.2004.03.044 [31] N. Seko, A. Katakai, S. Hasegawa, M. Tamada, N. Kasai, H. Takeda, T. Sugo and K. Saito, “Aquaculture of Ura- nium in Seawater by a Fabric-Adsorbent Submerged Sys- tem,” Nuclear Technology, Vol. 144, No. 2, 2003, pp. 274-278. [32] N. Seko, M. Tamada and F. Yoshii, “Current Status of Adsorbents for Metal Ions with Radiation Grafting and Crosslinking Technique,” Nuclear Instrument and Meth- ods in Physics Research Section B: Beam Interactions with Materials and Atoms, Vol. 236, No. 1, 2005, pp. 21-29. doi:10.1016/j.nimb.2005.03.244 [33] H. Kawakita, K. Uezu, S. Tsuneda, K. Saito, M. Tamada and T. Sugo, “Recovery of Sb(V) Using a Functional- Ligand-Containing Porous Hollow-Fiber Membrane Pre- pared by Radiation-Induced Graft Polymerization,” Hy- drometallurgy, Vol. 81, No. 3-4, 2006, pp. 190-196. doi:10.1016/j.hydromet.2005.12.010 [34] A. W. Eckert, D. Grobe and U. Rothe, “Surface-Modifi- cation of Polystylene-Microtitre Plates via Grafting of Glycidylmethacrylate and Coating of Poly-Glycidyl- methacrylate,” Biomaterials, Vol. 21, No. 5, 2000, pp. 441-447. doi:10.1016/S0142-9612(99)00098-8 [35] H. Hoshina, N. Seko, Y. Ueki and M. Tamada, “Synthesis of Graft Adsorbent with N-methyl-D-glucamine for Bo- ron Adsorption,” Journal of Ion Exchange, Vol. 18, No. 1-2, 2007, pp. 236-239. [36] C. A. Flemming and J. T. Trevors, “Copper Toxicity and Chemistry in the Environment: A Review,” Water Air Soil Pollution, Vol. 44, No. 1-2, 1989, pp. 143-158. doi:10.1007/BF00228784 [37] A. Sekine, N. Seko, M. Tamada and Y. Suzuki, “Biode- gradable Metal Adsorbent Synthesized by Graft Polym- erization onto Nonwoven Cotton Fabric,” Radiation Physics and Chemistry, Vol. 79, No. 1, 2010, pp. 16-21. doi:10.1016/j.radphyschem.2009.08.007 [38] M. M. Nasef, H. Saidi and K. Z. M. Dahlan, “Kinetic Investigations of Graft Copolymerization of Sodium Sty- rene Sulfonate onto Electron Beam Irradiated Poly(Vi- nylidene Fluoride) Films,” Radiation Physics and Chem- istry, Vol. 80, No. 1, 2011, pp. 66-75. doi:10.1016/j.radphyschem.2010.08.010 [39] N. Seko, L. T. Bang and M. Tamada, “Synthesis of Amine-Type Adsorbents with Emulsion Graft Polymeri- zation of Glycidyl Methacrylate,” Nuclear Instrument and Methods in Physics Research Section B: Beam Interac- tions with Materials and Atoms, Vol. 265, No. 1, 2007, pp. 146-149. doi:10.1016/j.nimb.2007.08.041
|