 Journal of Biosciences and Medicines, 2016, 4, 57-64 Published Online March 2016 in SciRes. http://www.scirp.org/journal/jbm http://dx.doi.org/10.4236/jbm.2016.43010 How to cite this paper: Li, X., Gao, Y.T., Li, P.F., Tian, F., Wang, J. and Wang, C. F. (2016) The Function of miR-31 Expression in Differentiation of Neural Stem Cells. Journal of Biosciences and Medicines, 4, 57-64. http://dx.doi.org/10.4236/jbm.2016.43010 The Function of miR-31 Expression in Differentiation of Neural Stem Cells Xiao Li1,2, Yuantao Gao3, Pengfei Li1,2, Feng Tian1,2, Jing Wang1,2, Chunfang Wang1,2* 1Experi men tal Animal Cent er, Shanxi Medical Univer sit y , Shanxi, China 2Shanxi Key Laboratory of Laboratory Animal and Animal Mo del of Human Disea ses, Sha nx i Medical Uni ver sit y, Shanxi, China 3Nanchang Univer sit y Qu een Mary School, Nanchang, China Received 7 February 2016; accepted 10 March 2016; published 17 March 2016 Abstract Spinal co rd in jury (SCI) is a severe comp li cat ion of ac ute spin al i njury , it can lead to axon al deg e- neration and necrosis of neurons, causing dama g e cross section below the movement, then loss of sensory, re flex an d au t on omou s c on trol fu ncti on be low th e d am ag e sec tion. No wadays , the r e is increasing evidenc e th at t r an sp l ant at io n of neural stem cells can help to rep air spinal co rd inju ry after sp inal c o rd in jury. The aim of thi s study was to res ea rc h th e func tio n of miR-31 expre ssion in differenti ation of neural s tem cel ls. M ir-31 was t ransfect ed the mimic s and i nhibit or in to n eu r al stem cells. By the morphol ogic al observa tion, th e cel l over expressio n of miR-31 was closer to NSCs. With the RT-PCR, H b9 and STM N1 wer e up-regulate when miR-31 was inh ibited, and Ne sti n was d own -regulate. When miR-31 was over expre ssion , the ex pres sion of Nes tin and H b9 wa s up-regulate. These findin gs su ggest th a t miR-31 may play a significant rol e in prol ifera tion and differenti ation of neu ral st em cell and ar e po ten ti al t arg ets fo r therapeu tic inte rventio ns follow ing spinal cord i njury . Keywords Spinal Cord Injur y, m icr oRNA, Neural S tem Cells, Transpl antation , Mou se 1. Introduction There is a wide variety of reasons happened to spinal cord injury (SCI). The most common cause for spinal cord is incident trauma. The clinical treatment for SCI lacks effective and safe strategies which have become a severe socioeconomically issue. Recent researches have shown that nerve stem cells (NSCs) replacement is an effective strategy for the treatment of SCI. (Paul Lu et al., 2014; Suzuki SO et al., 2003) [1] [2]. How induce more NSCs to motor neuron cells (MNs) is the vital part. MicroRNAs are a novel class of short, 18 - 25 nucleotide-long non-coding small RNAs. It play an important regulatory role in gene expression by binding to the 3’untranslated region of target mRNAs transcripts for de- *Corresponding author.  X. Li et al. gradation or inhibiting protein translation to determine a serial of vital life process such as cell differentiation, tissue repair and embryonic development. Individual miRNA can be inhibited multiple target genes simulta- neously to regulated complex physiological and pathological process. In recent years, a lot of studies have con- firmed microRNA may be involved in the regulation of differentiation in NSCs. (Maged M harrza, 2014; Chao Shi Niu, 2013; Eyal Mor, 2013) [3]-[5]. Therefore, it is very important to study how microRNAs regulated rele- vant signaling pathway to affect protein synthesis. Our previous study certify miR-31 is significant difference between NSCs and MNs which is highly expressed in NSCs and low expressed in MNs. (Hongen Wei, 2010) [6] It had been also reported to be an embryonic stem-specific microRNA. So we think that miR-31 may play an important role in maintaining NSCs in an undifferentiated state, but the concrete mechanism for miR-31 partici- pate in is unclear. To directly address the role of miR-31 in neuronal development, miRNA Mimics and miRNA Mimics control are induced NSCs to observe the expression changes of those genes to analysis the signaling pathway of miR-31 regulated. 2. Materials and Met ho d s 2.1. Animals Embryonic day 14 FVB mouse were used. This study was performed with permission of the local animal use and care committee in accordance with the applicable and governmental regulations. 2.2. Isolation, Culture and Identification of NSCs Spinal cords obtained from E14 mouse were collected in sterile D-Hank’s balanced salt solution and imme- diately processed for tissue culture. Spinal cords were mechanically separated from the surrounding connective tissue using sterile instruments under a dissecting microscope. Then discarded spinal meninges and triturated surplus ages by pipette to dissociate cells. Dispersed cells ere centrifuged at 1000 rpm for 10 min. the cells’ pel- let was resuspended in KnockOut™ DMEM/F12 supplemented with StemPro® Neural Supplement, EGF and FGFb (Bibco) and was plated in 25 cm2 Cell Culture Flask, and incubated in a CO2 Incubator of 5% CO2 at 37˚C. The medium was changed for half dose every 3d. The cells were observed every day. When the clonal globes were formatted detected by immunofluorescence. Cells culture were fixed in cold 4% paraformaldehyde and washed 3 times with PBS at 25˚C. Immunocytoche- mistry was carried out using standard protocols. Cell nuclei were counterstained with hochest 33342. The first antibodies was rabbit-anti-Nestin, 1:500. The second antibodies was goat-a nti-rabbit IgG-FITC conjugate, 1:200. Nuclei were stained using Hochest 33258 via immunocytochemistry. 2.3. Transfection To investigate the efficacy of RNAiMAX, the cells were seeded at 1 × 106 cells/well in 6 well plates, and after 4h incubation at 37˚C to adhere to the glass glide, the cells were transfected with 30 pM siRNA. The transfec- tion reagent was Lipofectamine RNAiMAX (Invitrogen) and Block-iTTM Alexa FluorR Red Lot No: 1477915 (invitrogen) were used according to the manufacturers’ instructions. After 24 h, 48 h and 72 h incubation at 37˚C the cells were observed by fluorescence microscope. Cells were seeded at a density of 1 × 106 cells/well in 6 well plates. After 4 h incubation at 37˚C, during which time the cells had adhered to the plastic, the cells were transfected with 30 pM siRNA. The transfection reagents were Lipofectamine® RNAiMAX, mmu-miR-31-5p mirVanaTM miRNA mimic, mirVanaTM miRNA mimic Negative Control, mmu-miR-31 Anti-miRTM miRNA inhibitor, Anti-miRTM Negative Control (Am- bion). Operations were used according to the manufacturers’ instructions. After 72 h incubation at 37˚C the cells were used immunohistochemical method to detect ChAT expression in cells. 2.4. Isolation of RNA for qRT-PCR After 72 h we isolated total RNA and analyzed by real-time PCR. Total RNA was extracted using the mirVana miRNA Isolation Kit (Ambion, Austin, TX, USA) according to the manufacturer’s protocol. The purified RNA was quantified by determining the absorbance at 260 nm using an UV spectrophotometer (Nanodrop, Thermo Scientific, USA). Total RNA (2 μg) was reverse transcribed into cDNA in a total volume of 15 μL using the 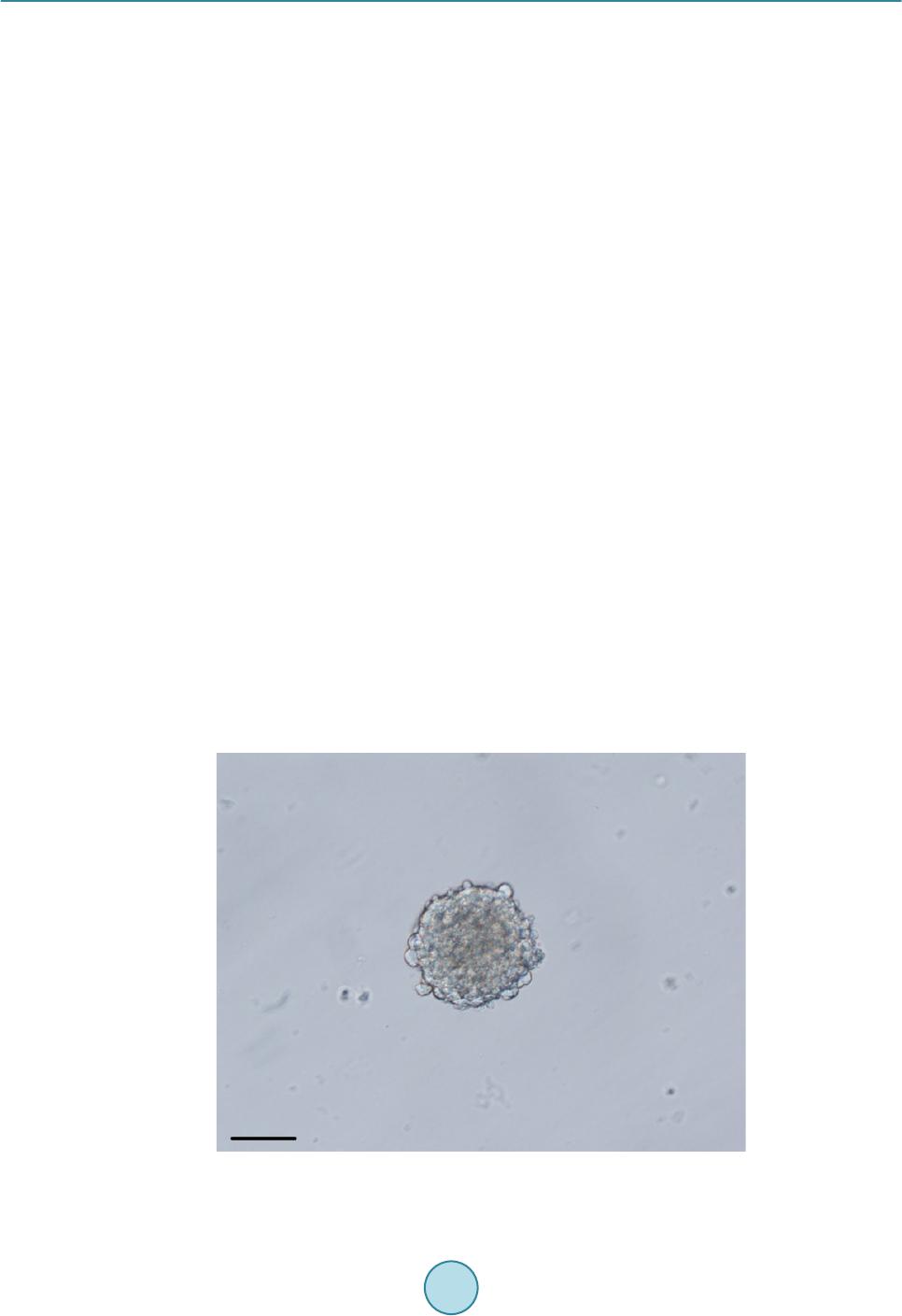 X. Li et al. Reverse Transcriptase M-MLV(RNase H-) (Takara)according to the manufacturer’s protocol. Each miRNA cDNA (10 ng) was obtained using the TaqMan Micro RNA Reverse Transcription Kit (Applied Bio systems) with the miRNA being reverse transcribed from the target miRNA using TaqMan Micro RNA Assays. 2.5. Quantification of miRNA Expression miRNA expression was measured using real-time RT-PCR on the Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems). U6 (Assay ID: 001973, Applied Biosystems) was used for internal control. Real- time PCR assay for miR-31 (Assay ID: 000185, Applied Biosystems) was used according to the manufacturer’s instructions. Real-time conditions: 50˚C for 2 min, 95˚C for 10 min, 40 cycles of 95˚C for 15 sec, 60˚C for 1 min on an Applied Biosystems 7300 thermocycler (Applied Biosystems). All samples were run in duplicates. In addition, real-time PCR analyses for Hb-9, Nestin and Stmn1were carried out using a SYBR Green PCR Kit (Invitrogen SYBR® Select Master Mix). All experiments were performed in duplicate. Copy numbers of cDNA for Hb-9, Nestin and Stmn1 were standardized to those of Rpl-19 for the same sample. Primer sequences for Hb-9 were as follows: 5’-CGAGACTCAGGTGAAGATTTGGT-3’ (forward) and 5’-CTGCTCTTTGG- CCTTTTTGC-3’ (reverse); and for Stmn1, 5’-AGAAGCCGATGT AGGACCGTATAG-3’ (forward) and 5’- TCCCCTTGAGCCCCTAAAA-3’(reverse); and for Nestin, 5’-GGTCACTGT CGCCGCTACTC-3’ (forward) and 5’-AAGCGGACGTGGAGCACTA-3’ (reverse); and for Rpl-19, 5’-ATCCGCAAGCCTGT GACTGT-3’ (forward) and 5’-TCGGGCCAGGGTGTTT TT-3’ (reverse). 3. Result 3.1. Isolation and Identification of NSCs After cultured 7 - 10 days, it can be observed number of neurospheres and volume was increased (Figure 1). Immunocytochemistry of Nestin display that the green fluorescence could be observed in spheres (Figure 2). 3.2. Transfection of NSCs When transfection was succeed the positive cells was observed red fluorescence by fluorescence microscope. Five fields were randomly selected from slide to count positive cells and total cell numbers to calculate transfec- tion efficiency. After 24 h, 48 h and 72 h, the transfection efficiency was 20% - 30%, 40% - 55% and 60% - 75%, respectively (Figure 3(E)). After one day, the cells added differentiation solution were observed a large number of death. Figure 1. Observed under the light microscope, visible spherical neural stem cells, refraction and strong, clear boundaries. Scale bar = 50 μm. 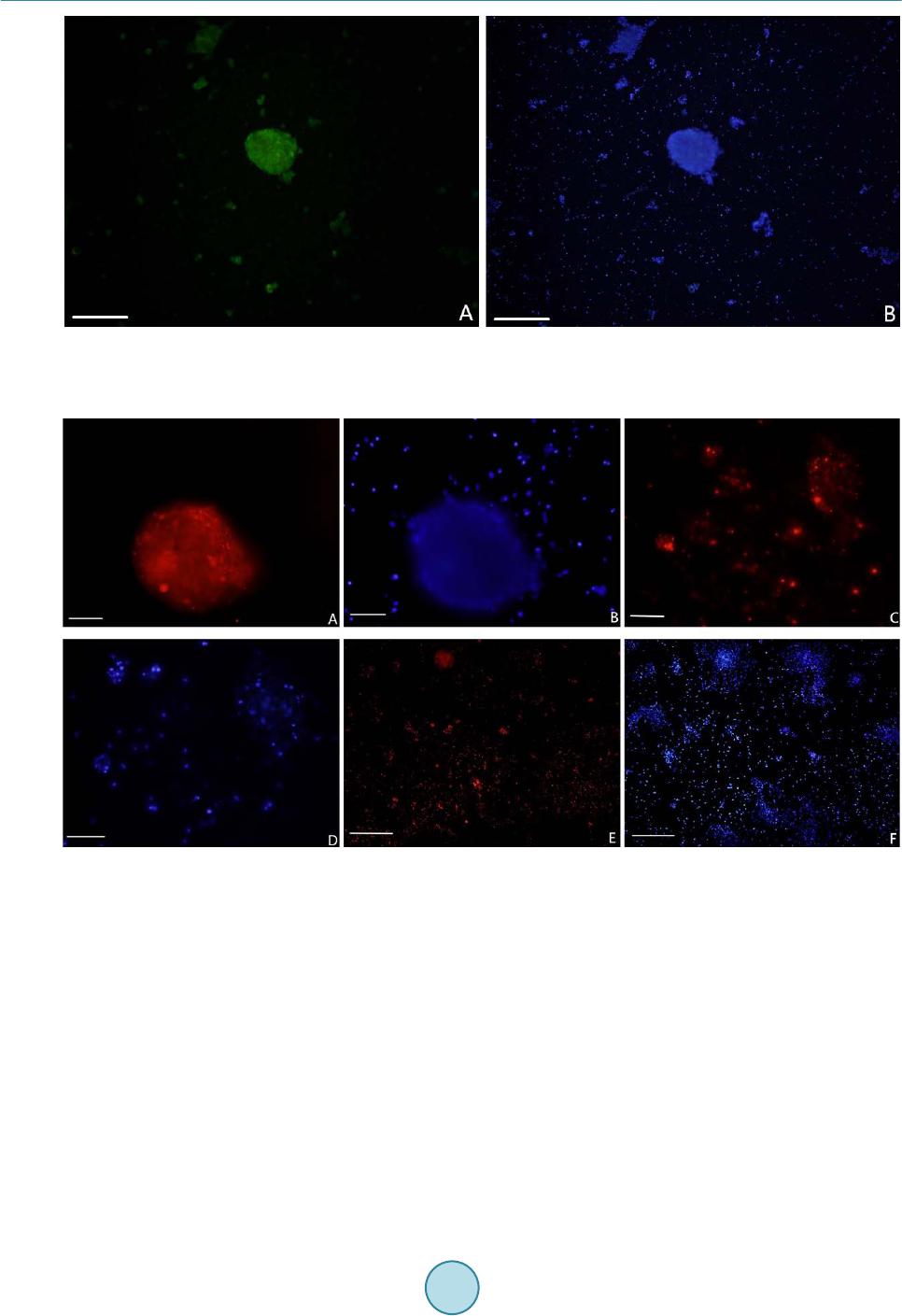 X. Li et al. Figure 2. The photos of neural stem cell identification. (A) Immunofluorescent staining indicated that Nestin was stained in the cytoplasm with a clear nuclear boundary. Scale bar = 100 μm. (B) Immunofluorescent staining of Hochest indicated that the blue areas were nucleus. Scale bar = 100 μm. Figure 3. The transfection efficiency of RNAiMAX. As we can see in the photo, this effect will last with the time going on. (A) and (B) The first day, only a few of cells transfected successfully. Scale bar = 50 μm. (C) an d (D) The second day, more cells was transfected successfully. Scale bar = 50 μm. (E) and (F) At the third day the efficiency is the highest. Scale bar = 100 μm. 3.3. Expression of ChAT After miR-31 were induced to differentiate into NSCs, ChAT stain was employed. The red fluorescence was seen under fluorescent microscope was ChAT positive cells. Compared with each group, the inhibitor group can emit stronger fluorescence (Figure 4(B)), and the mimics group was significantly lower than inhibitor group (Figure 4(A)). 3.4. Expression of miRNA and mRNA In Figure 5, RT-PCR assessment showed that expression of interference group was 0.006 and the interference control group was 0.181 higher than interference group; the highest is over-expression group, the expression was 21.081 higher than the over-expression control group was 0.726. 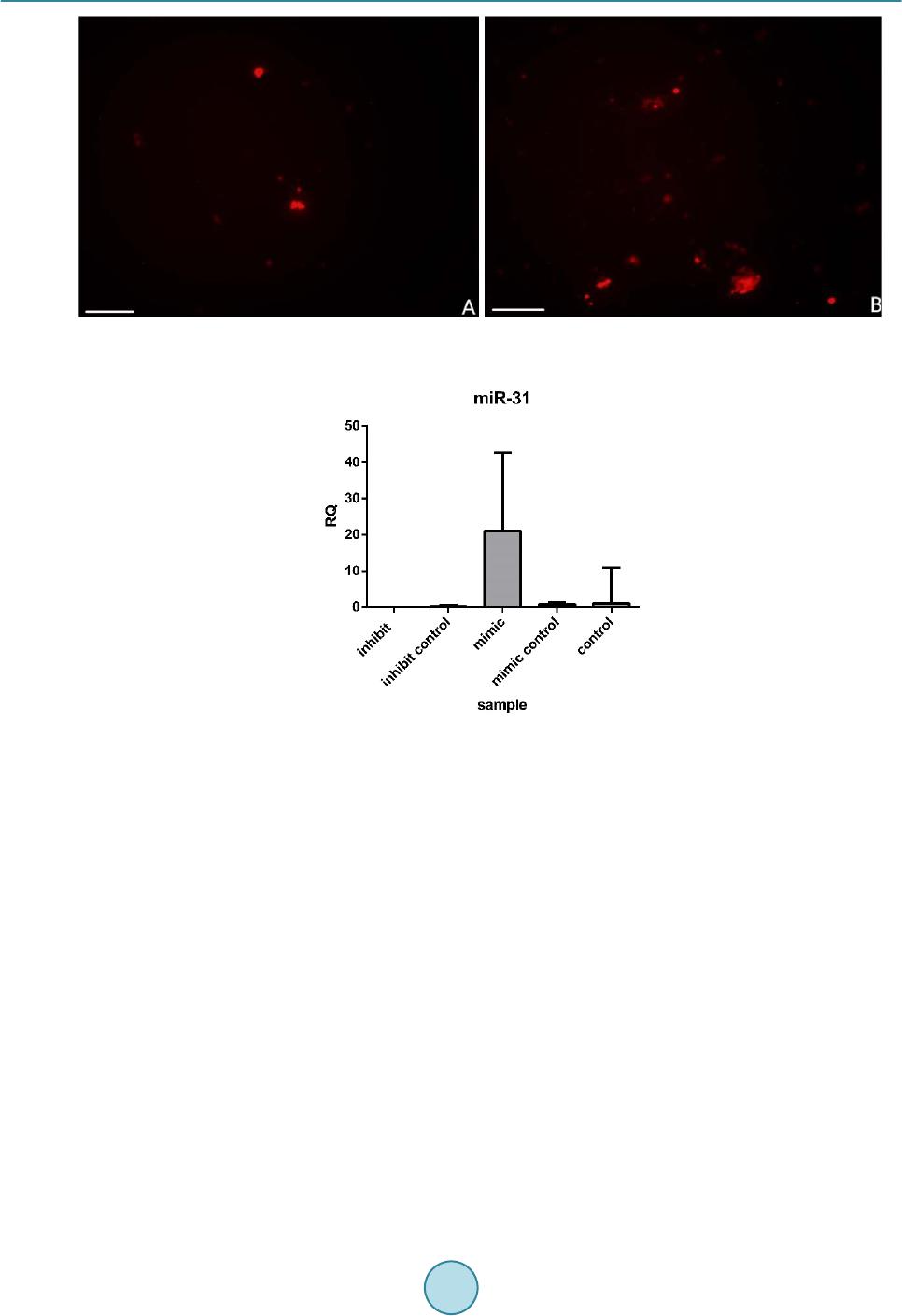 X. Li et al. Figure 4. ChAT immunocytochemical identification. (A) There was weak positive expression in the mimics group. (B) A strongly positive expression was shown in the inhibitor group. Scale bar = 100 μm. Figure 5. The results of induction efficiency. Compared with control group, the expression of miR-31 in mimics group was 0.006, and the expression of miR-31 in inhibitor group was 21.081. As shown in the Figure 6, the expression of genes were different among groups. In interference group, hb9 and stmn1 were up -regulated and Nestin was down-regulated. In over-expression group, Nestin, hb9 and stmn1 were up-regulated. 4. Discussion MicroRNAs play an important role in gene regulated, it binds to complementary sites specifically on the 3’-untranslated regions of the target mRNAs to induce cleavage or repression of translation. In this way, miR-31 regulated protein synthesis to impress protein functional expression. It can be participated in many signaling pathway to control the development process of the individual and the physiological and pathological process. There are many studies about miRNA already, but their specific roles in physiological process are exploring. Stem cell therapy is currently one of the promising approaches as many studies with a strong potential to promote functional recovery after SCI. It was being reported that transplantation therapy of stem cells has very big effect for spinal cord injury mouse. In our previous study, we found that injected NSCs from spinal to lesion location and it will promote the recovery, BBB score of treatment group obviously surpassed to the injury group. How to make more cells differentiated into neurons that had a positive effect on the functional outcome is the vital problem. Previous studies have shown that the level of miR-31 expression in NSCs was much higher than that in MNs. It also had been reported that miR-31 is an embryonic stem cell specific microRNA. So, miR-31 may be play an important role in maintaining NSCs in an undifferentiated state.  X. Li et al. Figure 6. The results of relative gene expression. So far, it is well accepted that quantity research of miR-31 related to cancer. Compared with normal tissues, miR-31 up-regulated at head and neck cancer (Wong et al, 2008) [7], colorectal cancer (motoyama et al, 2009; wang CJ et al, 2009) [8] [9], lung cancer (Liu X et al, 2010) [10] and so on; and down-regulated at breast cancer (Valastyan S et al, 2009) [11], prostatic cancer (Schaefer A et al, 2010) [12], gastric cancer (Guo J et al, 2009; Zhang Y et al, 2009) [13] [14 ]. Although there are a lot of research about miR-31, most of them are from the study of the process of disease. miRNA regulated signaling pathway to affect a series of protein in a biological processes. However, the effect that signaling pathways of miR-31 have yet to be fully defined. The NSCs from spinal cord is a kind of NSCs, but its homology is better than other NSCs. Cell fate decisions can be regulated, so we hope that by regulate the expression of miR-31 in the spinal cord make the NSCs proli- feration instead of NSCs injection treatment after spinal cord injury. We picked Nestin as the biomarker of NSCs and high expression at motor neurons hb9 and Stmn1. The goal of the study is to detect the expression of Nestin, hb9 and stmn1 after NSCs transfected miR-31 mimics, then we analyzed to try to explain the function of miR-31 in regulating NSCs functions in vitro. Nestin is an intermediate filament protein, and it is expressed in dividing cells during the early stages of de- velopment in the CNS and peripheral nervous system. Therefore, it is widely used as a neuronal stem cell mark- er. It has been reported that the Nestin knockout mice were significantly smaller than their littermates, most of them will die at around 3 weeks after birth, and the survivor was sterile. Furthermore, a fraction of Nes-/-embryos already dead after E8.5 because neural tube defects.(Park D, 2010) [15] In our study, we found that the expres- sion level of Nestin increased 2.72 times compared with control group after transfected miR-31 mimics, and it d o wn-regulated 0.609 times when we transfected miR-31 inhibited. It illustrate that cells closer to stem cell state when miR-31 is overexpression. Stathmin 1 (STMN1) is a microtubule-depolymerizing molecule that possess the capacity to bind tubulin and interfere with microtubule dynamics. It can take part in the process of proliferation and differentiation of motor neurons. (Yamada K, 2010) [16]. Sylvie Ozon was proved that STMN1 also particip ate in development of cen- tral nervous system. (Ozon S, 2002) [17] From this research we are aware that stmn1 was up-regulate 2.831 times when we inhibited miR-31, and it demonstrate that NSCs will differentiate into motor neurons when miR-31 was inhibited. Mohamed proved that STMN1 is a potentially target gene, over expression of miR-31 in KF-TX cells will reduced STMN1 expression. But in our experiment, STMN1 was no significant changes when miR-31 was over expression. (Hassan MK, 2015) [18]. The homeobox gene Hb9 is expressed selectively by motor neurons in the developing vertebrate CNS. But in our study, its expressions all up-regulated weather we transfected mimics or inhibit. What gives rise to this situ- ation change has been an outstanding issue. We herein investigated the role of miR-31 in the differentiation of NSCs. By controlling expression of miR-31, we found that it is consistent miR-31 maintaining NSCs in an undifferentiated state. Meantime, there are some different also. However, further studies are needed to explain the changes. Firstly, we will take western blot to test the protein level of related genes to determine the effect of miR-31. Secondly, we will detect the ex- pression of other genes continually to study the signaling pathway of miR-31. The current studies of miRNAs signa l pathways have also made some progress, a variety of signaling pathways are regulated by one miRNA. Therefore, we may use the mediating role of signaling pathways of miRNAs to guide cell differentiation, pro-  X. Li et al. vide a new reference for the treatment of diseases. Acknowledgements The study was supported by a grant from the The National Natural Science Foundation of China (81371384) and by the Experimental Animals Special Fundation in Shanxi Province (2014k15, 2014k06). References [1] Lu , P., Woodruff, G., Wang, Y.Z., Hunt , L.G.M., Wu, D., Bo ehle, E., Ahmad , R., Poplawski, G., Brock, J., Goldstein, L.S.B. and Tuszynski, M.H. (2014) Long-Distance Axonal Growth from Human Induced Pluripotent Stem Cells after Spinal Cord Injury. Neuron, 83, 789-796. http://dx.doi.org/10.1016/j.neuron.2014.07.014 [2] Suzuki, S.O. and Goldman, J.E. (2003) Multiple Cell Populations in the Early Postnatal Subventricular Zone Take Dis- tinct Migratory P athways: A Dynamic Study of Glial and Neuronal Progenitor Migration. J Neurosci, 23, 4240-42 50. [3] Harraz, M.M., Xu, J.-C. and Guiberson, N. (2014) miR-223 Regulates the Differentiation of Immature Neurons. Mol Cell Ther., 2, 1-15. http://dx.doi.org/10.1186/2052-8426-2-18 [4] Niu, C.S., Yang, Y. and Cheng, C.-D. (2013) mi R-134 Regulates the Proliferation and Invasion of Glioblastoma Cells by Reducing Nanog Expression. 42, 1533-1540. [5] Mor, E., Kano, S.-I., Colantuoni, C., Sawa, A., et al. (2013) microRNA-382 Expression Is Elevated in the Olfactory Neruoepithelium of Schizophrenia Patients. 55, 1-10. [6] Wei , H.E., Wang, C.F. , Zhang, C.S., Li, P.F. , Wang, F. and Zhang, Z.Y. (2010) Comparative Profiling of microRNA Expression between Neural Stem Cells and Motor Neurons in Embryonic Spinal Cord in Rat . Int. J. Devl Neuroscience, 28, 545-551. http://dx.doi.org/10.1016/j.ijdevneu.2010.04.007 [7] Wong, T.S., Liu, X.B., Wong, B.Y., Ng, R.W., Yuen, A.P. and Wei, W.L. (2008) MaturemiR-184 as Potential Onco- genic microRNA of Squamous Cell Carcinoma of Tongue. Clin Cancer Res, 14, 2588-2592. http://dx.doi.org/10.1158/1078-0432.CCR-07-0666 [8] Motoyama, K., Inoue, H., Takatsuno, Y., Tanaka, F., Mimori, K., Uetake, H., Sugihara, K. and Mori , M. (2009) Over - and Under-Expressed microRNAs in Human Colorectal Cancer. Int J Oncol, 34, 1069-1075. [9] Wan g, C.J., Zhou , Z.G., Wang, L., Yang, L., Zhou, B., Gu, J., Chen, H.Y. and S un , X.F. (2009) Clinicopathological Significance of microRNA-31, -143 and -145 Expression in Colorectal Cancer. Dis Markers, 26, 27-34. http://dx.doi.org/10.1155/2009/921907 [10] Liu , X., Sempere, L.F., Ouyang, H., Memoli, V.A., Andrew, A.S., Luo, Y., Demidenko, E., Korc, M., Shi, W., Preis, M., Dragnev, K.H., Li, H., D ir enzo, J., Bak, M., Freemantle, S.J., Kauppinen, S. and Dmitrovsky, E. (2010) Micro- RNA-31 Functions as an Oncogenic microRNA in Mouse and Human Lung Cancer Cells by Repressing Specific Tu- mor Suppressors. J Clin Invest, 120 , 1298-1309. http://dx.doi.org/10.1172/JCI39566 [11] Val astyan, S., Benaich, N., Chang, A., Reinhardt, F. and Weinberg, R.A. (2009) Concomitant Suppression of Three Target Genes Can Explain the Impact of a microRNA on Metastasis. Genes Dev., 23, 2592 -25 97. http://dx.doi.org/10.1101/gad.1832709 [12] Sch aefer, A., Jung, M., Mollenkopf, H.J., Wagner, I., Stephan, C., Jentzmik, F., Miller, K., Lein, M., Kristiansen, G. and Jung, K. (2010) Diagnostic and Prognostic Implications of microRNA Profiling in Prostate Carcinoma. Int J Can- cer, 126, 1166-1176 . [13] Gu o, J., Miao, Y., Xiao, B., Huan, R., Jiang, Z., Meng, D. and Wang, Y. (2009) Differential Expression of microRNA Species in Human Gastric Cancer Versus Non-Tumorous Tissues. J Gastroenterol Hepatol, 24, 652-657. http://dx.doi.org/10.1111/j.1440-1746.2008.05666.x [14] Zhang, Y., Guo, J., Li, D., Xiao, B., Miao, Y., Jiang, Z. and Zh uo , H. (2010) D own -Regulation of miR-31 Expression in Gastric Cancer Tissues and Its Clinical Significance. Med Oncol, 27, 685-689. http://dx.doi.org/10.1007/s12032-009-9269-x [15] P ark, D., Xiang, A.P., Mao, F.F., Zhang, L., Di, C.G., Liu, X.M., Shao, Y., Ma, B.F., Lee, J.H., Ha, K.S., Walton, N. and Lahn , B.T. (2010) Nestin Is Required for the Proper Self-Renewal of Neural Stem Cells. Stem Cells, 28, 2162 - 2171. http://dx.doi.org/10.1002/stem.541 [16] Yamad a, K., Matsuzaki, S., Hattori, T., Kuwahara, R., Taniguchi, M., Hashimoto, H., Shintani, N., Baba, A., Kuma- moto, N., Yamada, K., Yoshikawa, T., Katayama, T. and Tohyama, M. (2010) Increased Stathmin1 Expression in the Dentate Gyrus of Mice Causes Abnormal Axonal Arborizations. PLoS One, 5, e8596. http://dx.doi.org/10.1371/journal.pone.0008596 [17] Ozon , S., Guichet, A., Gavet, O., Roth, S. and Sobel, A. (2002) Drosophila Stathmin: A Microtubule-Destabilizing Factor  X. Li et al. Involved in Nervous System Formation. Mol Biol Cell, 13, 698-710. http://dx.doi.org/10.1091/mbc.01-07-0362 [18] Hassan, M.K., Watari, H., Mitamura, T., Mohamed, Z., El-Khamisy, S.F., Ohba, Y. and Sakur agi, N. (2015) P18/Stath- min1 Is Regulated by miR-31 in Ovarian Cancer in Response to Taxane. Oncosci ence, 2, 29 4 -308. http://dx.doi.org/10.18632/oncoscience.143
|