 Open Access Library Journal How to cite this paper: Cui, T.T., Wang, C., Shan, C.S. and Wu, P. (2014) Optimization of the Extraction Technology of Chlo- rogenic Acid in Honeysuckle by Response Surface Method. Open Access Library Journal, 1: e941. http://dx.doi.org/10.4236/oalib.1100941 Optimization of the Extraction Technology of Chlorogenic Acid in Honeysuckle by Response Surface Method Tingting Cui1*, Chao Wang2, Changsong Shan1, Peng Wu1* 1College of Food Science and Technology, Shandong Agriculture University, Taian, China 2College of Horticulture Science and Engineering, Shandong Agricultural University, Taian, China Email: *13954847 828@163. co m, *157538415 19@163.co m Received 3 June 2014; re vi sed 13 July 2014; ac cept ed 23 August 2014 Copyright © 2014 by authors and OALib . This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativ ecommon s.org/l icens es/by/4.0/ Abstract The ultrasound-assisted extraction of chlorogenic acid from honeysuc kle was modeled using re- sponse surface methodology. A three-level -thr e e-factor Box–Behnken design was employed to op- timize three extraction variables, including extraction temp er ature (X1), ethanol concentration (X2), and soak time (X3), for the achievement of high extraction yield of the chlorogenic acid. The optimized conditions are extract temperature of 80˚C, ethanol concentration of 70.37%, soak time of 9.35 h. Under this optimized conditions, the experimental yiel d of chlorogenic acid is 1.918%, which is well matched with the predicted yield of 1.929%. Keywords Design-Expert Soft wa re, Lonicera Japonica, Chlorogenic Ac id , Ext rac ti o n Technology Op tim izati on Subject Areas: Analytical Chemistry, Food Science & Technology 1. Introduction Honeysuckle, the flower-bud or flower of Lonicera japonica Thund which also c a lle d double flower, tastes fresh and sweet. It is widely used for the treatment of tumors, bacterial dysentery, cold, pain, sores, carbuncles, furun- cles, swelling and fever disease caused by influenza virus [1]. Studies have been found that honeysuckle con- tains many kind s of antiox idant and anti-tumor compounds such as chlorogenic acid, flavonoid, triterp enoid sa- ponins, polysaccharides and volatile oil [2]. Chlorogenic acid, as the maj o r bio ac ti ve c ons t ituent o f ho ne ys uc kl e, has been shown the function of anti-bacterial anti-inflammatory, liver-protection, hypolipidemic effect, detoxi- *  T. T. Cui et al. OALibJ | DOI:10.4236/oalib.1100941 2 September 2014 | Volume 1 | cation and anti-mutagenic and it is b enefit to the immun e system pharmacolo gical activities [3]. So it is impor- tant to study the effective extraction of chlorogenic acid from honeysuckle. Design -Expert, as the most comprehensive, affinity design software, has functions such as statistical a nalysis of test data; establishing the mathematical model, fit curve equation, prediction and optimizatio n of the test re- sults. Besides, two-dimensional contour map and 3D graphics also can be drawn by it [4]. Present, the design software has been widely used in all kinds of experimental design and analysis. Wu Jiahui studied enzyme-as- sisted extraction of lentinan by using this soft wa r e [5]; the corn fertilizer production mushroom mycelium was studied by Lee S. through response surface method [6]. As a new extraction technology, it is widely u sed in the extr ac tion of natural p ro ducts, hea t sensiti ve material, particularly. In addition, ultrasonic extraction can protect active material; short extraction time, and improve ex- traction efficiency. Assisted by the response surface design of ultrasonic extraction method not only improves the chlorogenic a cid yield but also simplifies the experiment pro c e ss [7] [8 ]. In order to seek the best extraction method of chlorogenic acid in Lonicera japonica to provide theoretical reference, the single factor and center combining with experiment and the software of Box-Behn ken ce nter was adopted in this research. 2. Experimental 2.1. Materials and Chemicals Purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, Chi- na), Chlorogenic acid (J & K, purity is 98%) was dissolved in 95% ethanol and stored at −20˚C. Other reagents were analytical grade and purchased from Tianjin Chemical Reagent Factory (Tianjin, China). Distilled water was used t hroughout the whole expe riment. Hone ysuckl e used in thi s stud y was harve sted fro m Xint ai T ai’an (T ai’a n China ). Dri ed hone ysuck le cr ushed in Micro P lant Grinding Machine and t hen sieved through a wire mesh to re move the midrib materials. Repre- sentative 1.000 g samples were weighed on an electronic analytical balance TW 223L from the Shimadzu Japan Instruments Co., Ltd. 2.2. Apparatus and Equipments Sonication of samples was done using an Ultrasonic Generator 2510E-DTH (245 watts, 42 kHz) purchased from Branson Ultrasonic Corporation in USA. An automatically adjustable HH-4 Constant Temperature Water Bath from Guohua Electric Appliance Co., Ltd. was used to control the extraction te mperat ure. A labora tory thermo- meter was used to monitor the effectiveness of the temperature control. The crude extracts were centrifuged in a X1R High Speed Multifuge from Beijing Aoheng Technology Co., Ltd. and then filtered. The filtered extracts were analyzed by an UV 2430 spectrophotometer from Shimadzu Japan Instruments Co., Ltd. 2.3. Ultrasonic Extraction One gram of dried Honeysuckle powder was weighted accurately to a flask, and then 30 mL ethanol was added to the Erlenmeyer flask which was sealed with silver paper. The powder was extracted in an ultrasonic generator for different extraction time and temperature at various ethanol concentrations. After ultrasonic treatment, the extracted slurry was centrifuged at 3000 rpm for 20 min to collect the s upernatant and th en transfer to fla sk vo- lumetric. 2.4. Determination of Chlorogenic Acid Yield The total chlorogenic acid content in the supernatant was determined using Chlorogenic acid-sta nda rd curve method with a slight modification. And then the percentage of chlorogenic acid yield is calculated as follows: 3 0.0081 10 52.533 100% AVN YM − −×× × = × (1) In the above formula: Y is percentage chlorogenic acid yield (%), A is absorbance value of sa mple so luti on, V is volume (mL) of extract supernatant, N is dilution ratio, and M is the quantit y of honeysu ckle ( g).  T. T. Cui et al. OALibJ | DOI:10.4236/oalib.1100941 3 September 2014 | Volume 1 | 2.5. Experimental Design A thr ee -level-thr ee -factor, Box-Be hnke n Design (BBD) was employed to determine the best combination of ex- traction variables for the chlorogenic acid compounds based on the results of preliminary single-factor-test [9] [10]. E xtraction temperature (X1) , ethanol concentration (X2), and soak time of raw material (X3) were the inde- pendent variables, and their coded and uncoded levels were presented in Table 1. Extraction yield (Y) taken a s the response for t he de s ign experiment was give n in Table 2 . 2.6. Statistical Analysis Each test was repeated three times, and calculated the averaged. SPSS software version 16.0 and Sigma Plot 10.0 was used to evaluate the analysis of variance (ANOVA) and draw the fi gures, respectively. And then the trend charts, responds surface and contour plots of various facto rs on the chlorogenic acid yield were draw. 3. Results 3.1. Optimization of Maximum Absorption Wavelength for Chlorogenic Acid According to the method of 2.4, the maximum absorption wavelength of chlorogenic acid was 324.3 nm and the standard curve equation was y = 52.533x + 0.0081, R2 = 0.9997. As can be seen from Figure 1, the results sho we d that the chlorogenic acid concentrat ion within 0 - 0.02 mg/mL meet good linear range relationship. 3.2. Result of Single-Fa cto r-Test 3.2.1. Effect of Ethanol Concentration on Ex t rac ti on Yield of Chlorogenic Acid Extraction process was carried out at different ethanol concentration of 50%, 60%, 70%, 80% and 90%, while other parameters were as following: extraction time 50 min, soak time 6 h and extraction temperature 70˚C. The Table 1. Independent variables and their levels for Box-Behnken design. Independent variables Levels −1 0 1 Ext r action temp erature (X1) (˚C) 60 70 80 Etha nol concentration (X2) (%) 60 70 80 Soak time (X3) (h) 6 9 12 Table 2 . E f fect of different response s urface design of extraction rate of chlorogenic acid. Run X1 Extraction temp er atu re (˚C) X2 Ethanol concentration (%) X3 Soak time (h) Y Extraction yiel d (%) 1 70 60 6 1.634 2 60 60 9 1.594 3 60 80 9 1.446 4 70 60 12 1.574 5 70 70 9 1.893 6 70 80 6 1.494 7 70 70 9 1.910 8 80 80 9 1.767 9 60 70 6 1.533 10 60 70 12 1.475 11 80 70 12 1.806 12 80 60 9 1.733 13 70 70 9 1.904 14 80 70 6 1.713 15 70 80 12 1.534 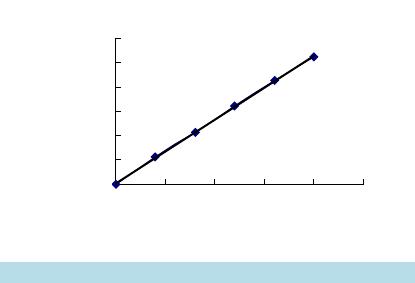 T. T. Cui et al. OALibJ | DOI:10.4236/oalib.1100941 4 September 2014 | Volume 1 | Figure 1. The curve of standard chlorogenic acid sample. effect of ethanol concentration on extraction yield of chlorogenic acid is shown in Figure 2(a ). In the initial stage, along with the ethanol concentration increased from 50% to 70%, the extraction yield of chlorogenic acid increased rapidly; while ethanol concentration greater than 70% chlorogenic acid extraction yield was showing slow decreasing trend, and peak at 70% ethanol concentration. This is because the increase of ethanol concentra- tion leads to enhanced ma ss transfer dyna mics, solvents and honeysuckle getting full acc ess, and then the co n- tents of chlorogenic acid dissolved increased [11]. When the ethanol concentration reached a certain level, some of chlorogenic acid was difficult to be dissolved by high co ncentrat ion o f ethanol, and al so lead to the increase of the alcohol-soluble imp urity co ntent, r es ultin g in a loss o f chlo ro genic acid co nte nt [1 2]. Moreover, the great- er of ethanol concentration, the more difficult to refine chlo rogenic ac id and it will cause wasted and t he cost of production increased. Therefore, the ethanol concentration of 70% is good for the chlorogenic acid extraction. 3.2.2. Effect of Extraction Tempera tu re on Extraction Yield of Chlorogenic Acid Extraction process was carried out using extraction temperature from 40 to 80˚C, while other parameters were as following: Ethanol concentration 50% (v/v), soak time 6 h and extr action t ime 30 min. As shown in Figure 2(b), extraction temperature has obvious effects on yield of chlorogenic acid. When extraction temperature increased, the extraction yield increased rapidly and reached a maximum at 70˚C, when extraction temperature went above 70˚C, the extraction yield starte d to decrea se. At initiall y, extractio n yield increasi ng with the rising o f temper a- ture may be that elevated temperature accelerated the chlorogenic acid chemical bond rupture and speeded mo- lecular motion, so that a large number of chlorogenic acid in cell dissolution into the soluti on [13]. whe n hea ti n g temperature greater than 70˚C, high tempe rature cause d t he des truct io n of c hlor ogenic acid structure, accelerated the degradation reaction, and lost chlorogenic acid activity, and then Chlorogenic acid content is rapidly reduced [14]. Therefore, 70˚C is favorable for extracting the chlorogenic acid. 3.2.3. Effect of Extraction Time on Extraction Yield of Chlorogenic Ac id Extraction process was carried out using extraction time from 20 to 60 min, while other parameters were as fol- lo win g: ethano l c once ntr a tion 5 0% (v/ v) , soak time 6 h and extraction temperature 70˚C. T he experiment results showed that 50 min is the optimum extraction time of the chloro genic a cid, as sho wn in Figure 2(c). When ex- traction time increased, the cell walls of honeysuckle got fully fall apart and chlorogenic acid got into material liquid diffusio n so that the extraction yield is relativel y rapid [15]. Dur ing lon g extract ion t ime, hone ysuckl e lo- cal overheating was prone to cause thermal decomposition of chlorogenic acid, because of the unstable chemical bonds of chlorogenic acid molecular, such as eater bond, unsaturated bond and polyphenol [16]. And then the honeysuckle content was decreased. Therefore, 50 min is favorable for extracting the chlorogenic acid. 3.2.4. Effect of Soak Time on Extraction Yield of Chlorogenic Acid Extraction process was carried out at different soak time of 3 h, 6 h, 9 h, 12 h, and 15 h, while other parameters were as following: ethanol concentration 50% (v/v), extraction time 50 min and extraction temperature 70˚C. The experiment results were shown in Figure 2(d). When soak time increased, the extraction yield was rela- tively rapid and reached a maximum at 9 h, and then decreased as the extraction proceeds. This is because the chlorogenic acid can soluble in organic solvents, such ethanol and so on [17]. Increasing the immersion time y = 52.533x + 0.0081 R2 = 0.9997 0.000 0.200 0.400 0.600 0.800 1.000 1.200 0.000 0.005 0.010 0.015 0.020 0.025 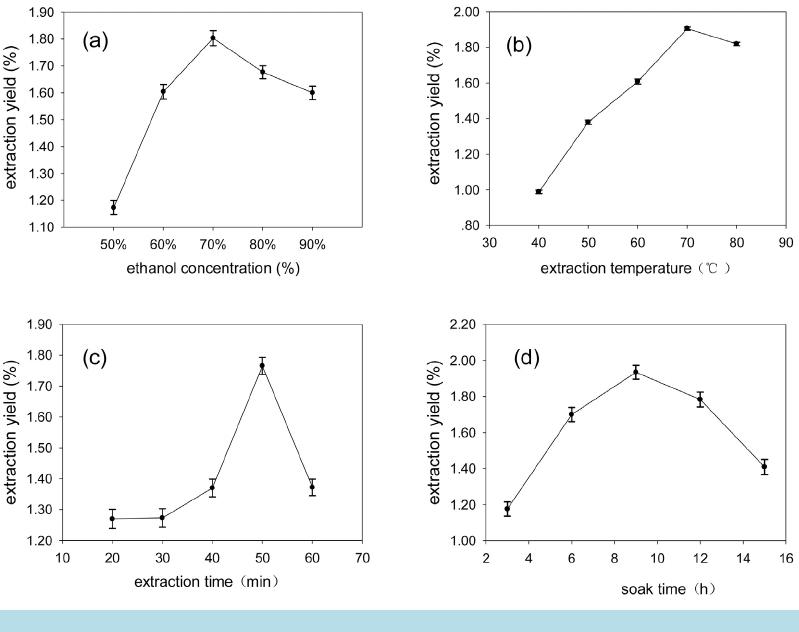 T. T. Cui et al. OALibJ | DOI:10.4236/oalib.1100941 5 September 2014 | Volume 1 | Figure 2. Effect of differe nt extract ion variables on ext raction yield. made full chlorogenic acid dissolution, so chlorogenic acid content increased. However, once again increasing the immersion ti me increased the lead to impurities in soluti on, and the leaching of impurities ad sorbed portion of chlorogenic acid, resulting in loss of yield lower [18]. Therefore, 9 h is favorable for extracting the chloro- genic acid. 3.3. Optimization of Extraction Parameters for Chlorogenic Acid Table 2 showed the process variables and experimental data of 15 runs containing 3 replicates at center point. B y ap pl yi ng multiple regression analysis on the experimental data, the model for the response variable could be expressed by the following quadr atic po lynomial equation in the form of coded values: 1 2312 222 1323 123 y9.79065 0.10483X0.19496X0.20017X0.000456XX 0.001263XX 0.000834XX0.000971X 0.001700X 0.019246X =−+ +++ + +−−− (2) Analysis of variance (ANOVA) for the model was shown in Table 3. The determination coefficient (R2 = 0.9963) indicated that only 0.37% of the total variations were not explained by the model. For a good statistical model, the adjusted determination coefficient (R2adj) should be clo se to d eter mination co efficie nt (R2) . As s h o wn in Table 3, R2adj (0.9896) was close to R2. Moreover, R2pred (0.9462) was in reasonable agreement with R2adj and the results confirmed that the model was highly significant. At the same time, a relatively low value of coeffi- cient of variation (CV) (0.99) indicates a better precision and reliability of the experimental values. Therefore, the model is adequate for prediction in the range of experimental variables. As can be seen from Table 4, the lack of fittest determines whether the selected model is adequate to explain the experimental data, or whether another model should be reselected. The significance of each coefficient measured using p-value and F-value is listed i n Table 4. S maller p -value and greater F-value mean that the cor- responding variables would be more significant. The p-value of the model is less than 0.0001, which indicates that the model is si gnificant and c an be used to op timize the extraction variables. The one independent variables (X1), interaction terms(X1X2, X1X3) and three quadratic terms (X12, X22 and X32) significantly (P < 0.01) affect  T. T. Cui et al. OALibJ | DOI:10.4236/oalib.1100941 6 September 2014 | Volume 1 | Table 3 . The correlation analysis of model. R2 0.9963 R2adj 0.9896 R2pre d 0.9 462 C.V. % 0.9900 Table 4 . Significan ce test of regression co efficients. Source Sum of squa r es Degree of freedom Mean squ are F-value P-value (Pr > F) Mod el 0 .3700000 9 0.0410 0000 1 49.66 < 0.00 01 X1 0.1200 000 1 0.12000000 4 33.31 < 0.00 01 X2 0.0110 000 1 0.01100000 3 9.49 0 .0015 X3 0.0000 251 1 0.00002500 0.092 0.7738 X1X2 0.0083410 1 0.0083 4100 3 0.59 0 .0026 X1X3 0.0057500 1 0.0057 5000 2 1.09 0 .0059 X2X3 0.0025050 1 0.0025 0500 9.18 0.0291 X12 0.0350 000 1 0.03500000 1 27.82 < 0.00 01 X22 0.1100 000 1 0.1 100000 0 391 .67 <0 .0001 X32 0.1100 000 1 0.1 100000 0 406 .29 <0.00 01 residual 0.0013630 5 0.0002727 0 Lack of fit 0.0012 190 3 0.0004062 0 5.61 0.1549 Pure error 0.0 001447 2 0.0000723 5 Total error 0.3 700000 14 the extraction yield within a 99% confidence interval. Meanwhile, extraction time (X1) is the most significant factor affecting the extraction yield. 3D response surface and 2D contour plots are the graphical representations of regression equation and they are very useful to judge the relationship between independent and dependent variables. Different shapes of the contour plo ts indicate whet her the mutual interactio ns between the variables are significant or not. Circular con- tour plot means the interactions between the corresponding variables are negligible, while elliptical contour suggests the interactions between the corresponding variables are significant. The three-dimensional representa- tion of the r esponse surfaces and two-dimensional contours generated by the model are shown in Figures 3-5. In these three variables, when two variables are depicted in three-dimensional surface plots, the third variable is fixed at zero level. It is fou nd in Figures 3-5 that all the three response surfaces are convex in shape, which in- dicates that the ranges of variables were chosen properly. As sho wn in Figure 3, extraction yield increased rapidly when extraction temperature (X1) and ethanol con- centration (X2) increased in t he range of 60˚C - 75˚C and 6 5% - 75%, respectively; but beyond 75˚C and 75%, extraction yield decreased slightly. The results demonstrated that the effect of extraction temperature (X1) and ethanol concentration (X2) on extraction yield was significant and the results were in good agreement with the results in Table 4. Moreover, the elliptical contour plots in Figure 3 mean that there was a significant interac- tion between the two variables, which also agrees with the results in Table 4. As shown in Figure 4, both ex- traction temperature (X1) and soak time (X3) have quad ratic e ffect on extrac tio n yield . Ext ractio n yield increases at first and then decreased quickly with increasing of the two parameters, and a maximum extraction yield is achieved when extraction temperature (X1) and soak time(X3) are 70˚C and 9 h, respectively. It can be seen that the mutual interactions bet ween extraction temperature (X1) and soak time (X3) was si gnificant due to t he ellip- tical contour plots shown in Figure 4, which was also confirmed by the results in Table 4. It is obvious in Figure 5 that extraction yield increases slowly with the increasing of ethanol concentration (X2) and decreases slowly after 70%; while extraction yield increases rapidly with the increasing of soak time (X3) from 6 to 9 h and decreases rapidly after 9 h. The results suggested that the interactions between the two variables were not significant, which was in a gre ement with the contour plots in Figure 5 and t he results in Table 4.  T. T. Cui et al. OALibJ | DOI:10.4236/oalib.1100941 7 September 2014 | Volume 1 | Figure 3. Response su rface and contour plots showing effect of extraction temperature (X1) and ethanol concentration (X2). Figure 4. Response surface and contour plots showing effect of extraction temperature (X1) and soak time (X3). Figure 5. Respon se s urface and contour plots showing effect of ethanol concentration (X2) and soak time (X2). T. T. Cui et al. OALibJ | DOI:10.4236/oalib.1100941 8 September 2014 | Volume 1 | 3.4. Verification of the Model The suitabilit y of the model equation for p redicting the opti mum response values is tested using the selected o p- timum conditio ns. The optimum conditions are extraction te mperature (X1) of 80 ˚C, ethanol concentration (X2) of 70.37%, and soak time (X3) of 9.35 h, under which the predicted yield is 1.929%. However, considering the operab ility in actual prod uction, the optimum condition s are modified as following: extrac tion temperature (X1) of 80˚C, ethanol concentration (X2) of 70%, and soak time (X3) of 9 h, under which the experimental yield is 1.911% (n = 3). The theoretical prediction error is 1.13%, agreeing closely with the predicted yield. Conse- quentl y, ado pting the r espons e surface method t o seek the best co ndition o f extracti ng hone ysuckle chloroge nic acid is feasible, and has practical significance. 4. Conclusions and Discussion An ultrasonic-assisted extraction technology was performed for the extraction of chlorogenic acid from honey- suckle and optimized by RSM. Based on the single-factor-test, Box-Behnken design was used to evaluate and optimize the extraction variables (extraction temperature, ethanol concentration and soak time) for the extraction yield. The results showed that the variables (soak time and ethanol concentration) are significant and a high cor- relation of quadr atic model obtained is satisfactory and accurate to predict the extraction yield, which was simi- larity to the repo rted [19] [2 0]. The op timized conditions are as follows: extraction te mperature o f 80˚C, etha nol concentration of 70%, and soak time of 9 h, under which the experimental yield is 1.911% (n = 3), which is agreed closely with the predicted yield of 1.929%. Therefore, method for the extraction of chlorogenic acid plays an active guidance of theory. Chlorogenic acid is one of the main active substances of Lonicera japonica. In order to increase extraction yield of Chlorogenic acid, this experi ment test influence factors, such as soak time, which were rare reported. As shown in Figure 2(d), with the increase of soak time, chlorogenic acid yield increased; when the soak time is more than 9 h, extraction yield was slow down. This is because the chlorogenic acid can dissolve in organic solvent. Before extraction, the chlorogenic acid fully dissolve o ut after a certain a mount of time, and the exte n- sion of time not only lead to increased alcohol soluble impurities, but also the impurities can soak up some chlor ogenic ac id. Co mpar ed with t he honeys uckle witho ut soaki ng in related report , extract ion yields in the ex- periments greatly increased [21]. Yuan Fei [22] optimized the ultrasonic extraction process of chlorogenic acid in Lonicera japonica. The ultrasonic extraction conditions were that the soak time of 12 h and then ultrasonic extraction of 2 times; the chlorogenic acid yield after decompression enrichment was 2.110%, which higher 0.181% than this experi ment. This may be related to the extraction conditions and other objective factors. So we should take various factors into accounts during the experiment, and strictly control test conditions. Extraction times, as the influence factors of yield, should be tested during experiment, to determine the change trends on the yield of chlorogenic acid . Ultrasonic extraction not only can protect active substance from destruction, but also has many advantages, such as shorten extraction time, increase extraction efficiency, low cost and easy operation and so on. Hence, ul- trasonic extraction is widely used in natural products extraction, especially in the extraction of heat sensitive materials. Assisted by the response surface design of ultrasonic extraction method not only improves the chlo- roge nic acid yield, but also simplifies the experiment process. In recent years, the application of ultrasonic ex- traction tech nology is becoming more common in industrial extractio n o f chlorogenic a c id in Lonicera japonica. Combining response surface methodology and ultrasonic extraction not only improved the chlorogenic acid yield but also simpli fied the experiment pr ocess. In ord er to develop series ho neysuckle f unctional he alth pr od- ucts and ensure the steady development of the honeysuckle industry, deep processing and system development of this Lonicera japonica will provide scientific theoretical b a sis for it. Funding Supported by youth science and technology innovation foundation of Shandong Agricultural University (No.20120604). References [1] Chinese P harmacopoeia Commis s ion (2010) Chinese Pharmacopoeia. China Medical S cience Pres s, Beijing, 205 -206.  T. T. Cui et al. OALibJ | DOI:10.4236/oalib.1100941 9 September 2014 | Volume 1 | [2] Lee, E.J., Kim, J.S., Kim, H.P., et al. (2010) Phenolic Constituents from the Flowers Bud of Lonicera Japonica and Their 5-li-Poxygenase Inhibitory Activities. Food Chemistry, 120, 134-139. http://dx.doi.org/10.1016/j.foodchem.2009.09.088 [3] Bassoli, B.K., Cassolla, P., Borba-Mu rad, G.R., et al. (2008) Chlorogenic Acid Reduces the Plasma Glucose Peak in the Oral Gluco se Toleran ce Test. Ef fects on Hep atic Gluco se Rel eas e and Glycaemia. Cell Biochemistry and Function, 26, 320-328. http://dx.doi.org/10.1002/cbf.1444 [4] Macrco s, A.B., Ricardo , E.S., El iane, P.O., et al. (2008) Response Su rface Methodo logy (RSM) as a Tool for Optimi- zation in Analytical Chemistry. Talanta, 76, 965 -977. http://dx.doi.org/10.1016/j.talanta.2008.05.019 [5] Wu, J.H, Wan g, L., Ga o , F., et al. (2011) Study on En zyme-Assisted Extraction of Lentin an. Food and Fermentation Industries, 37, 20 1-205 (in Chinese). [6] Lee , S., Bae, H., Kim, N., et al. (2008 ) Optimization of Growth Condition of Lentinus Edodes Mycelium on Corn Processing Waste Using Respo nse Surface Analysis. Journal of Bioscience and Bioengineering, 105, 161 -163. [7] Huang, L., Tan g, J.L., Huang, J., et al. (2012). Research Progress in Application and Chlorogenic Acid Extraction. University Laboratory Study and Work, 113, 114 -11 7 (in Chinese). [8] Abraham, S.K. (1993) Protective Effects of Chlorogenic Acid, Curcuin in and a α-Carotene Against γ-Radiation In- duced in Vitro Chromosomal Damage. Mutation Research, 303, 10 -11. [9] Yong, L., Shoulian, W. and Miaochuan, L. (2013) Optimization of Ultrasonic Extraction of Phenolic Compounds from Euryale Ferox Seed Shells U sing Response Surface Method ology. Industrial Crops and Products, 49, 837 -848. http://dx.doi.org/10.1016/j.indcrop.2013.07.023 [10] Pinho, C., Melo, A., Mansilha, C. and Ferreira, I.M.P.L.V.O. (2011 ) Optimization of Conditions for Anthocyanin Hy- drolysis from Red Wine Using Response Surface Methodology (RSM). Journal of Agricultural and Food Chemistry, 59, 50-55. http://dx.doi.org/10.1021/jf103839j [11] Chen, W., Wang, W.P., Zhang, H.S. and Huang, Q. (2012) Optimization of Ultrasonic-Assisted Extraction of Water- Solub le Pol ysaccharid es fro m Boletus edulis Mycelia Using Response Surface Methodology. Carbohydrate Polymers, 87, 614-619. http://dx.doi.org/10.1016/j.carbpol.2011.08.029 [12] Carrera, C., Ruiz-Rodríguez, A., Palma, M. and Barroso, C.G. (2012) Ultrasound Assisted Extraction of Phenolic Compounds from Grapes. Analytica Chimica Acta, 732, 100 -104. http://dx.doi.org/10.1016/j.aca.2011.11.032 [13] Li, Q. and Fu, C. (2005) Application of Response Surface Methodology for Extraction Optimization of Germinant Pum pk in Seeds Pro te i n. Food Chemistry, 92, 701-706 . http://dx.doi.org/10.1016/j.foodchem.2004.08.042 [14] Sheng, Z.L., Wan, P.F., Dong, C.L. and Li, Y.H. (20 13 ) Optimization of Total Chlorogenic Content Extracted from Flos Populi Using Response Surface Methodology. Industrial Crops and Products, 43, 778-78 6. http://dx.doi.org/10.1016/j.indcrop.2012.08.020 [15] Ferreira, S.L., Bruns, R.E., Ferreira, H.S., Matosa, G.D., Davida, J.M. , Br andão, G.C. , et al. (2007) Box-Behnken De- sign : An Alternative for the Optimization of Analytical Meth ods. Analytical chemistry Acta, 597, 17 9-186. http://dx.doi.org/10.1016/j.aca.2007.07.011 [16] Spigno, G. and De Faveri, D.M. (2009) Microwave-Assisted Extraction of Tea Phenols: A Phenomenological Study. Journal of Food Engineering, 93, 210-21 7. http://dx.doi.org/10.1016/j.jfoodeng.2009.01.006 [17] Iwanhas, H., Negor o, Y., Iked a, A., Morishita, H. and Kid o , R. (1986) Inhibition by Chlorogenic Acid of H aematin - Catal ysed Retinoic Acid 5, 6-Epoxid atio n . The Bioche m ic al Jour nal, 239, 641 -646. [18] Zhang, L., Liu, J ., Zhang, P., Yan, S., He, X. and Chen , F. (2011 ) Ionic Liquid-Based Ultrasound-Assisted Extraction of Chlorogenic Acid from Lonicera japonica Thunb. Chromatographia, 73, 129-133. http://dx.doi.org/10.1007/s10337-010-1828-y [19] Merab et, S., Robert, D., Weber, J.V., Bouhelassa, M. a nd Benkhanouche, S. (2009) Photocatalytic Degradation of In- dole in UV/TiO2: Optimization and Modeling Using the Response Surface Methodology (RSM). Environmental Che- mistry Letters, 7, 45 -49. http://dx.doi.org/10.1007/s10311-008-01 37 -2 [20] Zhu, C. and Liu, X. (2013 ) Optimization of Extraction Process of Crude Polysaccharides from Pomegranate Peel by Response Surface Methodology. Carbohy dr ate P oly m e r s , 92, 1197-1202. http://dx.doi.org/10.1016/j.carbpol.2012.10.073 [21] Song, D., Lia n g, Y. and Yan g, G. (2010) Optimization on the Ultrasonic Treat ment E xtract in g P rocess for Chlorogenic Acid from Lonicera japonica Thumb by Orthogon al Design . Journal of Animal and Veterinary Medicine, 20 10, 3 7-40 (In Chinese). [22] Yuan, F. (2012) Orthogonal Test Design for Optimization of Ultrasonic Extraction Process of Chlorogenic Acid from Honeysuckle. Chinese Journal of Chinese Guide, 2012 , 913-91 6 (In Chinese).
|