 Pharmacology & Pharmacy, 2011, 2, 180-188 doi:10.4236/pp.2011.23026 Published Online July 2011 (http://www.scirp.org/journal/pp) Copyright © 2011 SciRes. PP Intranasal Delivery of Two Benzodiazepines, Midazolam and Diazepam, by a Microemulsion System Shafir Botner, Amnon C. Sintov* Department of Biomedical Engineering, Faculty of Engineering Sciences, Ben Gurion University of the Negev, Beer Sheva, Israel. Email: asintov@bgu.ac.il Received January 2nd, 2011; revised May 4th, 2011; accepted June 23th, 2011. ABSTRACT Nasal application of benzodiazepines might be an alternative to intravenous administration in acute clinical situations such as seizures emergencies. However, irritation and pain as well as symptoms like teary eyes, dizziness, discomfort, nasal drainage and bad taste usually accompany subject received midazolam and diazepam via the nasal route. The purpose of this study was to evaluate the use of a new alcohol-free microemulsion system as a carrier for diazepam or midazolam given intranasally. Midazolam (base) or diazepam was solubilized in the microemulsion to obtain a high drug concentration of 25 mg/g (2.5% by weight), to provide 2.5 mg drug in 100 µl spray (d ≈ 1.00 g/ml). The nasal ab- sorption of both drugs from the same microemulsion formulation (containing 20% aqueous phase) was found to be fairly rapid after administration of 0.4 mg/kg to rabbits. The absolute bioavailability of diazepam after intranasal ad- ministration using this formulation was 33.45% ± 12.36% and the tmax was 18.33 ± 23.09 min, which was twice longer than the tmax obtained after midazolam administration, 9.25 ± 6.75 min. The pharmacokinetic parameters of midazolam in W/O (20% water) microemulsion and their comparison with midazolam in O/W (50% water) microemulsion have shown that both formulations resulted in a relatively short time to reach the peak plasma level (tmax), that is, 9.25 ± 6.75 min and 6.75 ± 5.67 min, respectively. However, the peak plasma levels (Cmax) and the absolute bioavailability (FA) of midazolam were significantly higher after administration of the W/O formulation than those obtained after application of O/W formulation, i.e., 46.62 ± 17.38 µg/ml vs. 15.44 ± 4.00 µg/ml, and 35.19 % ± 11.83% vs. 19.83% ± 16.32%, re- spectively. Our results suggest that the new microemulsion system may be useful for getting rapid-onset of midazolam and diazepam following intranasal administration, resulting in reasonable peak plasma levels and bioavailability, but most importantly, providing a high measure of tolerability and comfort. Keywords: Microemulsion, Intranasal Drug Delivery, Benzodiazepines, Nasal Spray, Diazepam, Midazolam 1. Introduction Benzodiazepines are a group of psychoactive drugs with clinical effects like sedation, hypnotic, anxiolytic, anti- convulsant, muscle relaxant and amnesia. These thera- peutic properties make benzodiazepines useful in treating anxiety, insomnia, agitation, seizures, muscle spasms, alcohol withdrawal syndrome and as a premedication for various medical or dental procedures. Benzodiazepines have been the first line treatment of seizures, which are an emergency medical situation [1]. While untreated prolonged seizures increase the risk of mortality, mor- bidity and permanent brain damage, an early and rapid termination of the seizures by benzodiazepines is needed. Benzodiazepines exert their anticonvulsant effect by in- teracting with γ-aminobutric acid (GABA) receptors at the benzodiazepine binding site and allosterically modi- fying GABAA receptor current to enhance inhibition [2- 4]. Traditionally, intravenous and especially rectal diaze- pam (DZP), a highly lipophilic benzodiazepine, has been used as front line therapy. However, diazepam via intravenous and rectal routes have several drawbacks [5- 8]: 1) The establishment of an intravenous access is not practical in an emergency situation when the patient is not in a hospital. A highly qualified, trained medical person is required for this procedure; 2) The use of rectal diazepam results in variable plasma levels and fails to terminate 30% of seizures [9]. It is also socially embar- rassing, and although difficult to administer during con-  Intranasal Delivery of Two Benzodiazepines, Midazolam and Diazepam, by a Microemulsion System 181 vulsions can be used only in children and in a few cases in adolescents; 3) it is highly lipophilic and therefore has a large volume of distribution. Repeated doses are some- time needed and its accumulation may lead to compli- cations such as bradypnea and even respiratory arrest [10,11]. Diazepam is also available in tablets; however, the oral route is not accessible during seizures and cannot be used. Thus, an alternative fast-acting benzodiazepine delivery route, such as the nasal route, is needed. By the alternative route, diazepam should be easily administered by the surrounding people or caregivers and could dra- matically improve the management of out-of-hospital seizures as well as the patient recovery. The intranasal administration of diazepam was evaluated in several studies, in which supersaturated formulations were used with solvents such as a glycofurol/water mixture [7,12], glycofurol/polyethylene glycol 200 [13,14], propylene glycol/ethanol [15], and polyethylene glycol 300 [16]. Human studies [7,12], which were conducted on human volunteers reported that subjects rated nasal diazepam as causing considerable pain immediately following admini- stration. In addition, discomfort, nasal drainage and wat- ery eyes were also reported. Midazolam hydrochloride (MDZ-HCl) is a short act- ing, water soluble benzodiazepine. Its effectiveness on the CNS is dependent on the dose, route of administra- tion, and whether it is used concomitantly with other medications. Midazolam has also been used for rapid an- esthesia at emergency setting and as an agent for seda- tion prior to medical procedures. Because of its high solubility in aqueous solutions, MDZ-HCl can be used intravenously, intramuscularly, buccally, and intranasally. Although midazolam is marketed only in injectable and oral syrup formulations, there is increased interest in its administration via the nasal route and it is indeed the most extensively studied nasal benzodiazepine [17]. A survey research [18] among anesthesiologists showed that the most commonly used (>80%) sedative premedi- cant in children was midazolam, 8% of which have prac- ticed intranasal midazolam, apparently in “off-label” use, to premedicate pediatric patients preoperatively. The interest in intranasal drug delivery arises from the unique advantages presented by the nasal cavity such as: 1) A large surface area available for drug deposition and ab- sorption, 2) The nasal epithelium is thin and highly vas- cularized, 3) Absorbed substances are transported di- rectly into the systemic circulation thereby avoiding the first pass metabolic effect, and 4) In some cases, drug can be absorbed directly into the CNS by passing the tight blood brain barrier [19]. Intranasal midazolam has already been explored during the last decade and a number of clinical works revealed the potential of its administration via the nasal route [9-11,17,20-23]. Nev- ertheless, there are still issues waiting to be resolved. Most studies reported the use of various dilutions of a commercial midazolam, dripped by syringe into patient's nostrils. This use of aqueous solutions (usually employed for injections) is not optimal for intranasal administration from two main reasons: a) The acidic pH (pH ≈ 3) is too low for the nasal mucosal membrane and is therefore a potential irritant, and b) The solutions are too diluted to provide a considerably small volume for human nostril, namely, 100 - 150 µl of liquid at a time. Optimal for- mulation should contain at least 2.5 mg of midazolam in 100 µl solution. Although more concentrated MDZ-HCl solutions [17,21,26] resulted in a relatively high bio- availability in healthy volunteers, the researchers reported that irritation (“burning” sensation) and pain occurred in all subject received midazolam, as well as symptoms like teary eyes, dizziness, and bad taste. In the present paper, we propose the use of a new mi- croemulsion that in pre-clinical studies, seemed to have solved the problem of irritability following regular nasal benzodiazepine administrations. We have studied and compared the pharmacokinetic characteristics of diaze- pam and midazolam applied to rabbits in a microemul- sion formulation via the nasal route. The new microe- mulsion system did not contain alcohols or other irritant chemicals and is generally recognized as safe (GRAS) compounds. The purpose of this work was to evaluate the use of the new microemulsion formulation as a car- rier for diazepam or midazolam given via the nasal route. 2. Materials and Methods 2.1. Materials Midazolam (base) and diazepam were kindly donated by Rafa Laboratories Ltd., Jerusalem, Israel. Commercial midazolam HCl solution for injection (Midolam amps., 5 mg/ml or 0.5% wt/v, Rafa Laboratories, Israel) was pur- chased from a local pharmacy. Commercial diazepam solution for injection (Assival amps., 10 mg/2ml, Teva Group, Israel) was also purchased from a local pharmacy. Glyceryl oleate was obtained from Uniqema, Brombor- ough Pool, The Wirral, UK. Labrasol was obtained from Gattefosse, France. Isopropyl palmitate (IPP) and pro- pylene carbonate were purchased from Aldrich (Sigma- Aldrich Inc., St. Louis, MO). Acepromazine (10 mg/ml acepromazine maleate) was used from PromAce In- jectable, Fort Dodge-Animal Health (Iowa, USA). 2.2. Preparation of Microemulsions Generally, microemulsions were prepared by mixing La- brasol, glyceryl oleate (surfactants) and isopropyl palmi- tate (oil) with propylene carbonate (co-surfactant) and water. Appropriate quantities of midazolam (base) or di- C opyright © 2011 SciRes. PP  182 Intranasal Delivery of Two Benzodiazepines, Midazolam and Diazepam, by a Microemulsion System azepam were then solubilized in the microemulsion to reach a final concentration of 2.5% (wt/wt) of the desired drug. The monophasic formulations were formed after a short stirring at room temperature. The cosurfactant- surfactants (CoS/S) weight ratio was 1:5, and the surfac- tants; ratio was 1:3. 2.3. Construction of Phase Diagrams Pseudo-ternary phase diagrams of oil, distilled water, and co-surfactant (CoS)—surfactants (S) mixtures were con- structed at fixed CoS/S weight ratios. The weight ratio of the two surfactants, glyceryl oleate to Labrasol derivative, were fixed and kept constant. Phase diagrams were ob- tained by visual inspection of mixtures of the ingredients, which were pre-weighed into glass vials, titrated with water and stirred well at room temperature. As a con- venient method, the construction of the phase diagrams were done by drawing “water dilution lines” representing an increase of water content while decreasing CoS-S and oil levels. The water was titrated along dilution lines drawn from the water apex to the opposite surfactant side of the triangle. The line was arbitrarily denoted as the value of the line intersection with the surfactant scale (e.g., DL87 means line representing a surfactant-to-oil ratio of 87:13). In case turbidity appeared followed by a phase separation, the samples were considered as bi- phasic. In case monophasic, clear and transparent mix- tures were visualized, the samples were marked as points in the phase diagram. The area covered by these points was considered as the microemulsion region of exis- tence. 2.4. Dynamic Light Scattering (DLS) The hydrodynamic diameter spectrum of microemulsion nano-droplets was collected using CGS-3 Compact Go- niometer System (ALV GmbH, Langen, Germany). The laser power was 20 mW at the He-Ne laser line (632.8 nm). Correlograms were calculated by ALV/LSE 5003 correlator, which were collected at 60˚, during 10 s for 20 times, at 25˚C. Measurements were performed at per- manent angle of 60˚. The droplet size was calculated using the Stokes-Einstein relationship, and the analysis was based on regularization method as described by Provencher [24]. 2.5. Pharmacokinetic Study All animal procedures were performed in accordance with protocols reviewed and approved by the Institu- tional & Use Committee, Ben Gurion University of the Negev, which complies with the Israeli Law of Human Care and Use of Laboratory Animals. New Zealand white rabbits (HsdIf: NZW males, 2.0 - 3.5 kg body weight, Harlan, Jerusalem) were used in the experiments. The rabbits were housed individually with free access to food and water. A 12 h light/12 h dark cycle was held to keep a normal circadian rhythm in the animals. Nasal formulations or intravenous drug dosage forms were ad- ministered in a randomized cross-over design with a wash-out period of at least four days. After the animals had been tranquillized with 0.5 ml acepromazine, Ven- flon™ cannula (22 G, Poly Medicure Ltd., Faridabad, India) was inserted into the main artery of the rabbit ear. Each rabbit was weighed and the drug (MDZ or DZP) was nasally or intravenously administered. Microemul- sion containing 2.5% wt/wt (=25 mg/g) midazolam or diazepam was administered at a 0.4 mg/kg dose by nasal spraying (approx. 100 µl of microemulsion containing 2.5 mg of drug, approx. 50 µl in each nostril). For the purpose of comparison, MDZ at the same dosage was also applied in macro-emulsion formulation (same for- mulation without co-surfactant) and in a mixture of oil and surfactants (same formulation without an aqueous phase), all based on the same microemulsion’s compo- nents and their ratios. Commercial midazolam solution for injection (5 mg/ml) had first been diluted in sterile saline solution (×5) before administered intravenously at a 0.2 mg/kg dose (approx. 0.5 ml solution). Commercial diazepam solution for injection (5 mg/ml) had first been ×5 diluted in propylene glycol then administered intra- venously at a 0.2 mg/kg dose (approx. 0.5 ml solution). The exact application volume was determined according to the individual body weight. Spraying technique was developed by using a 100 µl syringe connected to MAD Nasal Drug Delivery Device (MAD 320, Wolfe Tory Medical, Inc., Salt Lake City, UT). Blood samples were collected at 0, 2, 5, 15, 30, 45, 60, 90, 120, and 180 min- utes after application in heparin-containing tubes. Plasma was obtained after centrifugation at 10,000 rpm for 10 minutes, and stored at –20˚C until analyzed for MDZ or DZP. Plasma drug concentrations were determined using LC /MS/MS method pre-developed in our laboratory. 2.6. Plasma Drug Determination A LC/MS/MS analysis was performed using a Reprosil C18-AQ 5 µm column (100 × 2 mm) (Dr. Maisch, Ger- many), equipped with a C18 guard column. The mobile phase consisted of a 33.3:66.7 v/v mixture of 1mM am- monium acetate buffer (eluent A) and methanol-acetoni- trile (20:80 v/v) (eluent B). The flow rate was 0.3 ml/min at ambient temperature. Detection was performed using an API 2000 instrument (MDX SCIEX, Concord, On- tario, Canada). The API 2000 ES source was tuned by infusing a standard solution of drug (1 µg/ml in methanol) into the source at a flow rate of 10 µL/min. The optimal parameters were: source temperature 550˚C, decluster- ing potential 96 eV, focusing potential 370 eV, entrance C opyright © 2011 SciRes. PP 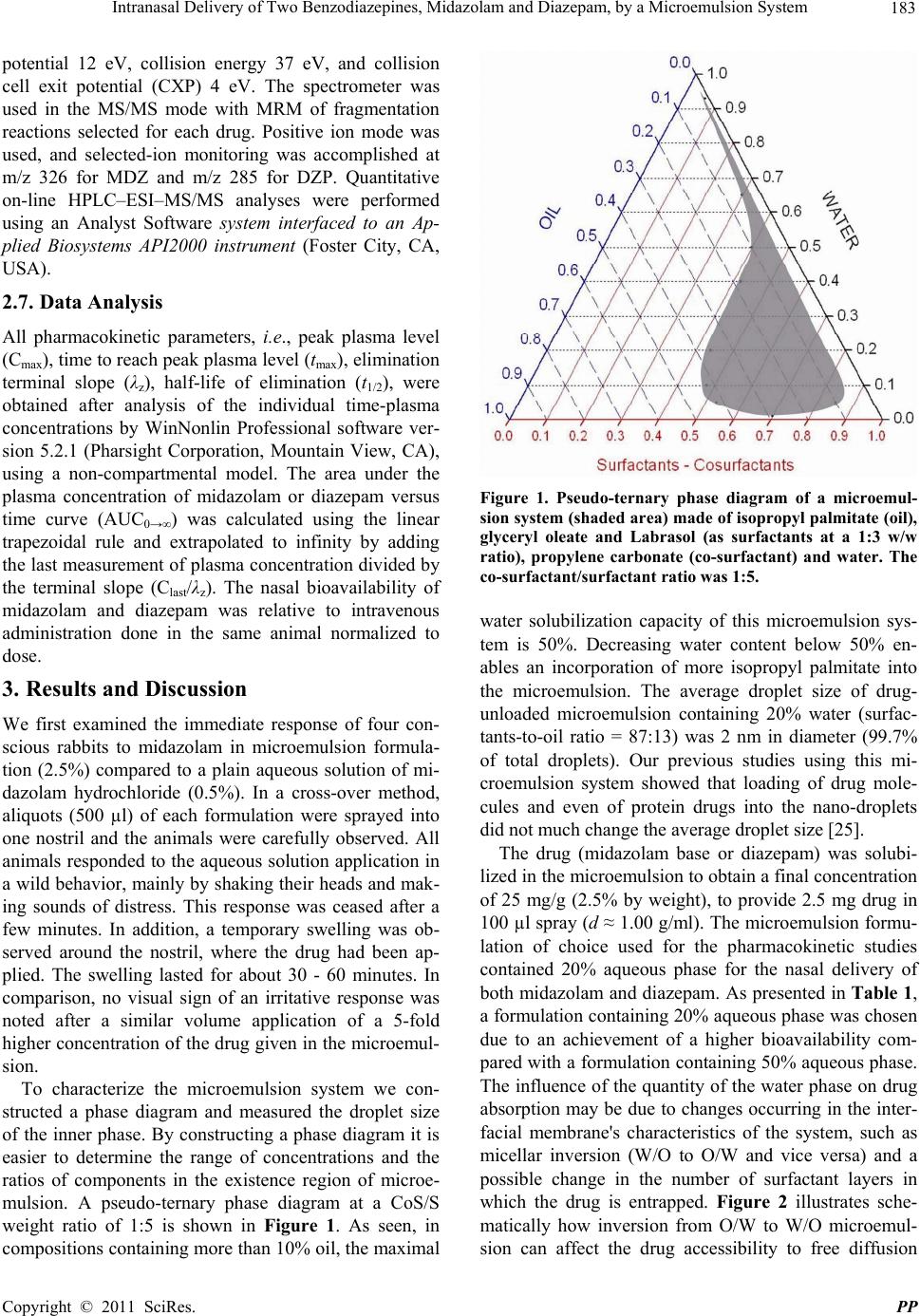 Intranasal Delivery of Two Benzodiazepines, Midazolam and Diazepam, by a Microemulsion System 183 potential 12 eV, collision energy 37 eV, and collision cell exit potential (CXP) 4 eV. The spectrometer was used in the MS/MS mode with MRM of fragmentation reactions selected for each drug. Positive ion mode was used, and selected-ion monitoring was accomplished at m/z 326 for MDZ and m/z 285 for DZP. Quantitative on-line HPLC–ESI–MS/MS analyses were performed using an Analyst Software system interfaced to an Ap- plied Biosystems API2000 instrument (Foster City, CA, USA). 2.7. Data Analysis All pharmacokinetic parameters, i.e., peak plasma level (Cmax), time to reach peak plasma level (tmax), elimination terminal slope (λz), half-life of elimination (t1/2), were obtained after analysis of the individual time-plasma concentrations by WinNonlin Professional software ver- sion 5.2.1 (Pharsight Corporation, Mountain View, CA), using a non-compartmental model. The area under the plasma concentration of midazolam or diazepam versus time curve (AUC0→∞) was calculated using the linear trapezoidal rule and extrapolated to infinity by adding the last measurement of plasma concentration divided by the terminal slope (Clast/λz). The nasal bioavailability of midazolam and diazepam was relative to intravenous administration done in the same animal normalized to dose. 3. Results and Discussion We first examined the immediate response of four con- scious rabbits to midazolam in microemulsion formula- tion (2.5%) compared to a plain aqueous solution of mi- dazolam hydrochloride (0.5%). In a cross-over method, aliquots (500 µl) of each formulation were sprayed into one nostril and the animals were carefully observed. All animals responded to the aqueous solution application in a wild behavior, mainly by shaking their heads and mak- ing sounds of distress. This response was ceased after a few minutes. In addition, a temporary swelling was ob- served around the nostril, where the drug had been ap- plied. The swelling lasted for about 30 - 60 minutes. In comparison, no visual sign of an irritative response was noted after a similar volume application of a 5-fold higher concentration of the drug given in the microemul- sion. To characterize the microemulsion system we con- structed a phase diagram and measured the droplet size of the inner phase. By constructing a phase diagram it is easier to determine the range of concentrations and the ratios of components in the existence region of microe- mulsion. A pseudo-ternary phase diagram at a CoS/S weight ratio of 1:5 is shown in Figure 1. As seen, in compositions containing more than 10% oil, the maximal Figure 1. Pseudo-ternary phase diagram of a microemul- sion system (shaded area) made of isopropyl palmitate (oil), glyceryl oleate and Labrasol (as surfactants at a 1:3 w/w ratio), propylene carbonate (co-surfactant) and water. The co-surfactant/surfactant ratio was 1:5. water solubilization capacity of this microemulsion sys- tem is 50%. Decreasing water content below 50% en- ables an incorporation of more isopropyl palmitate into the microemulsion. The average droplet size of drug- unloaded microemulsion containing 20% water (surfac- tants-to-oil ratio = 87:13) was 2 nm in diameter (99.7% of total droplets). Our previous studies using this mi- croemulsion system showed that loading of drug mole- cules and even of protein drugs into the nano-droplets did not much change the average droplet size [25]. The drug (midazolam base or diazepam) was solubi- lized in the microemulsion to obtain a final concentration of 25 mg/g (2.5% by weight), to provide 2.5 mg drug in 100 µl spray (d ≈ 1.00 g/ml). The microemulsion formu- lation of choice used for the pharmacokinetic studies contained 20% aqueous phase for the nasal delivery of both midazolam and diazepam. As presented in Table 1, a formulation containing 20% aqueous phase was chosen due to an achievement of a higher bioavailability com- pared with a formulation containing 50% aqueous phase. The influence of the quantity of the water phase on drug absorption may be due to changes occurring in the inter- facial membrane's characteristics of the system, such as micellar inversion (W/O to O/W and vice versa) and a possible change in the number of surfactant layers in which the drug is entrapped. Figure 2 illustrates sche- matically how inversion from O/W to W/O microemul- ion can affect the drug accessibility to free diffusion s C opyright © 2011 SciRes. PP  Intranasal Delivery of Two Benzodiazepines, Midazolam and Diazepam, by a Microemulsion System Copyright © 2011 SciRes. PP 184 Table 1. Mean pharmacokinetic parameters of midazolam after IV and IN administration to rabbits (total of 8 animals). Study 1, n = 4 (paired) Study 2, n = 4 (paired) PK parameter IV solution* 0.2 mg/kgIN microemulsion with 20% water 0.4 mg/kgIV solution** 0.2 mg/kg IN microemulsion with 50% water 0.4 mg/kg Cmax (μg/ml) 188.80 (±79.63) 46.62 (±17.38) 195.87 (±37.43) 15.44 (±4.00) tmax (min) 0 9.25 (±6.75) 0 6.75 (±5.67) z (min–1) 0.0207 (±0.0035) 0.0169 (±0.0041) 0.0394 (±0.0145) 0.0338 (±0.0158) Elimination t1/2 (min) 34.19 (±5.88) 43.15 (±11.63) 19.57 (±7.24) 28.32 (±22.86) AUC0–∞ (μg·min·ml–1) 3499 (±991) 2494 (±1098) 2050 (±334) 789 (±607) AUC0–∞/dose (μg·min·ml–1·D–1) 17.50 (±4.95) 6.23 (±2.74) 10.25 (±1.67) 1.97 (±1.51) FAa (%) ---- 35.19 (±11.83) ---- 19.83 (±16.32) *IV reference group for the IN group which received 20% water-containing microemulsion; **IV reference group for the IN group which received 50% wa- ter-containing microemulsion; aFA% = absolute bioavailability = (AUC0–∞ IN × Dose IV) × 100/(AUC0–∞ IV × Dose IN). mulations resulted in a relatively short time to reach the peak plasma level (tmax), 9.25 ± 6.75 min and 6.75 ± 5.67 min (t-test, p > 0.05), respectively. In contrast, the peak plasma levels (Cmax) and the absolute bioavailability (FA) of MDZ were significantly higher after administration of the W/O formulation than those obtained after applica- tion of O/W formulation, i.e., 46.62 ± 17.38 g/ml vs. 15.44 ± 4.00 µg/ml, and 35.19% ± 11.83% vs. 19.83% ± 16.32%, respectively (p < 0.05). It is to be noted that the average elimination half-life (t1/2) of MDZ in rabbits of Study 1 (Table 1) after IV administration was statisti- cally different compared with the average value obtained after IV administration to rabbits of study 2, i.e., 34.19 ± 5.88 min vs. 19.57 ± 7.24 min (t-test, p < 0.05), respec- tively. This difference was probably due to the relatively lower body weight of the animals in study 2. However, there was no statistically significant change in the half- lives obtained after IN administration to the same ani- mals in each study. In addition, no statistical difference was noted between half-lives of MDZ after IN admini- strations in both studies. Table 2 presents the pharma- cokinetic parameters of diazepam in a study involved three rabbits which received both IV and IN administra- tions. Figures 3 and 4 show the pharmacokinetic profiles of 0.4 mg/kg doses of MDZ (Figure 3) and DZP (Figure 4) applied intranasally to rabbits as compared to IV ad- ministrations of each drug at 0.2 mg/kg doses. The nasal administration of both drugs was carried out by the same microemulsion vehicle containing 20% aqueous phase and 25 mg/ml drug concentration. As shown in Table 2, the absolute bioavailability of DZP after IN administra- tion using this formulation was 33.45% ± 12.36% and the tmax was 18.33 ± 23.09 min, which was twice longer than the tmax obtained after MDZ administration (9.25 ± 6.75 min). The tmax values obtained in this study for di- azepam is in agreement with Gizurarson, et al. [13], who achieved peak levels after 18 ± 11 min in healthy hu- mans. The difference between tmax values of DZP and Figure 2. Schematic illustration (not to scale) of possible packing of midazolam in the nano-droplet’s membrane; up: W/O microemulsion; bottom: O/W microemulsion. from the interfacial membrane to the outer phase. The pharmacokinetic parameters of midazolam in W/O (20% water) microemulsion (Study 1) and their comparison with midazolam in O/W (50% water) microemulsion (Study 2) is presented in Table 1. As shown, both for- 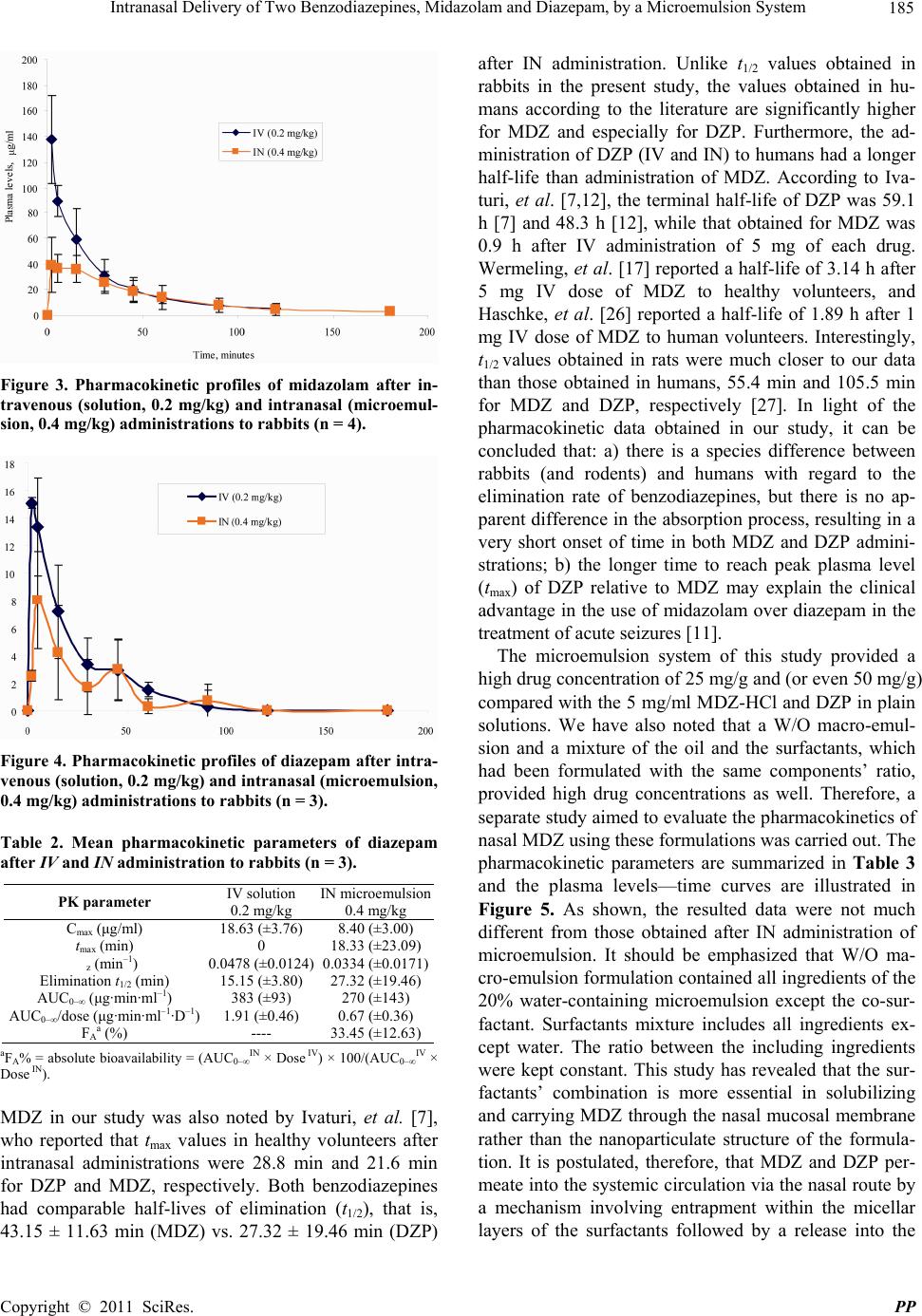 Intranasal Delivery of Two Benzodiazepines, Midazolam and Diazepam, by a Microemulsion System 185 Figure 3. Pharmacokinetic profiles of midazolam after in- travenous (solution, 0.2 mg/kg) and intranasal (microemul- sion, 0.4 mg/kg) administrations to rabbits (n = 4). Figure 4. Pharmacokinetic profiles of diazepam after intra- venous (solution, 0.2 mg/kg) and intranasal (microemulsion, 0.4 mg/kg) administrations to rabbits (n = 3). Table 2. Mean pharmacokinetic parameters of diazepam after IV and IN administration to rabbits (n = 3). PK parameter IV solution 0.2 mg/kg IN microemulsion 0.4 mg/kg Cmax (μg/ml) 18.63 (±3.76) 8.40 (±3.00) tmax (min) 0 18.33 (±23.09) z (min–1) 0.0478 (±0.0124) 0.0334 (±0.0171) Elimination t1/2 (min) 15.15 (±3.80) 27.32 (±19.46) AUC0–∞ (μg·min·ml–1) 383 (±93) 270 (±143) AUC0–∞/dose (μg·min·ml–1·D–1) 1.91 (±0.46) 0.67 (±0.36) FAa (%) ---- 33.45 (±12.63) aFA% = absolute bioavailability = (AUC0–∞ IN × Dose IV) × 100/(AUC0–∞ IV × Dose IN). MDZ in our study was also noted by Ivaturi, et al. [7], who reported that tmax values in healthy volunteers after intranasal administrations were 28.8 min and 21.6 min for DZP and MDZ, respectively. Both benzodiazepines had comparable half-lives of elimination (t1/2), that is, 43.15 ± 11.63 min (MDZ) vs. 27.32 ± 19.46 min (DZP) after IN administration. Unlike t1/2 values obtained in rabbits in the present study, the values obtained in hu- mans according to the literature are significantly higher for MDZ and especially for DZP. Furthermore, the ad- ministration of DZP (IV and IN) to humans had a longer half-life than administration of MDZ. According to Iva- turi, et al. [7,12], the terminal half-life of DZP was 59.1 h [7] and 48.3 h [12], while that obtained for MDZ was 0.9 h after IV administration of 5 mg of each drug. Wermeling, et al. [17] reported a half-life of 3.14 h after 5 mg IV dose of MDZ to healthy volunteers, and Haschke, et al. [26] reported a half-life of 1.89 h after 1 mg IV dose of MDZ to human volunteers. Interestingly, t1/2 values obtained in rats were much closer to our data than those obtained in humans, 55.4 min and 105.5 min for MDZ and DZP, respectively [27]. In light of the pharmacokinetic data obtained in our study, it can be concluded that: a) there is a species difference between rabbits (and rodents) and humans with regard to the elimination rate of benzodiazepines, but there is no ap- parent difference in the absorption process, resulting in a very short onset of time in both MDZ and DZP admini- strations; b) the longer time to reach peak plasma level (tmax) of DZP relative to MDZ may explain the clinical advantage in the use of midazolam over diazepam in the treatment of acute seizures [11]. The microemulsion system of this study provided a high drug concentration of 25 mg/g and (or even 50 mg/g) compared with the 5 mg/ml MDZ-HCl and DZP in plain solutions. We have also noted that a W/O macro-emul- sion and a mixture of the oil and the surfactants, which had been formulated with the same components’ ratio, provided high drug concentrations as well. Therefore, a separate study aimed to evaluate the pharmacokinetics of nasal MDZ using these formulations was carried out. The pharmacokinetic parameters are summarized in Table 3 and the plasma levels—time curves are illustrated in Figure 5. As shown, the resulted data were not much different from those obtained after IN administration of microemulsion. It should be emphasized that W/O ma- cro-emulsion formulation contained all ingredients of the 20% water-containing microemulsion except the co-sur- factant. Surfactants mixture includes all ingredients ex- cept water. The ratio between the including ingredients were kept constant. This study has revealed that the sur- factants’ combination is more essential in solubilizing and carrying MDZ through the nasal mucosal membrane rather than the nanoparticulate structure of the formula- tion. It is postulated, therefore, that MDZ and DZP per- meate into the systemic circulation via the nasal route by a mechanism involving entrapment within the micellar layers of the surfactants followed by a release into the C opyright © 2011 SciRes. PP 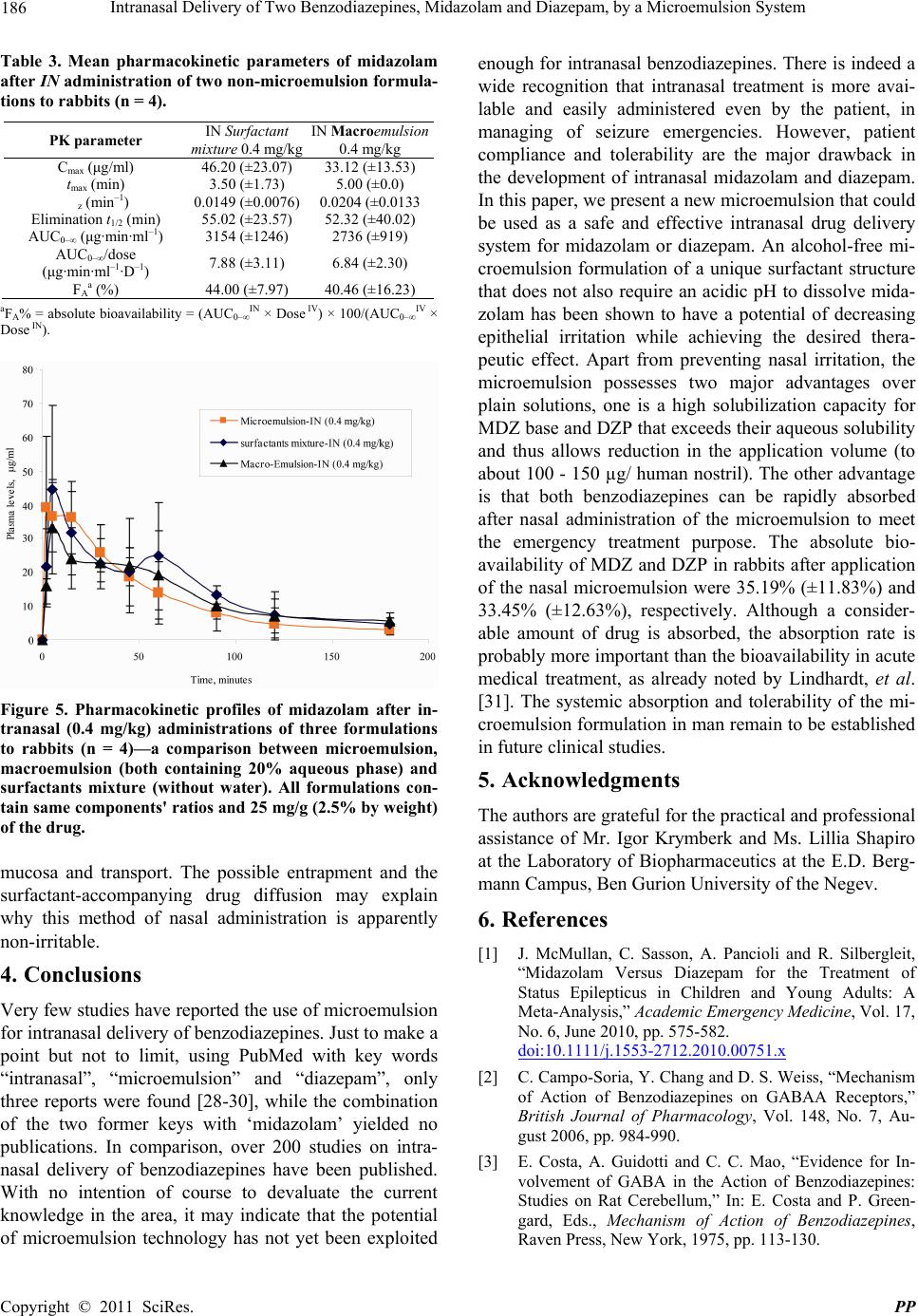 186 Intranasal Delivery of Two Benzodiazepines, Midazolam and Diazepam, by a Microemulsion System Table 3. Mean pharmacokinetic parameters of midazolam after IN administration of two non-microemulsion formula- tions to rabbits (n = 4). PK parameter IN Surfactant mixture 0.4 mg/kg IN Macroemulsion 0.4 mg/kg Cmax (μg/ml) 46.20 (±23.07) 33.12 (±13.53) tmax (min) 3.50 (±1.73) 5.00 (±0.0) z (min–1) 0.0149 (±0.0076) 0.0204 (±0.0133 Elimination t1/2 (min) 55.02 (±23.57) 52.32 (±40.02) AUC0–∞ (μg·min·ml–1) 3154 (±1246) 2736 (±919) AUC0–∞/dose (μg·min·ml–1·D–1) 7.88 (±3.11) 6.84 (±2.30) FAa (%) 44.00 (±7.97) 40.46 (±16.23) aFA% = absolute bioavailability = (AUC0–∞ IN × Dose IV) × 100/(AUC0–∞ IV × Dose IN). Figure 5. Pharmacokinetic profiles of midazolam after in- tranasal (0.4 mg/kg) administrations of three formulations to rabbits (n = 4)—a comparison between microemulsion, macroemulsion (both containing 20% aqueous phase) and surfactants mixture (without water). All formulations con- tain same components' ratios and 25 mg/g (2.5% by weight) of the drug. mucosa and transport. The possible entrapment and the surfactant-accompanying drug diffusion may explain why this method of nasal administration is apparently non-irritable. 4. Conclusions Very few studies have reported the use of microemulsion for intranasal delivery of benzodiazepines. Just to make a point but not to limit, using PubMed with key words “intranasal”, “microemulsion” and “diazepam”, only three reports were found [28-30], while the combination of the two former keys with ‘midazolam’ yielded no publications. In comparison, over 200 studies on intra- nasal delivery of benzodiazepines have been published. With no intention of course to devaluate the current knowledge in the area, it may indicate that the potential of microemulsion technology has not yet been exploited enough for intranasal benzodiazepines. There is indeed a wide recognition that intranasal treatment is more avai- lable and easily administered even by the patient, in managing of seizure emergencies. However, patient compliance and tolerability are the major drawback in the development of intranasal midazolam and diazepam. In this paper, we present a new microemulsion that could be used as a safe and effective intranasal drug delivery system for midazolam or diazepam. An alcohol-free mi- croemulsion formulation of a unique surfactant structure that does not also require an acidic pH to dissolve mida- zolam has been shown to have a potential of decreasing epithelial irritation while achieving the desired thera- peutic effect. Apart from preventing nasal irritation, the microemulsion possesses two major advantages over plain solutions, one is a high solubilization capacity for MDZ base and DZP that exceeds their aqueous solubility and thus allows reduction in the application volume (to about 100 - 150 µg/ human nostril). The other advantage is that both benzodiazepines can be rapidly absorbed after nasal administration of the microemulsion to meet the emergency treatment purpose. The absolute bio- availability of MDZ and DZP in rabbits after application of the nasal microemulsion were 35.19% (±11.83%) and 33.45% (±12.63%), respectively. Although a consider- able amount of drug is absorbed, the absorption rate is probably more important than the bioavailability in acute medical treatment, as already noted by Lindhardt, et al. [31]. The systemic absorption and tolerability of the mi- croemulsion formulation in man remain to be established in future clinical studies. 5. Acknowledgments The authors are grateful for the practical and professional assistance of Mr. Igor Krymberk and Ms. Lillia Shapiro at the Laboratory of Biopharmaceutics at the E.D. Berg- mann Campus, Ben Gurion University of the Negev. 6. References [1] J. McMullan, C. Sasson, A. Pancioli and R. Silbergleit, “Midazolam Versus Diazepam for the Treatment of Status Epilepticus in Children and Young Adults: A Meta-Analysis,” Academic Emergency Medicine, Vol. 17, No. 6, June 2010, pp. 575-582. doi:10.1111/j.1553-2712.2010.00751.x [2] C. Campo-Soria, Y. Chang and D. S. Weiss, “Mechanism of Action of Benzodiazepines on GABAA Receptors,” British Journal of Pharmacology, Vol. 148, No. 7, Au- gust 2006, pp. 984-990. [3] E. Costa, A. Guidotti and C. C. Mao, “Evidence for In- volvement of GABA in the Action of Benzodiazepines: Studies on Rat Cerebellum,” In: E. Costa and P. Green- gard, Eds., Mechanism of Action of Benzodiazepines, Raven Press, New York, 1975, pp. 113-130. C opyright © 2011 SciRes. PP  Intranasal Delivery of Two Benzodiazepines, Midazolam and Diazepam, by a Microemulsion System 187 [4] W. Haefely, A. Kulcsar and H. Moehler, “Possible In- volvement of GABA in the Central Actions of Benzodi- azepines,” Psychopharmacological Bulletin, Vol. 11, No. 4, October 1975, pp. 58-59. [5] G. J. de Haan, P. van der Geest, G. Doelman, E. Bertram and P. Edelbroek, “A Comparison of Midazolam Nasal Spray and Diazepam Rectal Solution for the Residential Treatment of Seizure Exacerbations,” Epilepsia, Vol. 51, No. 3, March 2010, pp. 478-482. doi:10.1111/j.1528-1167.2009.02333.x [6] B. K. Alldredge, A. M. Gelb, S. M. Isaacs, M. D. Corry, F. Allen, S. Ulrich, M. D. Gottwald, N. O’Neil, J. M. Neuhaus, M. R. Segal and D. H. Lowenstein, “A Com- parison of Lorazepam, Diazepam, and Placebo for the Treatment of Out-of-Hospital Status Epilepticus,” New England Journal of Medicine, Vol. 345, No. 9, August 2001, pp. 631-637. doi:10.1056/NEJMoa002141 [7] V. D. Ivaturi, J. R. Riss, R. L. Kriel and J. C. Cloyd, “Pharmacokinetics and Tolerability of Intranasal Diaze- pam and Midazolam in Healthy Adult Volunteers,” Acta Neurologica Scandinavica, Vol. 120, No. 5, November 2009, pp. 353-357. doi:10.1111/j.1600-0404.2009.01170.x [8] C. O'Dell, S. Shinnar, K. R. Ballaban-Gil, M. Hornick, M. Sigalova, H. Kang and S. L. Moshe, “Rectal Diazepam Gel in the Home Management of Seizures in Children,” Pediatric Neurology, Vol. 33, No. 3, September 2005, pp. 166-172. doi:10.1056/NEJMoa002141 [9] P. Mittal, R. Manohar and A. Rawat, “Comparative Study of Intranasal Midazolam and Intravenous Diazepam Se- dation for Procedures and Seizures,” Indian Journal of Pediatrics, Vol. 73, No. 11, November 2006, pp. 975-978. doi:10.1007/BF02758299 [10] E. Lahat, M. Goldman, J. Barr, T. Bistritzer and M. Berkovitch, “Comparison of Intranasal Midazolam with Intravenous Diazepam for Treating Febrile Seizures in Children: Prospective Randomised Study,” British Medi- cal Journal, Vol. 321, No. 7253, July 2000, pp. 83-86. doi:10.1136/bmj.321.7253.83 [11] M. Scheepers, B. Scheepers, M. Clarke, S. Comish and M. Ibitoye, “Is Intranasal Midazolam an Effective Rescue Medication in Adolescents and Adults with Severe Epi- lepsy?” Seizure, Vol. 9, No. 6, September 2000, pp. 417- 422. doi:10.1053/seiz.2000.0425 [12] V. D. Ivaturi, J. R. Riss, R. L. Kriel, R. A. Siegel and J. C. Cloyd, “Bioavailability and Tolerability of Intranasal Di- azepam in Healthy Adult Volunteers,” Epilepsy Research, Vol. 84, No. 2-3, April 2009, pp. 120-126. doi:10.1016/j.eplepsyres.2009.01.001 [13] S. Gizurarson, F. K. Gudbrandsson, H. Jonsson and E. Bechgaard, “Intranasal Administration of Diazepam Aim- ing at the Treatment of Acute Seizures: Clinical Trials in Healthy Volunteers,” Biological & Pharmaceutical Bul- letin, Vol. 22, No. 4, April 1999, pp. 425-427. [14] E. Bechgaard, S. Gizurarson and R. K. Hjortkjaer, “Phar- macokinetic and Pharmacodynamic Response after Intra- nasal Administration of Diazepam to Rabbits,” Journal of Pharmacy and Pharmacology, Vol. 49, No. 8, August 1997, pp. 747-750. doi:10.1111/j.2042-7158.1997.tb06105.x [15] L. Li, S. Gorukanti, Y. M. Choi and K.H. Kim, “Rapid- onset Intranasal Delivery of Anticonvulsants: Pharma- cokinetic and Pharmacodynamic Evaluation in Rabbits,” International Journal of Pharmaceutics, Vol. 199, No. 1, April 2000, pp. 65-76. doi:10.1016/S0378-5173(00)00373-2 [16] K. Lindhardt, S. Gizurarson, S. B. Stefansson, D. R. Olafsson and E. Bechgaard, “Electroencephalographic Effects and Serum Concentrations after Intranasal and In- travenous Administration of Diazepam to Healthy Volun- teers,” British Journal of Clinical Pharmacology, Vol. 52, No. 5, November 2001, pp. 521-527 doi:10.1046/j.0306-5251.2001.01486.x. [17] D. P. Wermeling, K. A. Record, T. H. Kelly, S. M. Archer, T. Clinch and A. C. Rudy, “Pharmacokinetics and Pharmacodynamics of a New Intranasal Midazolam Formulation in Healthy Volunteers,” Anesthesia & Anal- gesia, Vol. 103, No. 2, August 2006, pp. 344-349. doi:10.1213/01.ane.0000226150.90317.16 [18] Z. N. Kain, L. C. Mayes, C. Bell, S. Weisman, M. B. Hofstadter and S. Rimar, “Premedication in the United States: A Status Report,” Anesthesia & Analgesia, Vol. 84, No. 2, February 1997, pp. 427-432. doi:10.1213/00000539-199702000-00035 [19] M. I. Ugwoke, R. U. Agu, N. Verbeke and R. Kinget, “Nasal Mucoadhesive Drug Delivery: Background, Ap- plications, Trends and Future Perspectives,” Advanced Drug Delivery Reviews, Vol. 57, No. 11, November 2005, pp. 1640-1665. doi:10.1016/j.addr.2005.07.009 [20] M. Bhattacharyya, V. Kalra and S. Gulati, “Intranasal Midazolam vs Rectal Diazepam in Acute Childhood Sei- zures,” Pediatric Neurology, Vol. 34, No. 5, May 2006, pp. 355-359. doi:10.1016/j.pediatrneurol.2005.09.006 [21] P. D. Knoester, D. M. Jonker, R. T. Van Der Hoeven, T. A. Vermeij, P. M. Edelbroek, G. J. Brekelmans and G. J. de Haan, “Pharmacokinetics and Pharmacodynamics of Midazolam Administered as a Concentrated Intranasal spray. A Study in Healthy Volunteers,” British Journal of Clinical Pharmacology, Vol. 53, No. 5, May 2002, pp. 501-507. doi:10.1046/j.1365-2125.2002.01588.x [22] S. Bjorkman, G. Rigemar and J. Idvall, “Pharmacokinet- ics of Midazolam Given as an Intranasal Spray to Adult Surgical Patients,” British Journal of Anaesthesia, Vol. 79, No. 5, November 1997, pp. 575-580. [23] M. Holsti, B. L. Sill, S. D. Firth, F. M. Filloux, S. M. Joyce and R. A. Furnival, “Prehospital Intranasal Mida- zolam for the Treatment of Pediatric Seizures,” Pediatric Emergency Care, Vol. 23, No. 3, March 2007, pp. 148- 153. doi:10.1097/PEC.0b013e3180328c92 [24] S. W. Provencher, “CONTIN: A General Purpose Con- strained Regularization Program for Inverting Noisy Linear Algebraic and Integral Equations,” Computer Physics Communications, Vol. 27, No. 3, September 1982, pp. 229-242. doi:10.1016/0010-4655(82)90174-6 [25] A. C. Sintov, H. V. Levy and S. Botner, “Systemic Deliv- C opyright © 2011 SciRes. PP  188 Intranasal Delivery of Two Benzodiazepines, Midazolam and Diazepam, by a Microemulsion System ery of Insulin via the Nasal Route Using a New Microe- mulsion System: in vitro and in vivo Studies,” Journal of Controlled Release, Vol 148, No. 2, December 2010, pp. 168-176. doi:10.1016/j.jconrel.2010.08.004 [26] M. Haschke, K. Suter, S. Hofmann, R. Witschi, J. Froh- lich, G. Imanidis, J. Drewe, T. A. Briellmann, F. E. Dussy, S. Krahenbuhl and C. Surber, “Pharmacokinetics and Pharmacodynamics of Nasally Delivered Mida- zolam,” British Journal of Clinical Pharmacology, Vol. 69, No. 6, June 2010, pp. 607-616. doi:10.1111/j.1365-2125.2010.03611.x [27] A. Hoogerkamp, R. H. G. P. Arends, A. M. Bomers, J. W. Mandema, R. A. Voskuyl and M. Danhof, “Pharmacoki- netic/Pharmacodynamic Relationship of Benzodiazepines in the Direct Cortical Stimulation Model of Anticonvul- sant Effect,” The Journal of Pharmacology and Experi- mental Therapeutics, Vol. 279, No. 2, November 1996, pp. 803-812. [28] L. Li, I. Nandi and K. H. Kim, “Development of an Ethyl Laurate-Based Microemulsion for Rapid-Onset Intranasal Delivery of Diazepam,” International Journal of Phar- maceutics, Vol. 237, No. 1-2, April 2002, pp. 77-85. doi:10.1016/S0378-5173(02)00029-7 [29] P. Kaur and K. Kim, “Pharmacokinetics and Brain Up- take of Diazepam after Intravenous and Intranasal Ad- ministration in Rats and Rabbits,” International Journal of Pharmaceutics, Vol. 364, No. 1, November 2008, pp. 27-35. doi:10.1016/j.ijpharm.2008.07.030 [30] S. Porecha, T. Shah, V. Jogani, S. Naik and A. Misra, “Microemulsion Based Intranasal Delivery System for Treatment of Insomnia,” Drug Delivery, Vol. 16, No. 3, April 2009, pp. 128-134. doi:10.1080/10717540802560381 [31] K. Lindhardt, D. R. Olafsson, S. Gizurarson and E. Bechgaard, “Intranasal Bioavailability of Diazepam in Sheep Correlated to Rabbit and Man,” International Journal of Pharmaceutics, Vol. 231, No. 1, January 2002, pp. 67-72. doi:10.1016/S0378-5173(01)00872-9 C opyright © 2011 SciRes. PP
|