 America n Journal of Analy tic al Chemistry, 2011, 2, 383-391 doi:10.4236/ajac.2011. 23047 Published Online July 2011 (http://www.scirp.org/journal/ajac) Copyright © 2011 SciRes. AJAC Analytical Determination of Benzophenone-3 in Sunscreen Preparations Using Boron-D oped Diamond Electrodes Michelli Thomaz Laranjeira1, Fabio de Lima1, Silvio Cesar de Oliveira1, Valdir Souza Ferreira1, Robson Tadeu Soares de Oliveira*2 1Departamento de Química, Universidade Federal de Mato Grosso do Sul, Campo Grande, Brazil 2Instituto de Ciências Biológicas e Naturais-ICBN, Universidade Federal do Triângulo Mineiro, Uberaba, Brazil E-mail: rtsoj2003@yahoo.com.br Received October 17, 2010; revised November 17, 2010; accepted May 1, 2011 Abstrac A new electroanalytical procedure was developed for the determination of Benzophenone-3 (BENZO) in commercial sunscreen as the active ingredient. The procedure is based on the use of electrochemical methods as cyclic and square-wave voltammetry, with boron-doped diamond (BDD) electrodes. The reduction of BENZO in Britton-Robinson buffer (0.1 mol·L–1) using this type of electrode gives rise to one irreversible peak in –1.30 V (versus Ag/AgCl) in presence of cationic surfactant cetyltrimethylammonium bromide (CTABr). The proposed electrochemical method was successfully applied to the analysis of commercially available pharmaceu tical pr epar at ions. Keywords: Boron-Doped Diamond, Benzophenone-3 BENZO and Square-Wave Voltammetry 1. Introduction Benzophenone-3 (BENZO) (Figure 1) is the organic compound widely used in sunscreen agent that absorbs and dissipates ultraviolet radiation or in a variety of cos metic products [1]. BENZO also has been used as ultra violet stabilizer in p lastic surface coatings for food pack- aging to prevent polymer or food photodegradation [2] and is approved by the U.S. Food and Drug Adminstra- tion as an indirect food additive. The focus of pharmaceuticals and ingredients in per- sonal care products, including organic sunscreen agents, as environmental pollutants is increasing because these compounds may enter the aquatic environment not pri- marily as a result of manufacturing practices, but from Figure 1 . Chemical structure of ben zophenone-3. their steady and widespread use in human and veterinary dai ly a ctivities [3]. Furt her more, little is kno wn about the potential hazards associated with recurring human or ecologic exposures to these synthetic substances, many of which are bioactive. BENZO, one of these substances, has been detected in surface waters [4], drinking water, and wastewater [5]. The development of methods capa- ble of directly quantifying BENZO in commercial phar- maceutical preparations also becomes important in qual- ity control of sunscreens. The most often techniques observed in the literature for the determination sunscreen composition are high- performance liquid chromatography (HPLC) [6,7] and gas chromatography (GC) coupled with various detection methods such as ultraviolet (UV) [8] and mass spectro- metry [9]. However, electrochemical techniques are use- ful alternative methods widely used in pharmaceutical applications. They are usually easy and rapid to perform and are less expensive than chromatographic methods. In addition, the sensitivity of electrochemical methods is often greater t han that of spectrophotometric procedures. A few studies have reported the use of electroanalyti- cal methods to determine BENZO and related com- pounds. Vidal, et al. [10] applied chemically surface- modified carbon nanoparticles for the extraction and electrochemical deter mina tion of phenolic impurities such as BENZO (2-hydroxy-4-methoxybenzophenone).  M. T. LARANJEIRA ET AL. Copyright © 2011 SciRes. AJAC 384 The hydrophilic carbon nanoparticles were readily sus- pended and separated by centrifugation prior to deposi- tion onto suitable electrode surfaces and voltammetric analysis. Voltammetric peaks provide concentration in- formation over a 10 - 100 μM range and an estimated limit of detection of ca. 10 μM (or 2.3 ppm) for BENZO. Alternatively, analyte -free carbon nanoparticles immobi- lized at a graphite or glassy carbon electrode surface and directly immersed in analyte solution bind BENZO with an estimated Langmuir binding constants of mo l ·L−1 at pH 9.5 and it also give characteristic voltam- metric cathodic response for BE NZO with a linear range of ca. 1 - 120 μM. The estimated limit of detection is im- proved to ca. 5 μM (or 1.2 ppm) for BE NZO. Razak, et a l. [10] rep orted the use of differential pulse polarographic method for detection and trace determina- tion of benzophenone (the main impurity) in phenytoin powder. The method depends upon the polarographic activity of benzophenone in Britto n-Robinson buffer pH 5.6. The limit of detection was found to be 2.5 × 10−6 μg·mL−1. Phenytoin has been analysed polarographically after oxidation with alkaline permanganate to give ben- zophenone; the limit of detection was found to be 6 × 10−6 μg·mL−1. In a study performed by Cardoso, et al. [12], was proposed a methodology based on electro- chemical reduction for the simultaneous determinatio n of three sunscreen agents, namely 4-methylbenzylidene camphor (MBC), BENZO and 2-ethylhexyl-4-methox y - cinnamate (EHMC) by differential-pulse polarography (DP P). The hi ghest p eak cur rents and op timal separation of reduction peaks were obtained by using a supporting electrolyte consisted of Britton-Robinson buffer-me t h a - nol (8:2) solutio n at pH 4.0 a nd cationic surfactant 3.0 × 10−4 mol ·L−1 cetyltrimethylammonium bromide (CTABr). The methodology was validated using four commercial sunscreen preparations as a sample and the results sh- owed high recovery rates. The efficiency of the proposed methodology was demonstrated by com- paring the results obtained by DPP with those obtained by the highperformance liquid chromatography (HPLC) method. The procedures in electroanalysis strongly depend on working-electrode materials, increasing the interest in the development of new electrode materials. Carbon materi- als such as pyrolytic graphite, glassy carbon, and bo- ron-doped diamond (BDD) have been widely used for electrochemical applications [13-17]. It is well establi sh- ed that BDD electrodes have several advantages com- pared with other carbon surfaces. BDD electrodes have been extensively studied in recent years, both for their fundamental electrochemical properties [18-21] and its various applications [22,23]. The o utstanding electrochemical features of this mate- rial, including a wide potential window in aqueo us solu- tions [24,25], very low background current [26], weak adsorption for most types of organic molecules [27], high stability of response [28,29], and good electroactivity toward certain organic species all of which deactivate the surface of other conventional electrodes [30,31]—make this new material p ro mising fo r electr oanal ytical app lica - tions [32-37], electrosynthesis [38,39], and electroche- mical combustion [40-43], as well as for use as a sup- porting material in electrocatalysis [44-46]. Recent stu- dies reported in the literature have shown that several inorganic, organic and biomolecules can be satisfactorily determined with the use of BDD electrodes [32,47-49]. The combination of square-wave voltammetry (SWV) and BDD electrodes has proved to be an interesting and desirable alternative for the analytical determination of some organic molecules [49,50]. So, in view of the lack of a simple and direct electroanalytical method for the determinatio n of BENZO, the purpose of this investiga- tion is the development of an electrochemical method capable of directly quantifying BENZO in commercial pharmaceutical preparations available in sunscreen form, using cyclic voltammetry and SWV with BDD as the working electrode. 2. Experimental The stock solution of BENZO (Merck 99%) was pre- pared by direct weighing in order to obtain a solution 1.0 × 10−3 mol ·L −1 dissolved in methanol, followed by a di- lution to a concentration of 1.0 × 10−4 mol ·L −1. The ca- tionic cetyltrimethylammonium bromide (CTABr) (Acros, New Jersey, USA) surfactant was prepared to a concentration of 1% (m/V). The supporting electrolyte was pre- pared by dissolution of boric acid followed by dilution with acetic acid and phosphoric acid (all from Merck, Darmstadt, Germany) to a concentration of 0.04 mo l · L −1. The solutions had their pH adjusted by sodium hydroxide solution (Merck, Darmstadt, Germany). All reagents were of analytical grade. The deionized water was puri- fied with a Milli-Q plus system (Millipore, Bedford, MA, USA). The samples were prepared by di- rect weighing in a 15 mL beaker, followed by solubiliza- tion in 10 mL of methanol with sonication for 5 min. This solution was quantitatively transferred to a 25 mL volume tric flask and methanol was added to the mark. The use of methanol in the supporting electrolyte was necessary due to the low solubility of the sunscreen agents in aqueous me dium. The BDD electrodes were prepared in the Centre Suisse d’Electronique et de Microtechnique SA (CSEM), Neuchâtel, Switzerland, using the hot filament chemical vapor deposition (HF-CVD) technique with filament 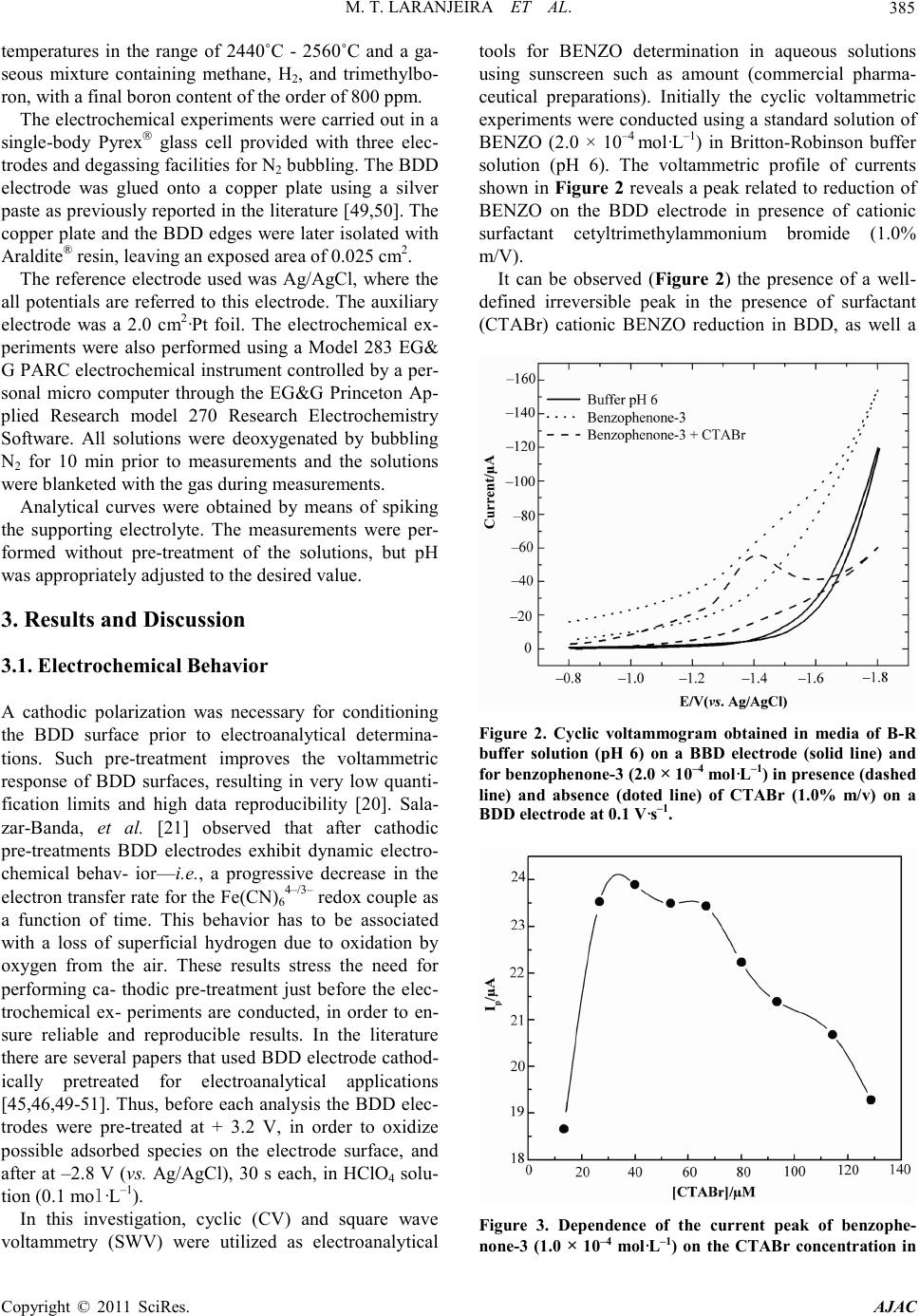 M. T. LARANJEIRA ET AL. Copyright © 2011 SciRes. AJAC 385 temperatures in the range of 2440˚C - 2560˚C and a ga- seous mixture containing methane, H2, and trimethylbo- ron, with a final boron content of the order of 800 ppm. The electrochemical experiments were carried out in a single-body Pyrex glass cell provided with three elec- trodes and degassing facilities for N 2 bub b li ng. The B D D electrode was glued onto a copper plate using a silver paste as previously reported in the literature [49,50]. T he copper plate and the BDD edges were later isolated with Araldite® resin, leaving an exposed area of 0.025 cm2. The reference electrode used was Ag/AgCl, where the all potentials are referred to this electrode. The auxiliary electrode was a 2.0 cm2·Pt foil. The electrochemical ex- periments were also performed using a Model 283 EG& G PARC electrochemical instrument controlled by a per- sonal micro computer through the EG&G Princeton Ap- plied Research model 270 Research Electrochemistry Software. All solutions were deoxygenated by bubbling N2 for 10 min prior to measurements and the solutions were blanketed with the ga s d uri n g mea s urements . Analytical curves were obtained by means of spiking the supporting electrolyte. The measurements were per- formed without pre-treatment of the solutions, but pH was appropriately adjusted to the desired value. 3. Resul t s and Discussion 3.1. Electrochemical Behavior A cathodic polarization was necessary for conditioning the BDD surface prior to electroanalytical determina- tions. Such pre-treatment improves the voltammetric response of BDD surfaces, resulting in very low quanti- fication limits and high data reproducibility [20]. Sala- zar-Banda, et al. [21] observed that after cathodic pre-treatments BDD electrodes exhibit dynamic electro- chemical behav- ior—i.e., a progressive decrease in the electron transfer rate for the Fe(CN)64–/3– redox couple as a function of time. This behavior has to be associated with a loss of superficial hydrogen due to oxidation by oxygen from the air. These results stress the need for performing ca- thodic pre-treatment just before the elec- trochemical ex- periments are conducted, in order to en- sure reliable and reproducible results. In the literature there are several papers that used BDD electrode cathod- ically pretreated for electroanalytical applications [45,46,49-51]. Thus, before each analysis the BDD elec- trodes were pre-treated at + 3.2 V, in order to oxidize possible adsorbed species on the electrode surface, and after at –2.8 V (vs. Ag/AgCl), 30 s each, in HClO4 solu- tion (0.1 mol·L–1). In this investigation, cyclic (CV) and square wave voltammetry (SWV) were utilized as electroanalytical tools for BENZO determination in aqueous solutions using sunscreen such as amount (commercial pharma- ceutical preparations). Initially the cyclic voltammetric experiments were conducted using a standard solution of BENZO (2.0 × 10–4 mo l ·L–1) in Britton-Robinson buffer solution (pH 6). The voltammetric profile of currents shown in Figure 2 reveals a peak related to reduction of BENZO on the BDD electrode in presence of cationic surfactant cetyltrimethylammonium bromide (1.0% m/V). It can be observed (Figure 2) the presence of a well- defined irreversible peak in the presence of surfactant (CTABr) cationic BENZO reduction in BDD, as well a Figure 2. Cyclic voltammogram obtained in media of B-R buffer solution (pH 6) on a BBD electrode (solid line) and for be nz op he n on e-3 (2.0 × 10–4 mol·L–1) in presence (d ashe d line) and absence (doted line) of CTABr (1.0% m/v) on a BDD electrode at 0 .1 V·s–1. Figure 3. Dependence of the current peak of benzo phe- none-3 (1.0 × 10–4 mol·L–1) on the CTABr concentration in 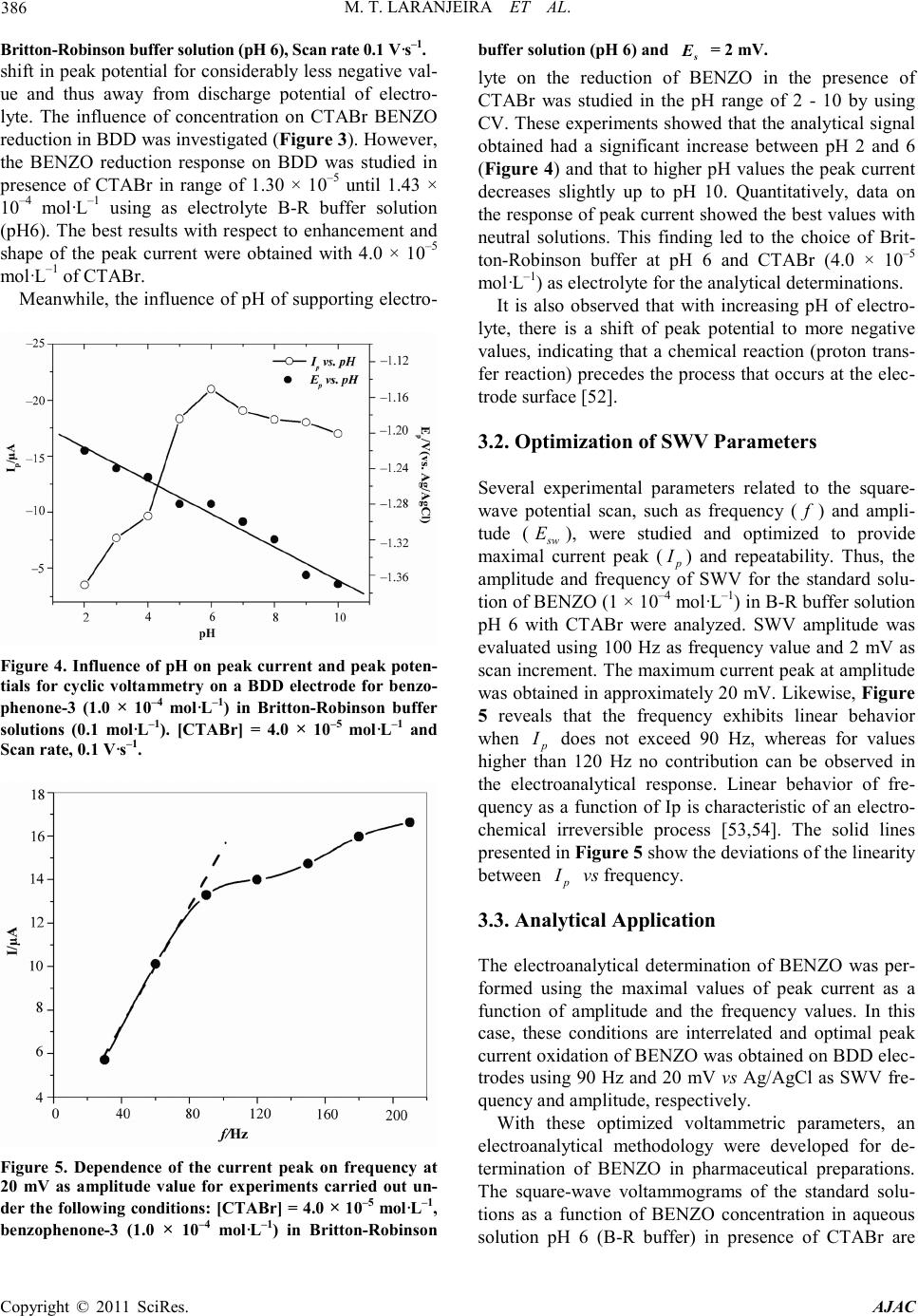 M. T. LARANJEIRA ET AL. Copyright © 2011 SciRes. AJAC 386 Britton-Robinson buffer solution (pH 6), Scan rate 0.1 V·s–1. shift in peak potential for considerably less negative val- ue and thus away from discharge potential of electro- lyte. The influence of concentration on CTABr BENZO reduction in BDD was inve stigated (Figure 3). However, the BENZO reduction response on BDD was studied in presence of CTABr in range of 1.30 × 10–5 until 1.43 × 10–4 mol·L–1 using as electrolyte B-R buffer solution (pH6). The best results with respect to enhancement a nd shape of the peak current were obtained with 4.0 × 10–5 mo l ·L–1 of CTABr. Meanwhile, the influence of pH of supporting electro- Figure 4. Influence of pH on peak current and pea k poten- tials for cyclic voltammetry on a BDD electrode for benzo- phenone-3 (1.0 × 10–4 mol·L–1) in Britton-Robinson buffer solutions (0.1 mol·L–1). [CTABr] = 4.0 × 10–5 mol·L–1 and Scan r ate, 0.1 V·s–1. Figure 5. Dependence of the current peak on frequency at 20 mV as amplitude value for experiments carried out un- der the following conditions: [CTABr] = 4.0 × 10–5 mol·L–1, benzophenone-3 (1.0 × 10–4 mol·L–1) in Britton-Robinson buffer solution (pH 6) and = 2 mV . lyte on the reduction of BENZO in the presence of CTABr was studied in the pH range of 2 - 10 by using CV. These experiments showed that the analytical signal obtained had a significant increase between pH 2 and 6 (Figure 4) and that to higher pH values the peak current decreases slightly up to pH 10. Quantitatively, data on the response of peak current showed the best values with neutral solutions. This finding led to the choice of Brit- ton-Robinson buffer at pH 6 and CTABr (4.0 × 10–5 mo l ·L–1) as electrol yte for the analytical determinatio ns. It is also observed that with increasing pH of electro- lyte, there is a shift of peak potential to more negative values, indicating that a chemical reaction (proton trans- fer reaction) precedes the process that occurs at the elec- trode surface [52]. 3.2. Optimization of SWV Parameters Several experimental parameters related to the square- wave potential scan, such as frequency ( ) and ampli- tude ( ), were studied and optimized to provide maximal current peak ( ) and repeatability. Thus, the amplitude and frequency of SWV for the standard solu- tion of BENZO (1 × 10–4 mol ·L–1) in B -R buffer solution pH 6 with CTABr were analyzed. SWV amplitude was evaluated using 100 Hz as frequency value and 2 mV as scan increment. The maximum current peak at amplitude was obtained in approximately 20 mV. Likewise, Figure 5 reveals that the frequency exhibits linear behavior when does not exceed 90 Hz, whereas for values higher than 120 Hz no contribution can be observed in the electroanalytical response. Linear behavior of fre- quency as a function of Ip is characteristic of an electro- chemical irreversible process [53,54]. The solid lines presented in Figure 5 show the deviations of the l inearity between vs frequency. 3.3. Analytical Application The electroanalytical determination of BENZO was per- formed using the maximal values of peak current as a function of amplitude and the frequency values. In this case, these conditions are interrelated and optimal peak current oxidatio n of BEN ZO was ob tained on BDD e lec- trodes using 90 Hz and 20 mV vs Ag/AgCl as SWV fre- quency and amplitude, respectively. With these optimized voltammetric parameters, an electroanalytical methodology were developed for de- termination of BENZO in pharmaceutical preparations. The square-wave voltammograms of the standard solu- tions as a function of BENZO concentration in aqueous solution pH 6 (B-R buffer) in presence of CTABr are 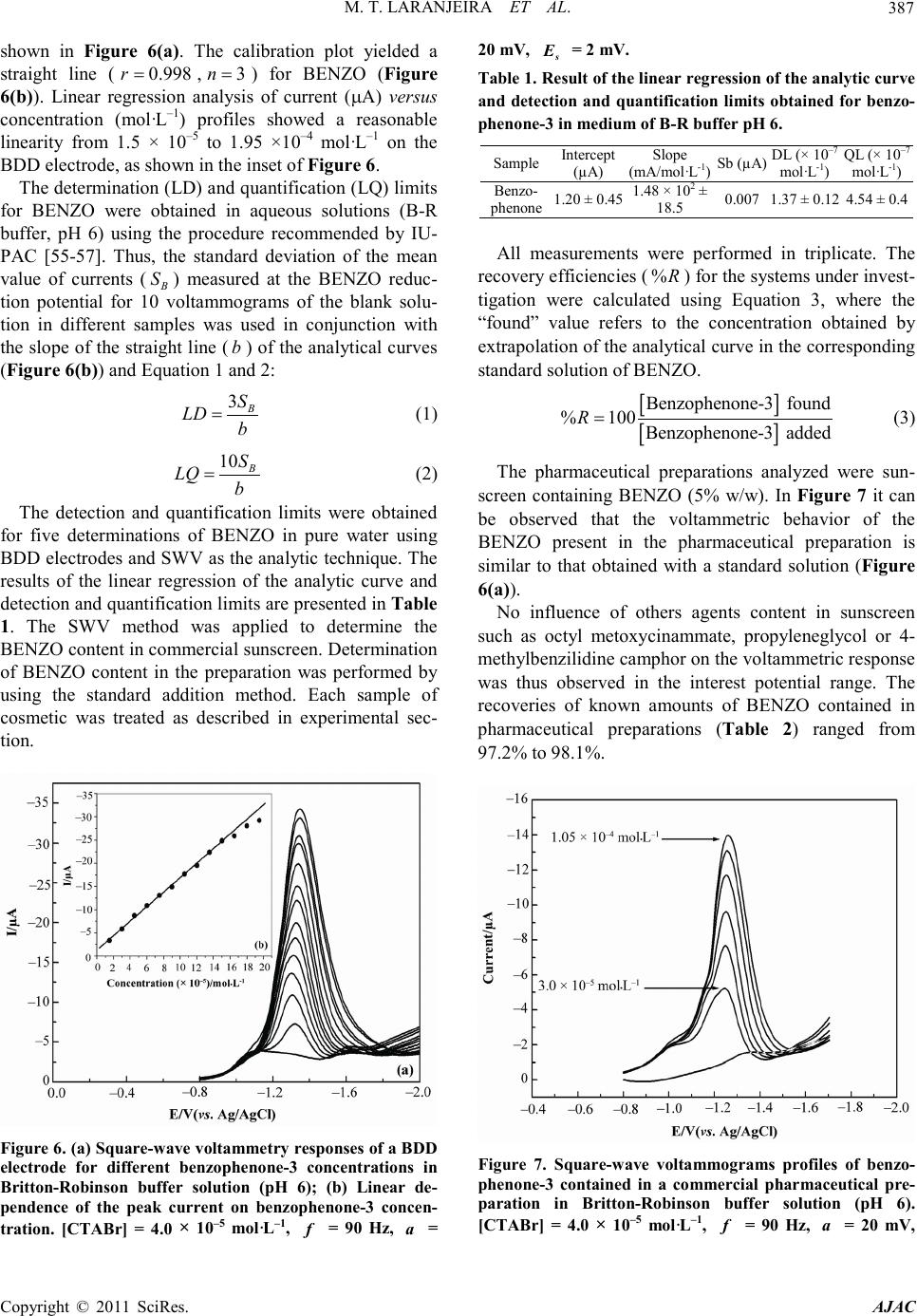 M. T. LARANJEIRA ET AL. Copyright © 2011 SciRes. AJAC 387 shown in Figure 6(a). The calibration plot yielded a straight line ( , ) for BENZO (Figure 6(b)). Linear regression analysis of current (µA) versus concentration (mol·L–1) profiles showed a reasonable linearity from 1.5 × 10–5 to 1.95 ×10–4 mo l·L–1 on the BDD electrode, as shown in the inset of Figure 6. The dete rmination (LD) a nd quantifica tion (LQ) li mits for BENZO were obtained in aqueous solutions (B-R buffer, pH 6) using the procedure recommended by IU- PAC [55-57]. Thus, the standard deviation of the mean value of currents ( ) measured at the BENZO reduc- tion potential for 10 voltammograms of the blank solu- tion in different samples was used in conjunction with the slope of the straight l ine ( ) of t he anal ytical c urves (Figure 6(b)) and Equation 1 and 2: (1) (2) The detection and quantification limits were obtained for five determinations of BENZO in pure water using BDD electrodes and SWV as the analytic technique. The results of the linear regression of the analytic curve and detection and quantification limits are presente d i n Table 1. The SWV method was applied to determine the BENZO content in commercial sunscreen. Determination of BENZO content in the preparation was performed by using the standard addition method. Each sample of cosmetic was treated as described in experimental sec- tion. Figure 6 . (a) Square-w ave v olta mmet ry res pons es of a B DD electrode for different benzophenone-3 concentrations in Britton-Robinson buffer solution (pH 6); (b) Linear de- pendence of the peak current on benzophenone-3 concen- tration. [CTABr] = 4.0 × 10–5 mol·L–1, = 90 Hz, = 20 mV, = 2 mV. Table 1 . Re sult of the l in ear regressio n of the a nal yti c curve and detection and qua ntification limits obtai ned for benzo- phenone-3 in medium of B-R buff er pH 6. Sample (µA) (mA/mol·L-1) Sb (µA) mol·L-1) mol·L-1) phenone 1.20 ± 0.45 18.5 0.007 1.37 ± 0.12 4.54 ± 0.4 All measurements were performed in triplicate. The recovery efficiencies ( ) for the s yste ms unde r invest- tigation were calculated using Equation 3, where the “found” value refers to the concentration obtained by extrapolation o f the ana lyti cal cur ve in the c or resp ond ing standard solution of BENZO. [ ] [ ] Benzophenone-3 found %100 Benzophenone-3 added R= (3) The pharmaceutical preparations analyzed were sun- screen containing BENZO (5% w/w). In Figure 7 it can be observed that the voltammetric behavior of the BENZO present in the pharmaceutical preparation is similar to that obtained with a standard solution (Figure 6(a)). No influence of others agents content in sunscreen such as octyl metoxycinammate, propyleneglycol or 4- methylbenzilid ine camphor on the vo ltammetric response was thus observed in the interest potential range. The recoveries of known amounts of BENZO contained in pharmaceutical preparations (Table 2) ranged from 97.2% to 98.1%. Figure 7. Square-wave voltammograms profiles of benzo- phenone-3 contained in a commercial pharmaceutical pre- paration in Britton-Robinson buffer solution (pH 6). [CTABr] = 4.0 × 10–5 mol·L–1, = 90 Hz, = 20 mV,  M. T. LARANJEIRA ET AL. Copyright © 2011 SciRes. AJAC 388 = 2 mV . Table 2 . Rec overies of benzop henone-3 samples in commer- cial cosmetic preparations (nominal conc. 5.0% w/w) using square wave voltammetry experiments carried out using the following conditions: = 90 Hz, = 20 mV and = 2 mV ( = 3). Lotion Added (mol·L–1 benzophenone-3) Found (mol·L–1 benzophenone-3) Recovery (%) R.S.D (%) A 1.00 × 10–5 9.81 × 10–6 98.1 0.8 B 1.00 × 10–5 9.72 × 10–6 97.2 1.1 C 1.0 × 10–5 9.78 × 10–6 97.5 0.9 4. Conclusions In this investigation, BENZO was found to provide a reductive peak when cyclic and square-wave voltam- metry experiments where conducted using BDD elec- trodes. Based on these experiments, an electroanalytical method for the determination of BENZO in water and commercial sunscreen was developed. The electro- chemical responses of pharmaceutical preparations were identical to those of standard BENZO and no influence of others agents content in sunscreen on the voltammet- ric responses was observed. BENZO recoveries values ranged from 97.2% to 98.1% demonstrate the elevated efficiency of the methodology. Consequently, given it’s easily of use, high sensitivity, and brevity, the method proposed can be successfully used to determine trace amounts of BENZO in several commercial products. 5. Acknowledgements The authors thank the Brazilian Research Funding Insti- tutions CNPq, Capes, Fundect and Fapemig for financial support. 6. References [1] H. Gonzalez, A. Abrot, O. Larko and A. M. Wennberg, “Percutaneous Absorption of the Sunscreen Benzophe- none-3 after Repeated Whole-Body Applications, with and without Ultraviolet Irradiation,” British Journal of Dermatology, Vol. 154, No. 2, 2006, pp .337-340. [2] T. Suzuki, S. Kitamura, R. Khota, K. Sugihara, N. Fujimoto and S. Ohta, “Estrogenic and Antiandrogenic Activities of 17 Benzophenone Derivatives Used as UV Stabilizers and Sunscreens,” Toxicology and Applied Pharmacology, Vol. 203, No. 1, 2005, pp. 9-17. doi:10.1016/j.taap.2004.07.005 [3] C. G. Daughton, “Environmental Stewardship and Drugs as Pollutants,” Lancet, Vol. 360, No. 9339, 2002, pp. 1035-1036. doi:10.1016/S0140-6736( 02)11 176-7 [4] M. E. Balmer, H. R. Buser, M. D. Muller and T. Poiger, “Occurren ce of Some Organic UV Filter s in Wastewater, in Surface Waters, and in Fish from Swiss Lakes,” Envi- ronmental Science & Technology, Vol. 39, No. 4, 2005, pp. 953-962. doi:10.1021/es040055r [5] G. A. Lo rai ne and M. E. Pet ti grove, “S easonal Variations in Concentrations of Pharmaceuticals and Personal Care Products in Drinking Water and Reclaimed Wastewater in Southern California,” Environmental Science & Tech- nology, Vol. 40, No. 3, 2006, pp. 687 -695. doi:10.1021/es051380x [6] S. C. Rastogi and G. H. Jensen, “Identification of UV Filters in Sunscreen Products by High-Performance Liq- uid Chromatography-Diode Array Detectio n,” Journal of Chromatography A, Vol. 828, No. 1-2, 1998, pp. 311-316. doi:10.1016/S0021-9673(98)0078 4-5 [7] V. Vanquerp, C. Rodriguez, C. Coiffard, L. J. M. Co- iffard and Y. D. Roeck-Holt zhauer, “High-Performance Liquid Chromatographic Method for the Comparison of the Photostability of Five Sunscreen Agent s ,” Journal of Chromatography A, Vol. 832, No. 1-2, 1999, pp. 273-277. doi:10.1016/S0021-9673(98)0092 8-5 [8] A. Chisvert, M. C. Pascual-Marti and A. Salvador, “De- termination of UV-Filters in Sunscreens by HPLC,” Fresenius Journ al of Analytical Chemistry, Vol. 369, No. 7-8, 2001, pp. 638-641. doi:10.1007/s002160100701 [9] T. Felix, B. J. Hall and J. S. Brodbelt, “Determination of Benzophenone-3 and Metabolites in Water and Human Urine by Solid-Phase Microextraction and Quadrupole Ion Tra p GC -MS,” Analytica Chimica Acta, Vol. 371, No. 2-3, 1998, pp. 195-203. doi:10.1016/S0003-2670(98)00293-1 [10] V. Lorena, C. Alberto, C. Antonio, P. Elefteria, L. Alexei, A. Fernan do, J. Karen, A. H. James and M. Fra nk, “Che- mically Surface-Modified Carbon Nanoparticle Carrier for Phenolic Pollutants: Extraction and Electrochemical Determination of Benzophenone-3 and Triclosan,” Ana- lytica Chimica Acta, Vol. 616, No. 1, 2008, pp. 28-35. doi:10.1016/j.aca.2008.04.011 [11] A. O. Razak, A. A. Gazy and A. M. Wahbi, “Polarogra- phic Determination of Phenytoin and Benzophenone (as Impurity) in Pharmaceutical Preparatio ns,” Journa l Pha r- maceutical and Biomedical Analysis, Vol. 28 , No. 3-4, 2002, pp . 613-619. doi:10.1016/S0731-7085(01)00669-0 [12] J. C. Cardoso, B. M. L. Armondes, J. B. G. Júnior and V. S. Ferreira, “Simultaneous Electrochemical Determina- tion of Three Sunscreens Using Cetyltrimethylammonium Bromide,” Colloids and Surfaces B: Biointerfaces, Vol. 63, No. 1, 2008, pp . 34-40. doi:10.1016/j.colsurfb.2007.11.001 [13] A. J. Wain, J. D. Wadhawan and R. G. Compton, “Elec- trochemical Studies of Vitamin K1 Microdroplets: Elec- trocatalytic Hydrogen Evolution,” Chemical Physics And Physical C hemistry, Vol. 4, No. 9, 2003, pp. 974-982. [14] J. M. Gong and X. Q. Lin, “Electrochemical Determina- tion of Serotonin and the Competitive Adsorption with Dopamine at 5,5-Ditetradecyl-2-(2-trimethylammonioeth- yl)-1,3-dioxane Bromide Lipid Film Modified by Glassy  M. T. LARANJEIRA ET AL. Copyright © 2011 SciRes. AJAC 389 Carbon Electrode,” Anaytica Scien ces, Vol. 20, No. 6, 2004, pp. 905-909. doi:10.2116/analsci.20.905 [15] H. B. Suffredini, S. A. S. Machado and L. A. Avaca, “The Water Decomposition Reaction on Boron-Doped Diamond Electrode,” Journal Brazilian Chemical Society, Vol. 15, No. 1, 2004 , pp. 16-21. doi:10.1590/S0103-50532004000100004 [16] R. Bellagamba, P. A. Michaud, C. Comninellis and N. Vatistas, “Electro-Combustion of Polyacrylates with Bo- ronDoped Diamond Anodes,” Electrochemistry Com- muication, Vol. 4, N o. 2, 2002, pp. 171-176. doi:10.1016/S1388-2481(01)00302-2 [17] J. S. Foord and C. H. Goeting, “Electrochemically Con- trolled Modification of CVD Diamond Surfaces,” Dia- mond and Related Materials, V ol. 13, No. 4-8, 2004, pp. 1054-1058. doi:10.1016/j.diamond.2003.12.016 [18] M. C. Granger and G. M. Swain, “The Influence of Sur- face Interactions on the Reversibility of Ferri/Ferrocya- nide at Boron-Doped Diamond Thin-Film Electrodes,” Journal of th e Electrochemical Society, Vol. 146, No. 12, 1999, pp . 4551-4558. doi:10.1149/1.1392673 [19] I. Duo, C. Lévy-Clément, A. Fujishima and C. Comni- nellis, “Electron Transfer Kinetics on Boron-Doped Dia- mond Part I: Influence of Anodic Treatment,” Journal of Applied Electrochemistry, Vol. 34, No. 9, 2004, pp. 935- 943. doi:10.1023/B:JACH.0000040525.76264.16 [20] H. B. Suffredini, V. A. Pedrosa, L. Codognoto, S. A. S. Machado, R. C. Rocha-Filho and L. A. Avaca, “Enhan- ced Electrochemical Response of Boron-Doped Diamond Electrodes Brought on by a Cathodic Surface Pre-Treat- ment,” Electrochimica Acta, Vol. 49, No. 22-23, 2004, pp. 4021-4026. doi:10.1016/j.electacta.2004.01.082 [21] G. R. Salazar -Banda, L. S. Andrade, P. A. P. Nascente, P. S. Pizani, R. C. Rocha-Filho and L. A. Avaca, “On the Changing Electrochemical Behaviour of Boron-Doped Diamond Surfaces with Time after Cathodic Pre-Treat- ments,” Electrochimica Acta, Vol. 51, No. 22, 2006, pp. 4612-4619. doi:10.1016/j.electacta.2005.12.039 [22] M. S. Saha, T. Furuta and Y. Nishiki, “Conversion of Carbon Dioxide to Peroxycarbo nate at Boron-Doped Dia- mond Electrode,” Electrochemistry Communication, Vol. 6, No. 2, 2004, pp. 201-204. doi:10.1016/j.elecom.2003.11.014 [23] J. Iniesta, P. A. Michaud, M. Panizza and C. Comninellis, “Electrochemical Oxidation of 3-Methylpyridine at a Bo- ron-Doped Diamond Electrode: Application to Elec- troorganic Synthesis and Wastewater Treat ment,” Elec- trochemistry Communication, Vol. 3, No. 7, 2001, pp. 346- 351. doi:10.1016/S1388-2481( 01)00 174-6 [24] J. W. Strojek, M. C. Granger, T. Dallas, M. W. Holtz and G. M. Swain, “Enhanced Sign al-to-Background Ratios in Voltammetric Measurements Made at Diamond Thin- Film Electrochemical Interfaces,” Analytical Chemistry, Vol. 68, No. 13, 1996, pp. 2031-2037. doi:10.1021/ac9506847 [25] M. Hupert, A. Muck, J. Wang, J. Stotter, Z. Cvackova, S. Haymond, Y. Show and G. M. Swain, “Conductive Di- amond Thin-Films in Electrochemistry,” Diamond and Related Materials, Vol. 12, No. 10-11, 2003, pp. 1940- 1949. doi:10.1016/S0925-9635(03)00260-7 [26] Y. Yano, D. A. Tryk, K. Hashimoto and A. Fujishima, “Electrochemical Behavior of Highly Conductive Boron- Doped Diamond Electrodes for Oxygen Reduction in Alkaline Solution,” Journal of the Electrochemical Soci- ety, Vol. 145, No. 6, 1998, pp. 1870-1876. doi:10.1149/1.1838569 [27] N. Vinokur, B. Miller, Y. Avyigal and R. Kalisk, “Elec- trochemical Behavior of Boron-Doped Diamond Elec- trodes,” Journal of the Electroch emica l So ciety, Vol. 143, No. 10, 1996, pp. L238-L240. doi:10.1149/1.1837157 [28] T. N. Rao, Y. Yagi, T. Miwa, D. A. Tryk and A. Fujishi- ma, “Electroch emical Oxi dation of NADH at Highly Bo- ron-Doped Diamond Electrodes,” Analytical Chemistry, Vol. 71, No. 13 , 1999, pp. 2506-251 1. doi:10.1021/ac981376m [29] E. Popa, H. Notsu, T. Miwa, D. A. Tryk and A. Fujishi- ma, “Selective Elect r oche mical Detection of Do pamine in the Presence of Ascorbic Acid at Anodized Diamond Thin Film Electrodes,” El ectrochemistry and Solid-State Letters, Vol. 2, No. 1, 1999, pp. 49-51. doi:10.1149/1.1390730 [30] J. Iniesta, P. A. Michaud, M. Panizza, C. Cerisola, A. Aldaz and C. Comniniellis, “Electrochemical Oxidation of Phenol at Boron-Doped Diamond Electrode,” Ele- ctrochimica Acta, Vol. 46, No. 23, 2001, pp. 3573-3578. doi:10.1016/S0013-4686(01)00630-2 [31] M. C. Granger, J. S. Xu, J.W. Strojek and G. M. Swain, “Polycrystalline Diamond Electrodes: Basic Properties and Applications as Amperometric Detectors in Flow In- jection Analysis and Liquid Chromatography,” Analytica Chimica Acta, Vol. 397, No. 1-3, 199 9, pp. 145-161. doi:10.1016/S0003-2670(99)00400-6 [32] V. A. Pedrosa, L. Codognoto and L. A. Avaca, “Electro- analytical Determination of 4-Nitrophenol by Square Wave Voltammetry on Diamond Electrodes,” Journal of the Brazilian Chemical Society, Vol. 14, No. 4, 2003, pp. 530-535. doi:10.1590/S0103-50532003000400007 [33] V. A. Pedrosa, L. Codognoto and L. A. Avaca, “Is the Boron-Doped Diamond Electrode a Suitable Substitute for Mercury in Pesticide Analyses? A Comparative Study of 4-Nitrophenol Quantification in Pure and Natural Wa- ters,” Journal of Electroanalytical Chemistry, Vol. 573, 2004, pp. 11-18. [34] V. A. Pedrosa, H. B. Suffredini, L. Codognoto, S. T. Ta- nimoto, S. A. S. Machado and L. A. Avaca, “Carbon Surfaces for Electrochemical Applications. A Compara- tive Study,” Analytical Letters, Vol. 38, 2005, pp. 1115- 1125. [35] L. Codognoto, S. A. S. Machad o and L. A. Ava ca, “Sq u ar e Wave Vo ltammetry on B oron-doped Diamond Electrodes for Analytical Determination,” Diamond and Related Materials, Vol. 11, No. 9, 2002. pp. 1670-1675. doi:10.1016/S0925-9635(02)00134-6 [36] G. W. Muna, N. Tasheva and G. M. Swa in “Electro-Oxi- dation and Amperometric Detection of Chlorinated Phe- nols at Boron-Doped Diamond Electrodes: A Compari-  M. T. LARANJEIRA ET AL. Copyright © 2011 SciRes. AJAC 390 son of Microcrystalline and Nanocrystalline Thin Films,” Environmental Science & Technology, Vol. 38, No. 13, 2004, pp . 3674-3682. d oi:10.1021/es034656e [37] C. E. Banks, M. E. Hyde, P. Tomcik, R. Jacobs and R. G. Comptom, “Cadmium Detection via Boron-Doped Di- amond Electrodes: Surfactant Inhibited Stripping Vol- tammetr y,” Talanta, Vol. 62, No. 2, 2004, pp.279-286. doi:10.1016/j.talanta.2003.07.008 [38] J. In iesta, P. A. Mi chau d, M . Panizza and C. Comninellis, “Electrochemical Oxidation of 3-Methylpyridine at a Boron-Doped Diamond Electrode: Application to Elec- troorganic Synthesis and Wastewater Treatment,” Elec- trochemistry Communication, Vol. 3, No. 7, 2001, pp. 346-351. doi:10.1016/S1388-2481(01)00174-6 [39] P. A. Michaud, E. Mahé, W. Haenni, A. Perret and C. Comninellis, “Preparation of Peroxodisulfuric Acid Us- ing Boron-Doped Diamond Thin Film Electrode,” Elec- trochemistry and Solid-S tate Letters, V ol. 3, No. 2, 2000, pp. 77-79. doi:10.1149/1.1390963 [40] R. T. S. Ol iveira, G. R. Salazar-Banda, M. C. Santos, M. L. Calegaro, D. W. Miwa, S. A. S. Machado and L. A. Avaca, “Electrochemical Oxidation of Benzene on Bo- ron-Doped Diamond El ectrodes,” Chemosphere, Vol. 66, No. 11, 2007 , pp. 2152-2158. doi:10.1016/j.chemosphere.2006.09.024 [41] E. Brillas, B. Boye, I. Sires, J. A. Garrido, R. M. Rodri- guez, C. Arias, P. L. Cabot and C. Comninellis, “Elec- trochemical Destruction of Chlorophenoxy Herb icides by Anodic Oxidation and Electro-Fenton Using a Boron- Doped Diamond Electrode,” Electrochimica Acta, Vol. 49, No. 25, 2004, pp. 4487-4496. doi :1 0.1016/j.electacta.2004.05.006 [42] J. F. Zhi, H. B. Wang, T. Nakashima, T. N. Rao and A. Fujishima, “Electrochemical Incineration of Organic Pollutants on Boron-Doped Diamond Electrode. Evi- dence for Direct Electrochemical Oxidation Pathway,” Journal of Physical Ch emistry B, Vol. 107, No. 48, 2003, pp. 1338 9-13395. doi:10.1021/jp030279g [43] R. Bellagamba, P. A. Michaud, C. Comninellis and N. Vatistas, “Electro-Combustion of Polyacrylates with Bo- ron-Doped Diamond Anodes,” Electrochemistry Com- munication, Vol. 4, No. 2, 2002, pp. 171-176. doi:10.1016/S1388-2481(01)00302-2 [44] F. Montilla, E. Morallon, I. Duo, C. Comninellis and J. L. Vazquez, “Platinum Particles Deposited on Synthetic Boron-Doped Diamond Surfaces. Application to Metha- nol Oxidation,” El ectrochimi ca Acta, Vol. 48, No. 25 -26, 2003, pp . 3891-3897. doi:10.1016/S0013-4686(03)00526-7 [45] G. R. Salazar-Banda, H. B. Suffredini and L. A. Avaca, “Improved Stability of PtOx Sol-Gel Modified Diamond Electrodes Covered with a Nafion Film,” Journal of the Brazilian Chemical Society, Vol. 16, No. 5, 2005, pp. 903-906. doi:10.1590/S0103-5053 200500060 0003 [46] H. B. S u ffredi ni , G.R. S alazar-Ban da, S. T. Tanimoto, M. L. Calegaro, S. A. S. Machado and L. A. Avaca, “AFM Studies and Electrochemical Characterization of Boron- Doped Diamond Surfaces Modified with Metal Oxid e s by the Sol-Gel Metho d,” Journal of the Brazilian Chemical Soci ety, Vol. 17, N o. 2, 2006, pp. 257-264. doi:10.1590/S0103-5053200600020 0007 [47] C. Terashima, T. N . Rao, B. V. Sarada, Y. Kubota and A. Fujishima, “Direct Electrochemical Oxidation of Disul- fides at Anodically Pretreated Boron-Doped Diamond Electrodes,” Analytical Chemistry, Vol. 75, No. 7, 2003, pp. 1564 -1572. doi:10.1021/ac020583q [48] T. N. Rao, T. A. Ivandini, C. Terashima, B. V. Sarada and A. Fujishima, “Applications of Bare and Modified Diamond Electrodes in Electro analysis,” New Diamond Frontier Carbon Technology, Vol. 13, 2003, pp.79-88. [49] R. T. S. Oliveira, G. R. Salazar-Banda, S. C . Oliveira, V . S. Ferreira and L. A. Avaca, “Electroanalytical Determi- nation of Lidocaine in Pharmaceutical Preparations Using Boron- Doped Diamond Electrodes,” Electroanalysis, Vol. 19, No. 11 , 2007, pp. 1189-119 4. doi:10.1002/elan.200603840 [50] R. T. S. Oliveira, G. R. Salazar-Band a, S. A. S. Machado and L. A. Avaca, “Electroanalytical Determination of N- Nitrosamines in Aqueous Solution Using a Boron-Doped Diamond Electrode,” Electroanalysis, Vol. 20, No. 4, 2008, pp . 396- 401. doi:10.1002/elan.200704055 [51] G. P as to r -Moreno and D. J. Riley, “The Influence o f Sur- face Preparation on the Electrochemistry of Boron-Doped Diamond: A Study of the Reduction of 1,4-Benzoquinone in Acetonitrile,” Electrochemistry Communication, Vol. 4, No. 2, 2002, pp. 218-221. doi:10.1002/elan.200704055 [52] A. Arr anz, L. Dolar a, S. F. Betõno, J. M. Moreda, A. Cid and J. F. Arranz, “Electroanalytical Study and Square Wave Voltammetric Techniques for the Determination of β-Blocker Timolol at the Mercury Electrode,” Analytica Chimi ca Acta, Vol. 389, No. 1-3, 1999, pp . 225-232. doi:10.1016/S0003-2670(99)00214-7 [53] M. Lovrić, K. Šebojka and W. Murray, “Adsorption Ef- fects in Square-Wave Voltammetry of Totally Irreversi- ble Redox Reactio ns,” Electrochimica Acta, Vol. 33, No. 6, 1988, pp. 739-744. doi:10.1016/S0013-4686(98)80002-9 [54] M. Lovrić and K. Šebojka, “Square-Wave Voltammetry of an Adsorbed Reactant,” Journal of Electronalytical Chemistry and Interf acial Electr ochemistry, Vol. 248, No. 2, 1988, pp. 239-253. doi:10.1016/0022-0728(88)85089-7 [55] R. Q. Thompson, M. Porter, C. Stuver, H. B. Halsall, W. R. Heineman, E. Buckley and M. R. Smyth, “Zeptomole Detection Limit for Alkaline Phosphatase Using 4-Aminophenyl-Phosphate, Amperometric Det ection , and an Optimal Buffer System,” Analytica Chimica Acta, Vol. 271, No. 2, 1993 , pp. 223-229. doi:10.1016/0003-2670( 93)80 049-Q [56] L. A. Currie, “International Recommendations Offered on Analytical Detection and Quantification Concepts and Nomenclature,” Analytica Chimica Acta, Vol. 391, No. 2, 1999, p. 103. doi:10.1016/S0003-2670(99)00103-8 [57] J. Mocak, A. M. Bond, S. Mitchell and G. Scollary, “A Statistical Overview of Standard (IUPAC and ACS) and New Procedures for Determining the Limits of Detection and Quantification: Application to Voltammetric and  M. T. LARANJEIRA ET AL. Copyright © 2011 SciRes. AJAC 391 Stripping Techniqu es,” Pure and Applied Chemistry, Vol. 69, No. 2, 1997, pp. 297-328. doi:10.1351/pac199769020297
|