 Pharmacology & Pharmacy, 2011, 2, 116-121 doi:10.4236/pp.2011.23015 Published Online July 2011 (http://www.scirp.org/journal/pp) Copyright © 2011 SciRes. PP Morphological and Functional Alterations in Human Red Blood Cells Treated with Titanium Citrate Gugliotta Tiziana, De Luca Grazia, Romano Pietro, Rigano Caterina, Romano Orazio, Scuteri Adriana, Romano Leonardo Department of Scienze della Vita “M. Malpighi”, Sezione di Fisiologia generale e Farmacologia, Facoltà di Scienze MM.FF.NN., Università degli Studi di Messina, Messina, Italy. Email: tiziana_gugliot@ yahoo.it Received February 24th, 2011; revised May 2nd, 2011; accepted June 10th, 2011. ABSTRACT The morphological and functional effects of titanium (Ti) citrate on human erythrocytes were studied by scanning elec- tron microscope (SEM), sulphate uptake via band 3 protein and by determining the reduced and oxidised glutathione (GSH and GSSG, respectively) concentrations. The rate constant for sulphate uptake was significantly lower after Ti citrate treatment. Ti citrate (0.001 and 0.0025 mM) significantly decreased erythrocyte GSH and increa sed GSSG con- centrations. At 0.005 mM Ti citrate, the intracellular GSH could not be tested due to significant cellular damag e. SEM of erythrocytes treated with 0.001 mM and 0.0025 mM Ti citrate showed structural membrane defects but almost nor- mal cellular diameters. At even higher Ti citrate concen trations (0.005 mM), erythrocytes showed obvious morphologi- cal alteration and shape changes compromising the cells physiological functions. In conclusion, although the Ti con- centrations used in our experiments are physiologically high, the cumulative effect of prolonged exposure to much lower doses of Ti, as might occur during total hip replacement, should be considered for further experimental testing. Keywords: A nion Transport, Band 3 Protein, Erythrocytes, E ry throcyte Membran e, GSH, Titanium Citrate 1. Introduction Titanium (Ti) is largely present in nature in the form of oxides and it is contained in different minerals. It is the ninth element in order of diffusion and the fourth in order of abundance among the metals, preceded only by aluminum, iron and magnesium. Modern extraction technologies have allowed the use of Ti both in the aereonautic-spatial industry as well as some medical fields, particularly orthopedics, cardio-vascular surgery and dental surgery. Among the many qualities that make Ti useful for implantation, are its long-term hard- ness and biocompatibility. The success of using pure Ti in medicine is due to its unique physical and chemical characteristics, not readily found in other metals, such as its X-ray transparency that helps in diagnosing the state of prosthesis conservation. Ti compounds are currently the subject of much bio- logical research [1,2]. A recent study has investigated Ti compounds as anticancer agents because they are easily disseminated throughout the body [3]. One the few studies showed that osteoblast-like cells respond to titanium particles through increased expression of the proinflammatory cytokine interleukin-6 in a process re- quiring phagocytosis and intracellular signaling path- ways. Other than this specific research, very limited data are available on the toxic effects of soluble Ti (IV) complexes in erythrocytes and in particular the effect on band 3 protein. Band 3 protein is the most abundant membrane protein in human erythrocytes, being pre- sent at approximately 1 million copies per cell [4], and it facilitates the electroneutral exchange of Cl– and 3 HCO across the membrane. Three anion exchanger isoforms are known, AE1, AE2 and AE3, which differ in their tissue expression. AE1 is found in erythrocytes and in the kidney, AE2 is found in a wide variety of tissue and AE3 is found in the brain, the retina and the heart. All the anion ex- changer family consist of two domains, an N-terminal cytoplasmic domain that contains binding sites for glycolytic enzymes and haemoglobin and a C-terminal  Morphological and Functional Alterations in Human Red Blood Cells Treated with Titanium Citrate 117 membrane domain [5,6]. The membrane domain is highly conserved, it spans the lipid bilayer 12 - 14 times and mediates anion transport. The cytoplasmic domain is anchored to the cytoskeleton and plays a structural role by connecting the cytoskeleton to the membrane [7,8]. Band 3 protein is one of the main phosphorylated membrane proteins. It is phosphorylated mainly at three sites in the cytoplasmic domain, the most impor- tant being tyrosine-8, followed by tyrosine-21 and ty- rosine-46 [9]. Band 3 protein phosphorylation is ac- companied by rigidification of the membrane skeleton, suggesting the visco-elastic properties of human eryth- rocytes may be regulated by band 3 tyrosine phos- phorylation [10]. The aim of our study was, therefore, to expose hu- man erythrocytes to different concentrations of Ti cit- rate (IV) in order to study: 1) its effects on the mecha- nisms of anionic transport mediated by band 3 protein, 2) its oxidative effects by the determination of -SH groups and intracellular concentrations of GSH and GSSG and 3) its action on the erythrocyte membrane by scanning electron microscopy (SEM). 2. Materials and Methods 2.1. Erythrocyte Preparation Human blood was obtained, after informed consent, from 30 healthy volunteers. The blood was collected in heap- rinnized tubes, washed in 150 mM NaCl, 25 mM HEPES, pH 7.40 and centrifuged three times for 5 min at 3000 rpm. At each step the buffy coat was carefully removed and the cells were suspended at 3% hematocrit and in- cubated for 1 h at 25˚C in the following medium: 140 mM choline chloride, 10 mM NaCl, 25 mM HEPES, 10 mM glucose, pH 7.4, ± increasing concentrations of Ti citrate (0, 0.0005, 0.001, 0.0025 and 0.005 mM). After incubation, the suspensions were centrifuged and the ery- throcytes were divided into three aliquots. These were used to study sulphate kinetics, to indirectly verify the oxidative effects exercised by Ti citrate on the -SH groups of the membrane proteins, for measuring the in- tracellular concentrations of GSH and GSSG and to ob- serve the red blood cell shape by SEM. Ti citrate (IV) was obtained by mixing Ti III chloride with sodium citrate in the ratio 1:1.2, at pH 3. The Ti citrate solution was used after verifying the absence of precipitates and after solutions become completely clear, to avoid interference with our experimental protocol [11- 13]. 2.2. Sulphate Transport Measurement For kinetic studies, the erythrocytes were resuspended in 3% hematocrit and incubated at 25˚C in the following medium: 115 mM Na2SO4, 5 mM Na3(C6H5O7)2H2O, 5 mM KCl, 25 mM HEPES, 5 mM glucose increasing concentrations of Ti citrate (0, 0.001, 0.0025 and 0.005 mM), pH 7.40. At specified intervals, 5 ml samples of the suspension were removed and added to a test tube containing 5 M 4,4’-diisothiocyanato-stilbene-2,2’-di- sulfonate (DIDS) stopping medium and incubated on ice. DIDS binds specifically and irreversibly to the band 3 protein and inhibits sulphate transport in human red blood cells. In the same experiments, DIDS was added to the red blood cell suspension to give a final concentra- tion of 5 M. After withdrawal of the last sample, the erythrocytes were washed three times in a sulphate-free medium at 0˚C to remove extracellular sulphate. Cells were then lysed with distilled water and trichloroacetic acid. The membranes were removed by centrifugation and sulphate ions in the supernatant were precipitated by adding glyc- erol and distilled water (1:1), 4 mM NaCl and HCl (37%) (12:1), and 124 mM BaCl2 dihydrate, to obtain a homo- geneous barium sulphate precipitate. The intracellular sulphate concentration was measured by atomic absorp- tion spectrophotometry at 425 nm. Using a standard cur- ve, obtained by precipitating known sulphate concentra- tions, we converted the absorption to mM intracellular sulphate and calculated the rate constant in min-1 by a non-linear, least square, curve-fitting procedure applying the following equation: C(t) = C∞(1 – e–rt) + C0 where C0, Ct and C represent the intracellular sulphate concentrations measured at times 0, t and [14,15], e indicates nepero number ( 2.7182818) and r is a constant. 2.3. GSH Measurement The GSH concentration was measured in erythrocytes before and after treatment with 0.001 and 0.0025 mM Ti citrate using an immunodiagnostic assay [16], intended for the quantitative determination of glutathione in EDTA- blood. At 0.005 mM Ti citrate, the intracellular GSH was not possible to test because of damage to the erythrocyte membrane. In this assay, the sample was treated with a dilute so- lution of Ti citrate and divided into two aliquots: 1) The reduced fraction was measured by adding 50 l of the diluted sample, 100 l reaction buffer and 100 l deri- vatization solution. After incubation for 20 min at 60˚C, during which time GSH was converted to a fluorescent product, 100 l of the precipitation solution were added to remove higher molecular weight substances. The sam- ples were precipitated for 10 min at 2˚C - 8˚C and centri- fuged for 10 min at 6000 rpm, then 200 l of supernatant Copyright © 2011 SciRes. PP  118 Morphological and Functional Alterations in Human Red Blood Cells Treated with Titanium Citrate were added to 200 l of the reaction buffer in autosam- pler vials. 2) The total glutathione was measured by adding 50 l of the diluted sample, 20 l of the reduction solution, 100 l of the internal standard and 100 l of the derivatization solution. The sample was then handled like the reduced fraction. After that, 20 l of the super- natant were injected into the HPLC system. The separa- tion by HPLC followed an isocratic method at 30˚C us- ing a reversed-phase column in two runs. The chromatograms were scanned by a fluorescence detector and concentrations were calculated by integra- tion of the peak height by the external standard method for the reduced fraction and the internal standard method for the total glutathione fraction. The amount of oxidised glutathione was calculated by subtraction of: glutathione total – glutathione reduced 2.4. Preparation of Erythrocyte Membrane, Isolation of Band 3 Protein and Determination of Sulphydryl Groups Erythrocytes (with/without Ti treatment) were washed with an isotonic solution (150 mM NaCl, 25 mM HEPES, pH 7.40) and hemolysed by adding 20 volumes of cold hypotonic buffer (5 mM HEPES, pH 7.40). Membranes were obtained by centrifugation at 20,000 g for 30 min at 4˚C. The process was repeated with the same hypotonic buffer until the red blood cell membranes were almost free of hemoglobin [17]. One volume of red blood cell membrane was then incubated with nine volumes of 0.1 N NaOH for 30 min at 0˚C in the presence of 0.2 mM DTT (dithiothreitol) and 20 g/ml PMSF (phenylmethyl- sulfonyl fluoride). After incubation, samples were cen- trifuged at 20,000 g for 30 min at 4˚C. The pellet containing band 3 protein was washed three times with 5 mM HEPES, pH 7.40, and used for deter- mination of sulphydryl groups. Membrane with/without Ti treatment and containing band 3 protein was incu- bated with 0.3 ml of 20% SDS (Sodium Dodecyl Sul- phate) and 2.8 ml of 100 mM sodium phosphate, pH 8, for 25 min at 37˚C. The pellet suspension was further incubated with 0.1 ml of 10 mM DTNB (2-nitrobenzoic acid) in 100 mM sodium phoshate, pH 8, for 20 min at 37˚C. The sulphydryl group concentration was measured by atomic absorption spectrophotometry at 412 nm [18]. A standard curve was employed to calculate the concen- tration of thiol groups. 2.5. Scanning Electron Microscopy and Human Erythrocytes The sample preparation technique for electron micros- copy involved: fixation, dehydration, assembly, covering with gold and observation. To analyse the effect of Ti citrate on the morphology of human red blood cells, the samples were incubated in the presence or absence of increasing concentrations of Ti at 25˚C for 1h. Samples were then washed with physiological solution, 166 mM NaCl, and fixed overnight at 5˚C by adding one drop of each sample to plastic tubes containing 1 ml of 4% glu- taraldehyde in Sorensen's phosphate buffer 0.1 M to pH 7.4. Samples were washed three times in the same buffer for 30 min and then exposed to increasing concentrations (30%, 50%, 70%, 90% and 95%) of ethanol for about 30 min each. The process of dehydration was carried out with liquid carbon dioxide until the critical point was reached. Then samples were assembled on a particular type of glass using conductive silver paste and covered with a thin layer of gold (200 - 300 A) in appropriate sputtering. The samples were then ready for observation by SEM. 3. Statistical Analysis In order to test the existence of homogenity between un- treated (control) and Ti treated cells, we applied non- parametric permutation tests. This procedure is more conservative than homologue parametric tests for small samples [19]. 4. Results The experiments were carried out in order to highlight not only possible modifications of sulphate transport me- diated by band 3 protein, but also variations in the levels of GSH, the shape of human red blood cell membranes and oxidation of -SH groups. We used low concentra- tions (0.0005 and 0.001 mM) and high concentrations (0.0025 and 0.005 mM) of Ti citrate. Sulphate influx in the control red blood cells and in cells treated with in- creasing concentrations of Ti citrate are reported in Fig- ure 1. The rate constant of the Ti citrate (0.001 mM) treated sample was 0.026 ± 0.001 × min–1 (Table 1), compared to 0.039 ± 0.001 × min–1 in the control sample, a reduc- tion of 33% relative to the control. In Figure 1, the curve (■) represents the cells treated with an intermediate con- centration (0.0025 mM) of Ti citrate. The last curve shows an increase in the time necessary to reach the satu- ration equilibrium of sulphate. In Figure 1, the graph (▲) represents the sulphate in- flux in human red blood cells treated with 0.005 mM of Ti citrate. It shows a remarkable difference in the kinetic profile. In fact it can be seen that sulphate saturation equilibrium was reached suddenly, because band 3 pro- tein lost its function of anion transporter due to damage by the titanium. Figure 2 shows a progressive decrease in the percen- tage of sulphydryl groups in band 3 protein with in- C opyright © 2011 SciRes. PP 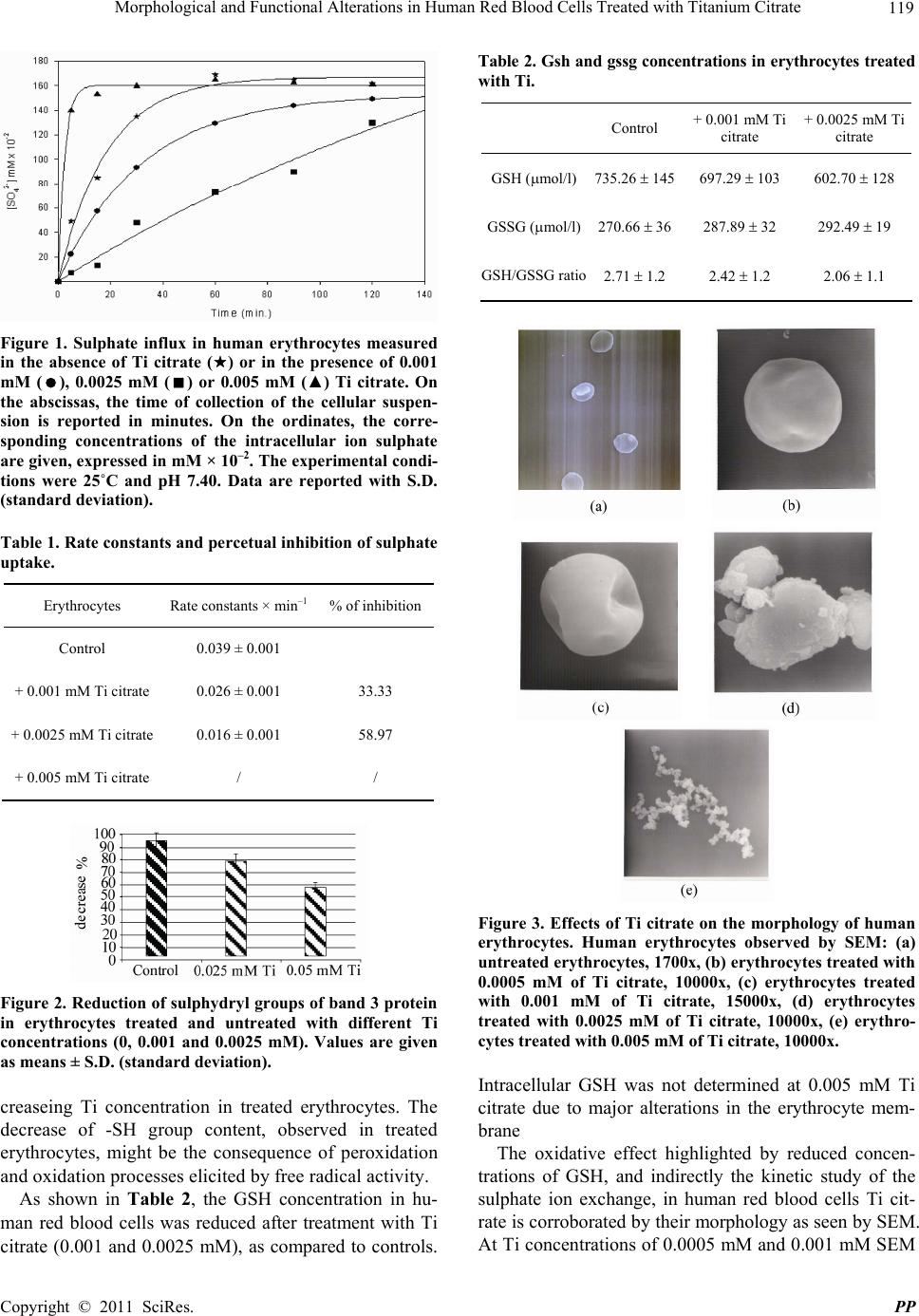 Morphological and Functional Alterations in Human Red Blood Cells Treated with Titanium Citrate 119 Figure 1. Sulphate influx in human erythrocytes measured in the absence of Ti citrate () or in the presence of 0.001 mM (), 0.0025 mM () or 0.005 mM (▲) Ti citrate. On the abscissas, the time of collection of the cellular suspen- sion is reported in minutes. On the ordinates, the corre- sponding concentrations of the intracellular ion sulphate are given, expressed in mM × 10–2. The experimental condi- tions were 25˚C and pH 7.40. Data are reported with S.D. (standard deviation). Table 1. Rate constants and percetual inhibition of sulphate uptake. Erythrocytes Rate constants × min–1 % of inhibition Control 0.039 ± 0.001 + 0.001 mM Ti citrate 0.026 ± 0.001 33.33 + 0.0025 mM Ti citrate 0.016 ± 0.001 58.97 + 0.005 mM Ti citrate / / Figure 2. Reduction of sulphydryl groups of band 3 protein in erythrocytes treated and untreated with different Ti concentrations (0, 0.001 and 0.0025 mM). Values are given as means ± S.D. (standard deviation). creaseing Ti concentration in treated erythrocytes. The decrease of -SH group content, observed in treated erythrocytes, might be the consequence of peroxidation and oxidation processes elicited by free radical activity. As shown in Table 2, the GSH concentration in hu- man red blood cells was reduced after treatment with Ti citrate (0.001 and 0.0025 mM), as compared to controls. Table 2. Gsh and gssg concentrations in erythrocytes treated with Ti. Control + 0.001 mM Ti citrate + 0.0025 mM Ti citrate GSH (mol/l)735.26 145697.29 103 602.70 128 GSSG (mol/l) 270.66 36287.89 32 292.49 19 GSH/GSSG ratio2.71 1.2 2.42 1.2 2.06 1.1 Figure 3. Effects of Ti citrate on the morphology of human erythrocytes. Human erythrocytes observed by SEM: (a) untreated erythrocytes, 1700x, (b) erythrocytes treated with 0.0005 mM of Ti citrate, 10000x, (c) erythrocytes treated with 0.001 mM of Ti citrate, 15000x, (d) erythrocytes treated with 0.0025 mM of Ti citrate, 10000x, (e) erythro- cytes treated with 0.005 mM of Ti citrate, 10000x. Intracellular GSH was not determined at 0.005 mM Ti citrate due to major alterations in the erythrocyte mem- brane The oxidative effect highlighted by reduced concen- trations of GSH, and indirectly the kinetic study of the sulphate ion exchange, in human red blood cells Ti cit- rate is corroborated by their morphology as seen by SEM. At Ti concentrations of 0.0005 mM and 0.001 mM SEM Copyright © 2011 SciRes. PP  120 Morphological and Functional Alterations in Human Red Blood Cells Treated with Titanium Citrate revealed small membrane alterations but an almost nor- mal cellular diameter (Figures 3(b) and (c)), compared with the control (Figure 3(a)). Increasing concentrations of Ti citrate (0.0025 and 0.005 mM) (Figures 3(d) and (e)) showed increased morphological damage of the erythrocyte population. 5. Discussions Band 3 protein mediates anion-exchange and acid-base equilibrium through the red blood cell membrane. It is an integral membrane protein crossing the bilayer lipid membrane many times. Because of its position, the pro- tein is continuously damaged by chemical agents and drugs circulating in the blood flow. Previous studies re- ported that some metals enhance degradation of band 3 protein after treatment with chemical substances likely to affect the sulphate permeability and modulate anion in- flux through the erythrocyte membranes [20]. In this set of experiments, sulphate uptake measured in control erythrocytes increased steeply at the intial stage reaching equilibrium in 30 minutes. This process was much slower across the membrane of Ti citrate treated cells. The decrease in the rate constant of influx in treated cells compared to control cells was more prominent after treatment with 0.0025 mM of Ti citrate (Figure 1). Ta- ble 1 indicates significant inhibition (33% and 58%) of rate constant sulphate influx across the red cell mem- brane after Ti citrate treatment. The structural integrity of band 3 protein has a close relationship with the func- tional aspects of this anion channel protein [15], and these results suggest a conformational change in the an- ion channel protein during Ti treatment making the transport sites more vulnerable to modification (Figures 3(a-e)). Marked degradation of membranes and band 3 protein resulted from using 0.005 mM of Ti citrate. In this case it was not possible to measure the rate constant in treated cells. This probably correlated with the gradual reduction in the percentage of sulphydryl groups (Figure 2) due to oxidative damage in the membrane because of a signifi- cant reduction in GSH (735 mol/l in control cells and 287 mol/l in Ti treated cells, Table 2). The GSH reduc- tion measured in Ti-treated erythrocytes may have al- tered the properties of hemoglobin and increased its ten- dency to aggregate, consequently modifying the hemo- globin-band 3 protein interaction [21]. In contrast, the binding of the chemical inhibitor DIDS at the anion binding site of band 3 protein is known to block the up- take of anion across the cell membrane. However, this does not deform the membrane. In fact, the GSH defi- ciency (Table 2) measured in Ti-treated erythrocytes al- tered the properties of haemoglobin and increased its tendency to aggregate, with consequent modification of the hemoglobin-band 3 protein interaction [21,22]. SEM of erythrocytes treated with increasing concen- trations of Ti revealed significant morphological differ- ences compared to untreated erythrocytes. In Figure 3, only a few of the erythrocytes exposed to lower concen- trations of Ti citrate (0.0005 and 0.001 mM) seem slight- ly deformed in shape compared to control cells. At high- er concentrations of Ti citrate (0.0025 mM), most of the erythrocytes showed morphological alterations. With 0.005 mM of Ti citrate the erythrocyte membranes were damaged and cells appeared smaller and more distorted. This is probably due to insertion of Ti citrate into the lipid bilayer of the erythrocyte membrane. In conclusion, although the Ti concentrations used in these experiments were unphysiologically high compared to Ti circulating, for example, after total hip replacement, our findings warrant examination of the effect of long- term exposure of erythrocytes to Ti and also check whe- ther the structural alterations induced by titanium citrate to human red blood cells can be extended to other cells affecting their functions. 6. References [1] Y. Kasai, R. Iida and A. Uccida, “Metal Concentrations in the Serum and Hair of Patients with Titanium Alloy Spinal Implants,” Spine, Vol. 28, No. 12, 2003, pp. 1320- 1326. doi:10.1097/00007632-200306150-00018 [2] C. G. Moon, H. S. Koth, D. Kluess, D. O’Commor, A. Mathur, G. A. Truskey, J. Rubin, D. X. F. Zhou and K. P. L. Sung, “Effects of Titanium Particle Size on Osteoblast Functions in Vitro and in Vivo,” Proceeding of the Na- tional academy of Sciences of the United States of Amer- ica, Vol. 102, No. 12, 2005, pp. 4578-4583. [3] F. Caruso and M. Rossi, “Antitumor Titanium Com- pounds,” Mini-Reviews in Medicinal Chemistry, Vol. 4, No. 1, 2004, pp. 49-60. doi:10.2174/1389557043487565 [4] N. Hamasaki and K. Okubo, “Band 3 Protein: Physiology, Function and Structure,” Cellular and Molecular Biology, Vol. 42, No. 7, 1996, pp. 1025-1039. [5] J. R. Casey and R. R. Kopito, “The Role of Cysteine Re- sidues in the Erythrocyte Plasma Membrane Anion Ex- change Protein,” The Journal of Biological Chemistry, Vol. 270, No. 15, 1995, pp. 8521-8527. doi:10.1074/jbc.270.15.8521 [6] A. Galtieri, E. Tellone, L. Romano, F. Misiti, E. Bellocco, S. Ficarra, A. Russo, D. Di Rosa, M. Castagnola, B. Giardina and I. Messana, “Band 3 Protein Function in Human Erythrocytes: Efffect of Oxygenation-Deoxige- nation,” Biochimica et Biophysica Acta, Vol. 1564, No. 1, 2002, pp. 214-218. doi:10.1016/S0005-2736(02)00454-6 [7] S. M. Blackman, E. J. Hustedt, C. E. Cobb and A. H. Beth, “Flexibility of the Cytoplasmic Domain of the An- ion Exchange Protein, Band 3, in Human Erythrocytes,” Biophysical Journal, Vol. 81, No. 6, 2001, pp. 3363-3376. doi:10.1016/S0006-3495(01)75969-3 C opyright © 2011 SciRes. PP  Morphological and Functional Alterations in Human Red Blood Cells Treated with Titanium Citrate Copyright © 2011 SciRes. PP 121 [8] J. Poole, “Red Cell Antigens on Band 3 and Glycophorin A,” Blood Reviews, Vol. 14, No. 1, 2000, pp. 31-43. doi:10.1054/blre.1999.0124 [9] D. Yannoukakos, C. Vasseur, J. P. Piau, H. Wajcman and E. Bursaux, “Phosphorilation Sites in Human Erythrocyte Band 3 Protein,” Biochimica et Biophysica Acta, Vol. 1061, No. 2, 1991, pp. 253-266. doi:10.1016/0005-2736(91)90291-F [10] A. Barbul, Y. Zipser, A. Nachles and R. Korenstein, “De- oxygenation and Elevation of Intracellular Magnesium Induce Tyrosine Phosphorilation of Band 3 in Human Erythrocytes,” FEBS Letters, Vol. 455, No. 1-2, 1999, pp. 87-91. doi:10.1016/S0014-5793(99)00822-4 [11] L. Messori, P. Orioli, V. Banholzer, I. Pais and P. Zatta, “Formation of Titanium (IV) Transferrin by Reaction of Human Serum Apotransferrin with Titanium Com- plexes,” FEBS Lettera, Vol. 442, No. 2, 1999, pp. 157- 161. doi:10.1016/S0014-5793(98)01651-2 [12] J. Shida, M. C. D. Trindade, S. B. Goodman, D. J. Schurman and R. L. Smith, “Induction of Interleukin-6 Release in Human Osteoblast-Like Cells Exposed to Ti- tanium Particles in Vitro,” Calcified Tissue International, Vol. 67, 2000, pp. 151-155. doi:10.1007/s00223001125 [13] M. Suwalsky, F. Villana, B. Norris, M. A. Soto, C. P. Sotomayor , L. Messori, P. Zatta, “Structural Effects of Titanium Citrate on the Human Erythrocyte Membrane,” Journal of Inorganic Biochemistry, Vol. 99, No. 3, 2005, pp. 764-770. doi:10.1016/j.jinorgbio.2004.12.003 [14] G. De Luca, T. Gugliotta, A. Scuteri, P. Romano, C. Rinaldi, A. Sidoti, A. Amato and L. Romano, “The Inter- action of Haemoglobin, Magnesium, Organic Phosphates and the Band 3 Protein in Nucleated and Anucleated Erythrocytes,” Cell Biochemistry and Function, Vol. 22, No. 3, 2004, pp. 179-186. doi:10.1002/cbf.1081 [15] L. Romano, A. Scuteri, T. Gugliotta, P. Romano, G. De Luca, A. Sidoti and A. Amato, “Sulphate Influx in the Erythrocytes of Normal, Diabetic and Hypertensive Pa- tients,” Cell Biology International, Vol. 26, No. 5, 2002, pp. 421-426. doi:10.1006/cbir.2002.0874 [16] S. M. Henning, J. Z. Zhang, R. W. McKee, M. E. Swend- seid and R. A. Jacob, “Glutathion Blood Levels and Other Oxidant Defense Indices in Men Fed Diets Low in Vitamin C,” Journal of Nutrition, Vol. 121, No. 12, 1991, pp. 1969-1975. [17] L. Romano, D. Peritore, E. Simone, A. Sidoti, F. Trischi- tta and P. Romano, “Chloride-Sulphate Exchange Chemi- cally Measured in Human Erythrocyte Ghosts,” Cell and Molecular Biology (Noisy-le-grand), Vol. 44, No. 2, 1998, pp. 351-355. [18] S. R. Sudipa, S. Gargi and B. Tuli, “Role of Sulfhydryl Groups in Band 3 in the Inhibition of Phoshate Transport Across Erythrocyte Membrane in Visceral Leishmania- sis,” Archives of Biochemistry and Biophysics, Vol. 436, No. 1, 2005, pp. 121-127. doi:10.1016/j.abb.2005.01.015 [19] F. Pesarin, “Multivariate Permutation Test,” Wiley, New York, 2001, pp. 37-61. [20] G. De Luca, T. Gugliotta, G. Parisi, P. Romano, A. Geraci, O. Romano, A. Scuteri and L. Romano, “Effects of Nickel on Human and Fish Red Blood Cells,” Biosci- ence Reports, Vol. 27, No. 4, 2007, pp. 265-273. doi:10.1007/s10540-007-9053-0 [21] C. Costagliola, L. Romano, P. Sorice and A. Di Benedetto, “Anemia and Chronic Renal Failure: The Pos- sible Role of the Oxidative State of Glutathione,” Neph- ron, Vol. 52, No. 1, 1989, pp. 11-14. doi:10.1159/000185575 [22] H. Pasaoğlu, A. Muhtaroğlu, M. Gűnes and C. Utas, “The Role of the Oxidative State of Glutathione and Gluta- thione-Related Enzymes in Anemia of Hemodialysis Pa- tients,” Clinical Biochemistry, Vol. 29, No. 6, 1996, pp. 567-572.
|