 Psychology 2011. Vol.2, No.4, 382-387 Copyright © 2011 SciRes. DOI:10.4236/psych.2011.24060 Romantic Breakups, Heartbreak and Bereavement* —Romantic Breakups Tiffany Field1,2 1Touch Research Institute, School of Medicine, University of Miami, Miami, USA; 2Fielding Graduate University, Santa Barbara, USA. Email: tfield@med.miami.edu Received March 26th, 2011; revised May 11th, 2011; accepted May 20th, 2011. This literature review suggests that romantic breakups may lead to bereavement symptoms including intrusive thoughts and attempts to suppress them and insomnia as well as morbidity factors including broken heart syn- drome and immune dysfunction. Although the broken heart syndrome has mimicked real heart attacks, an- giograms revealed no clogged arteries or permanent heart damage. Compromised immune function may result from reduced vagal activity and increased cortisol and catecholamines leading to increased inflammatory cyto- kines and decreased natural killer cell activity. The model proposed here is that romantic breakups result in the loss of a person as a regulator of stimulation and arousal modulation that can then lead to these physiological and biochemical effects. These data highlight the complexity of romantic breakups, heartbreak and bereavement and the need for multi-variable research on thes e systems both before and after the breakups occur. Keywords: Romantic Breakups, Heartbreak, Bereavement, Social Regulators Romantic breakups can be followed by symptoms of heart- break and bereavement (Prigerson & Jacobs, 2001; Raphael, Minkov, & Dobson, 2001; Davis, Shaver, & Vernon, 2003), typically, these symptoms have been associated with a loss from death, although they can also occur following other losses like divorce and romantic breakups (Prigerson & Jacobs, 2001; Davis et al., 2003). This review of research from these different literatures sug- gests that romantic breakups, like the losses following death or divorce, can lead to bereavement symptoms including intrusive thoughts, difficulty controlling intrusive thoughts and insomnia as well as heartbreak syndrome and compromised immune function. Studies on bereavement symptoms, heartbreak syn- drome and immune dysfunction are followed by a summary of research on romantic breakups and their symptoms. A potential underlying mechanism model is then presented, suggesting that it is the loss of the person as a regulator of stimulation and arousal modulation that can result in physiological and bio- chemical dysregulation including reduced vagal activity, in- creased cortisol and catecholamines and compromised immune function. Limitations of this literature are then suggested as well as future research including multi-variable studies that could assess these systems both before and after the breakups occur. Bereavement Symptoms Bereavement symptoms have varied cross-culturally, with more symptoms reported for non-Western cultures (Kleinman & Good, 1985), and the symptoms have differed even within religions. For example, Egyptian Muslims show intense grief, while Muslims in Bali do not (Wikan, 1988). Contradictory data include an international study that reported very similar symptoms across diverse cultures (Simon, VonKorff, Piccinelli, Fullerton, & Ormei, 1999). In that large sample study, sleep disturbances were among the most frequently reported symp- toms across cultures (Simon et al., 1999). Sleep Disturbances Sleep disturbances have been reported by as many as 43% of bereaved subjects in one sample (Bisconti, Bergeman, & Boker, 2004), and poor sleep has been noted in bereavement-related depression (McDermott, Prigerson, Reynolds, Houck, Dew, Hall et al., 1997; Hardison, Neimeyer, & Lichstein, 2005). In a study on college students, for example, insomnia was greater in bereaved versus non-bereaved groups (22% versus 17%) (Har- dison et al., 2005), with sleep onset insomnia being related to nighttime ruminations about the loss, and sleep maintenance insomnia being related to dreaming about the lost person. Ele- vated cortisol has also contributed to poor sleep including more REM sleep and less delta wave activity (Reynolds, Hoch, Buysse, Houck, Schlernitzauer, Pasternak et al., 1992), al- though it is not clear whether those EEG sleep changes pre- ceded or followed the depression. Intrusive Thoughts and Attempts to Control Intrusive Thoughts Intrusive images and attempts to control them are thought to contribute to the insomnia associated with bereavement. In- somnia, based on actigraphic recordings, for example, has re- sulted from unpleasant images (Nelson & Harvey, 2002). And, unpleasant images have been correlated with sleep onset la- *This research was supported by a merit Award (MH46586), NIH grants (AT00370 and HD056036) and Senior Research Scientist Awards (MH0033 and AT0011585) and a March of Dimes Grant (12-FY03-48) to Tiffany Field and funding from Johnson and Johnson P ediatric Institute to the Touch Research Institute.  T. FIELD 383 tency, with more of those images related to intimate relation- ships. Pre-sleep images have a lso been rated as less controllable than pre-sleep verbal thoughts, although more disengagement has been noted from images than verbal thoughts (Nelson & Harvey, 2003). Negative images have also been associated with higher heart rate, which is surprising given that negative verbal thoughts typically elicit greater cardiovascular responses than negative verbal images (Vrana, Cuthbert, & Lang, 1986). Attempts to suppress the images and thoughts often lead to dreams. In one study, participants were asked to think about a romantic “crush” or a “non-crush” (Wegner, Wenzlaff, & Ko- zak, 2004). Although there was no greater dreaming about the romantic “crush,” suppression enhanced eroticism of the “crush”. Thus, the increased accessibility of intrusive thoughts resulting from thought suppression transferred even to dreams. Potential Morbidity Factors Morbidity factors have also been associated with bereave- ment. And, romantic breakups may be a risk factor for the more serious complications associated with bereavement including broken heart syndrome (Wittstein, Thiemann, Lima, Baughman, Schulman, Gerstenblith et al., 2005) and endocrine and immune dysfunction (Frazier, Strauss, & Steinhauer, 2004). “Broken Heart” Syndrome The “broken heart” or heartbreak syndrome has been de- scribed as physical pain in the heart or chest after losing some- one. Although the heartache mimics symptoms of a real heart attack, those with broken heart syndrome typically recover faster (Wittstein et al., 2005). This condition has also been called stress cardiomyopathy or “takotsubo cardiomyopathy,” takotsubo being a fishing pot with a narrow neck and a wide base that is used to trap octopus in Japan, a shape that is similar to that of the left ventricle. Cardiac contractile abnormalities and heart failure have been recorded by several investigators, although angiograms have revealed no clogged arteries in heartbreak, unlike real heart attacks (Kawai, Suzuki, Yamagu- chi et al., 2000; Kurisu, Sato, Kawagoe, Masaharu, Yuji, Kenji et al., 2002; Villareal, Achari, Wilansky, & Wilson, 2001). Norepinephrine and epinephrine levels have also been ele- vated (7 - 34 times the normal levels) in individuals with bro- ken heart syndrome, but cardiac enzymes typically released from damaged heart muscle during real heart attacks were not noted (Wittstein et al., 2005). Echocardiograms suggested that although the left ventricle was contracting normally, there ap- peared to be a weakened contraction in the middle and upper portions of the heart muscle, and inverted T waves and pro- longed Q-T intervals which are often associated with stress were noted. Magnetic resonance imaging scans suggested that none of the broken heart syndrome patients suffered irreversible heart damage, and their recovery rates were faster (typically two months) than after real heart attacks (Akashi, Nakazawa, Sakakibara, Miyake, Koike, & Sasaka, 2003; Nyui, Yamanaka, Nakayama, Sawano, & Kawai, 2000). Potential underlying mechanisms offered for these effects in- clude: 1) increased catecholamines causing spasms in the coro- nary arteries (Wittstein et al., 2005); 2) multiple simultaneous spasms of the coronary arteries that would cause enough loss of blood flow to lead to the transient stunning of the heart (Kurisu et al., 2002); and 3) a failure of the arteries to provide adequate oxygen to the heart (Kawai et al., 2000). Most of these re- searchers have suggested, however, that all of these factors may be operating. Unfortunately, many of these studies were based on small samples, and although there are strong associations between increased heart rate and the release of catecholamines and the resultant cardiomyopathy, the relationships are only suggestive. The elevated catecholamines may simply be an epiphenomenon or a secondary response in the patients with the stress cardio- myopathy rather than an original cause. Nonetheless, elevated catechalomines are typically indicative of elevated stress and when prolonged can lead to endocrine and immune dysfunc- tion. Endocrine and Immune Dysfunction Decreased vagal activity and increased skin conductance have been associated with elevated stress (Frazier et al., 2004), and increased heart rate and blood pressure have been accom- panied by increased cortisol and norepinephrine levels, which when prolonged can have negative effects on the immune sys- tem (Uchino, Kiecolt-Glaser, & Glaser, 2000). This initial “fight-or-flight” mechanism is adaptive in mobilizing energy stores leading to increased inflammatory cytokines which ulti- mately mobilize antibodies as a defense against infection (Black, 2002). In this way, immune activity is initially en- hanced, but, over time, elevated stress hormones and cytokine activity can result in impaired immune function (Kiecolt-Glaser, McGuire, Robles, & Glaser, 2002). Compromised immune function occurs via the necrotic effects of stress hormones on the immune organs. Examples have been given of elevated inflammatory cytokines (IL-1, IL-2, IL-6 and PNF-alpha) ac- companying the depressed state (Leonard, 2006), as well as higher antibody titres to the Epstein-Barr virus and lower than normal natural killer cell activity (noted to kill bacterial, viral and cancer cells) following divorce (Powell, Lovallo, Matthews, Meyer, Midgley, Baum et al., 2002). Bereaved individuals have had profiles of high anxiety and depression scores, elevated cortisol and decreased natural killer cell activi ty which in some individuals lasted for as long as six months (Gerra, Monti, Panerai, Sacerdote, Anderlini, & Avanzini, 2003). These physiological and biochemical changes may contribute to the greater incidence of physical illnesses (following “betrayal”) (Freyd, Klest, & Allard, 2005) and heart disease (related to “broken hearts”) (Johnson & Grippo, 2006) in bereaved individuals. Romantic Breakups Although most adults are resilient following romantic breakups, some experience symptoms similar to those of be- reavement including intrusive thoughts, insomnia and depres- sion. In a study conducted by our group, university students who experienced romantic breakups had elevated scores on intrusive thoughts, difficulty controlling intrusive thoughts and insomnia scales (Field, Diego, Pelaez, Deeds, & Delgado, 2009) (see Table 1). In a regression on these data, scores on these scales contributed to 34% of the variance on breakup distress which was experienced by 58% of the students following ro- mantic breakups (see Table 2). Similarly, in a survey of more than 5000 internet respon- 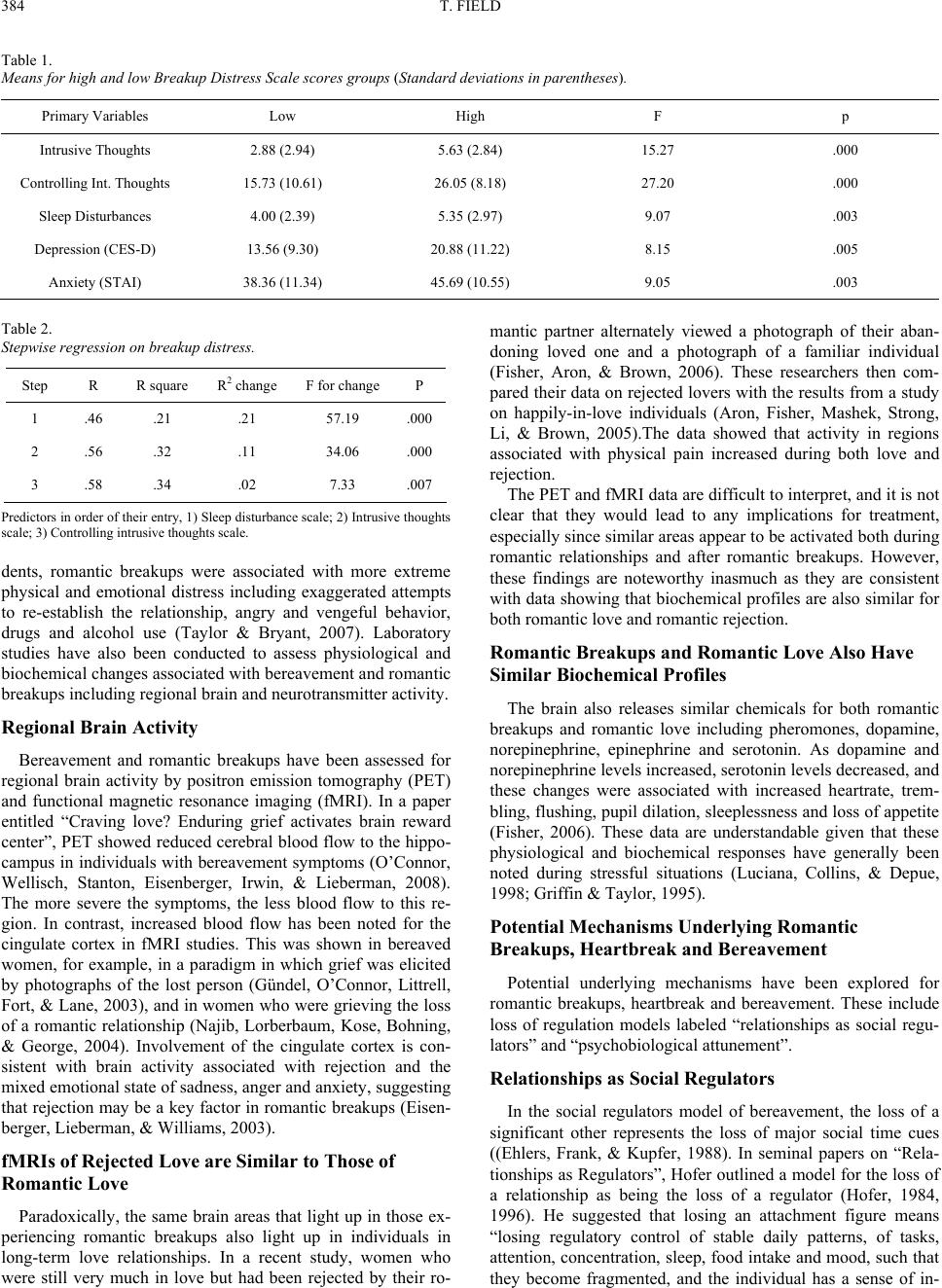 T. FIELD 384 Table 1. Means for high and low Br ea k up D i st r es s S ca l e s co r es g r ou ps (Standard devi at io ns i n p ar entheses). Primary V ariables Low High F p Intrusive Thoughts 2.88 (2.94) 5.63 (2.84) 15.27 .000 Controlling Int. Thoughts 15.73 (10.61) 26.05 (8.18) 27.20 .000 Sleep Distu r bances 4.00 (2.39) 5.35 (2.97) 9.07 .003 Depression (CES-D) 13.56 (9.30) 20.88 (11.22) 8.15 .005 Anxiety (ST AI) 38.36 (11.34) 45.69 (10.55) 9.05 .003 Table 2. Stepwise regression on breakup distress. Step R R square R2 change F for changeP 1 .46 .21 .21 57.19 .000 2 .56 .32 .11 34.06 .000 3 .58 .34 .02 7.33 .007 Predictors in order o f their en try, 1) Sleep distu rbance scale; 2 ) Intrusive thou ghts scale; 3) Controlling intr usive thoughts scale. dents, romantic breakups were associated with more extreme physical and emotional distress including exaggerated attempts to re-establish the relationship, angry and vengeful behavior, drugs and alcohol use (Taylor & Bryant, 2007). Laboratory studies have also been conducted to assess physiological and biochemical changes associated with bereavement and romantic breakups including regional brain and neurotransmitter activity. Regional Brain Activity Bereavement and romantic breakups have been assessed for regional brain activity by positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). In a paper entitled “Craving love? Enduring grief activates brain reward center”, PET showed reduced cerebral blood flow to the hippo- campus in individuals with bereavement symptoms (O’Connor, Wellisch, Stanton, Eisenberger, Irwin, & Lieberman, 2008). The more severe the symptoms, the less blood flow to this re- gion. In contrast, increased blood flow has been noted for the cingulate cortex in fMRI studies. This was shown in bereaved women, for example, in a paradigm in which grief was elicited by photographs of the lost person (Gündel, O’Connor, Littrell, Fort, & Lane, 2003), and in women who were grieving the loss of a romantic relationship (Najib, Lorberbaum, Kose, Bohning, & George, 2004). Involvement of the cingulate cortex is con- sistent with brain activity associated with rejection and the mixed emotional state of sadness, anger and anxiety, suggesting that rejection may be a key factor in romantic breakups (Eisen- berger, Lieberman, & Williams, 2003). fMRIs of Rejected Love are Similar to Those of Romantic Love Paradoxically, the same brain areas that light up in those ex- periencing romantic breakups also light up in individuals in long-term love relationships. In a recent study, women who were still very much in love but had been rejected by their ro- mantic partner alternately viewed a photograph of their aban- doning loved one and a photograph of a familiar individual (Fisher, Aron, & Brown, 2006). These researchers then com- pared their data on rejected lovers with the results from a study on happily-in-love individuals (Aron, Fisher, Mashek, Strong, Li, & Brown, 2005).The data showed that activity in regions associated with physical pain increased during both love and rejection. The PET and fMRI data are difficult to interpret, and it is not clear that they would lead to any implications for treatment, especially since similar areas appear to be activated both during romantic relationships and after romantic breakups. However, these findings are noteworthy inasmuch as they are consistent with data showing that biochemical profiles are also similar for both romantic love and romantic rej ection. Romantic Breakups and Romantic Love Also Have Similar Biochemical Profiles The brain also releases similar chemicals for both romantic breakups and romantic love including pheromones, dopamine, norepinephrine, epinephrine and serotonin. As dopamine and norepinephrine levels increased, serotonin levels decreased, and these changes were associated with increased heartrate, trem- bling, flushing, pupil dilation, sleeplessness and loss of appetite (Fisher, 2006). These data are understandable given that these physiological and biochemical responses have generally been noted during stressful situations (Luciana, Collins, & Depue, 1998; Griffin & Taylor, 1995). Potential Mechanisms Underlying Romantic Breakups, Heartbreak and Bereavement Potential underlying mechanisms have been explored for romantic breakups, heartbreak and bereavement. These include loss of regulation models labeled “relationships as social regu- lators” and “psychobiological attunement”. Relationships as Social Regulators In the social regulators model of bereavement, the loss of a significant other represents the loss of major social time cues ((Ehlers, Frank, & Kupfer, 1988). In seminal papers on “Rela- tionships as Regulators”, Hofer outlined a model for the loss of a relationship as being the loss of a regulator (Hofer, 1984, 1996). He suggested that losing an attachment figure means “losing regulatory control of stable daily patterns, of tasks, attention, concentration, sleep, food intake and mood, such that they become fragmented, and the individual has a sense of in-  T. FIELD 385 ternal disorganization”. Relationships can help maintain psychological and physio- logical equilibrium, as each person is associated with a st ate of psychological security and physiological calm for the other and serves to up- or down-regulate the partner’s psychophysiologi- cal arousal (Hofer, 1984, 1996; Depue & Morrone-Strupinsky, 2005; Sbarra & Hazan, 2009). This co-regulation is considered a property of the relationship (not a property of either individ- ual alone), and, it can occur through several senses (e.g. touch, smell, eye contact) and is thought to regulate and synchronize body rhythms. In the absence of the “co-regulator,” the psy- chological and physiological rhythms can become dysregulated, leading to dysphoria, restlessness/agitation, sleep disturbances, changes in appetite and decreased vagal tone (Sbarra & Hazan, 2008). Dysregulation can happen when a partner is absent, for ex- ample, during business trips and military deployments, as sleep disturbances have been noted during the travel period, and the individuals then return to a regulated state following reunion (Diamond, Hicks, & Otter-Henderson, 2008). It can also hap- pen during threat conditions that can be alleviated by holding the hand of one’s partner versus the hand of a stranger (Coan, Schaefer, & Davidson, 2006). The authors of the handholding study suggested that the threatened person “borrowed emo- tional and physiological stability from the partner”. Some have noted that even mental representations of one’s partner can be dysregulating following a breakup or loss, leading to intrusive thoughts and disturbing dreams (Uvnäs-Moberg, 1998). Most of the examples given have involved the partner de- creasing arousal levels rather than helping find a balance be- tween under- and over-arousal. Under-arousal could be equally disturbing as, for example, the sensory deprivation experienced by individuals who are in military combat and confined to light and sound-proof chambers (Hofer, 1984). Loss of Psychobiological Attunement A model called “psychobiological attunement” or “being on the same wavelength”, accommodates both the need for optimal stimulation and for arousal modulation (Field, 1985, 1996). In this model, each partner provides meaningful stimulation for the other and has a modulating influence on the other’s arousal level. Both over-stimulation and under-stimulation are aversive, and stimulation that brings or keeps an individual within an optimal arousal zone is considered reinforcing. Thus, the loss of a significant other means the loss of both activating and calm- ing stimulation. The individual experiencing the loss would be expected to fluctuate between one end of the continuum of under-stimulation and the other end of over-stimulation and not be able to modulate these levels to experience optimal arousal levels. Other terms used to describe this phenomenon were syn- chrony and sharing rhythms (Field, 1985, 1996). Synchrony is a term that is usually applied to the matching of physiological or physical activity rhythms by individuals in a close relationship. Examples of this can be seen in partners who are extremely close tending to coordinate their physical movements and ex- pressions while talking, as well as their cortisol cycles tending to be synchronized on weekends when they are together (Field, 1985, 1996). Thus, attunement or “being on the same wave- length” happens for both behavioral and physiological rhythms in adults who have a close relationship. Seemingly, the only way this could happen is if each partner of the dyad is sensitive and responsive to each other’s stimulation and arousal-modu- lation needs, as in a feedback loop, and each accordingly ad- justs his or her behavior to facilitate the behavioral and physio- logical synchrony of the couple. If and when the partner is not there to meet the needs for dif- ferent types and degrees of stimulation, dysregulation may occur including physiological disorganization such as decreased vagal activity (Frazier et al., 2004; Diego, Field, & Hernan- dez-Reif, 2007), and in some cases changes in immune function such as increased inflammatory cytokines (Leonard, 2006) and decreased natural killer cell activity (Powell et al., 2002). In our model, the loss of a loved one may result in this dysregulation simply because the source of stimulation and arousal modula- tion is no longer present (Field, 1985, 1996). Physical intimacy can enhance attunement (Fisher, 2004). Via touching, individuals can learn each other’s stimulation and arousal modulation needs. Although it is possible to self-regu- late in the absence of an intimate partner, it may not be as easy or effective. When a partner is no longer there and touch stimulation, for example, is missing, it may become necessary to find that type of stimulation from other activities until a new partner is found. Massage, yoga, and other forms of exercise, for example, may help avoid the physiological dysregulation and immune problems that can result from touch deprivation (Field, 2009). Limitations of This Research and Future Directions The intent of this review was to summarize the limited lit- erature on romantic breakup symptoms that are similar to those of the bereavement syndrome including intrusive thoughts, attempting to control intrusive thoughts and insomnia and more serious complications including heartbreak syndrome and im- mune dysfunction. Much of the discussion regarding romantic breakups, however, is mere speculation based on the bereave- ment and heartbreak syndrome literature. And, the bereavement and heartbreak syndrome literature has the problem that the data are derived primarily from loss related to death and di- vorce. These likely have commonalities with romantic breakups, but also major differences, as in divorce not only involving betrayal and rejection but also having to continue the relation- ship for family reasons and death involving a permanent loss. Even within the loss by divorce and the loss by death literatures, comparisons across studies are problematic given the different measures, the different intervals from the time of loss to the time of assessment, and the different age, ethnic and cultural groups assessed (among other potentially confounding vari- ables). Other nuisance factors are the small sample sizes and the measurement of only one or two variables. This is particularly problematic when the results appear paradoxical such as the fMRI data showing that the same region of the brain is acti- vated by romantic breakup and by romantic love and the same biochemical profile emerging (albeit from different studies). Without converging variables such as behavioral data, these findings are difficult to interpret. And, as already mentioned, they are not perhaps useful for informing potential treatment  T. FIELD 386 options. In brief, in any of these research areas, multi-variable studies would be more informative. Combining self-report, behavioral, physiological and biochemical measures in the same sample of individuals experiencing loss from the same cause would be optimal. Perhaps the greatest weakness of these literatures is that it is not clear what is happening in relationships that are then miss- ing when the loss occurs, whether by death, divorce or romantic breakups. Reviewing the “social regulators” and the “psychobi- ological attunement” models in this paper was meant to high- light how little we know about what changes occur from before to after the loss or what was critical about the relationship that was then missing after the loss occurred. This, of course, is always difficult given the longitudinal nature of the problem. However, convenience samples could be researched such as university students whose relationships tend to be short-lived, affording the opportunity to collect behavioral, physiological and biochemical data during the relationship and after the breakups. To address these questions, we are currently design- ing research to videotape interactions of university student cou- ples during their relationships and after their break-ups as well as recording their heartrate and assaying saliva samples for cortisol levels. Relationships between older couples, for exam- ple couples in assisted living, could also provide the opportu- nity for studies of long-term relationships that are “snuffed out” by the death of one partner. These are potential challenges for the very important problem of determining how to alleviate the significant social pain of loss, be it by death, d ivorce or roman - tic breakups. In a sense, each of these, no matter the cause, are romantic breakups that can be chronically painful and therapeu- tically costly. Summary In summary, romantic breakups, heartbreak syndrome and bereavement are complex behavioral, physiological and bio- chemical phenomena. Romantic breakups may be at risk for the symptom profile of bereavement including intrusive thoughts and attempts to suppress them as well as insomnia and more serious complications including broken heart syndrome and immune dysfunction. Although the broken heart syndrome mimics a real heart attack, it has been differentiated from heart attacks by angiograms revealing unclogged arteries and no permanent heart damage. Reduced vagal activity and increased cortisol and catecholamines are thought to lead to the associ- ated immune dysfunction including increased inflammatory cytokines and reduced natural killer cell activity. Potential un- derlying mechanisms for romantic breakups, heartbreak and bereavement effects include the loss of social regulators who provide optimal stimulation and arousal modulation. These data highlight the complexity of romantic breakups, heartbreak and bereavement and the need for further multi-variable research that is conducted both before and after the breakups. References Akashi, Y. J., Nakazawa, K., Sakaki bara, M., Miyake, F., Koike, H., & Sasaka, K. (2003). The clinical features of takotsubo cardiomyopathy. Quarterly Journal of Medic in e , 96, 563-573. Aron, A., Fisher, H., Mashek, D. J., Strong, G., Li, H., & Brown, L. L. (2005). Reward, motivation, and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology, 94, 327-337. doi:10.1152/jn.00838.2004 Bisconti, T. L., Bergeman, C. S., & Boker, S. M. (2004). Emotional well-being in recently bereaved widows: A dynamical systems ap- proach. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 59, 158-167. doi:10.1093/geronb/59.4.P158 Black, P. H. (2002). Stress and the inflammatory response: A review of neurogenic inflammation. Brain, Behavior, and Immunity, 16, 622-635. doi:10.1016/S0889-1591(02)00021-1 Coan, J. A., Schaefer, H. S., & Davidson, R. J. (2006). Lending a hand: Social regulation of the neural response to threat. Psychological Sci- ence, 17, 1032-1039. doi:10.1111/j.1467-9280.2006.01832.x Davis, D., Shaver, P. R., & Vernon, M. L. (2003). Physical, emotional and behavioral reactions to breaking up: The roles of gender, age, emotional involvement, and attachment style. Personality and Social Psychology Bulletin, 29, 871-884. doi:10.1177/0146167203029007006 Depue, R. A., & Morrone-Strupinksy, J. V. (2005). A neurobehavioral model of affiliative bonding: Implications for conceptualizing a hu- man trait of affiliation. Behavioral and Brain Sciences, 28, 313-395. doi:10.1017/S0140525X05000063 Diamond, L. M., Hicks, A. M., & Otter-Henderson, K. (2008). Every time you go away: Changes in affect, behavior, and physiology asso- ciated with travel-related separations from romantic partners. Journal of Personality and Social Psychology, 95, 385-403. doi:10.1037/0022-3514.95.2.385 Diego, M., Field, T., & Hernandez-Reif, M. (2007). Preterm infant massage consistently increases vagal activity and gastric motility. Acta Paediatrica, 96, 1588-1591. doi:10.1111/j.1651-2227.2007.00476.x Ehlers, C. L., Frank, E., & Kupfer, D. J. (1988). Social zeitgebers and biological rhythms: A unified approach to understanding the etiology of depression. Archives of General Psychiatry, 45, 948-952. Eisenberger, N. I., Lieberman, M. D., & Williams, K. D. (2003). Does rejection hurt? An fMRI study of social exclusion. Science, 302, 290-292. doi:10.1126/science.1089134 Faschingbauer, T., Zisook, S., & DeVaul, R. (1987). The Texas revised inventory of grief. In S. Zisook, (Ed.), Biopsychosocial aspects of bereavement (pp. 111-124). Washington, DC: American Psychiatric Press, Inc. Field, T. (1985). Attachment as psychobiological attunement: Being on the same wavelength. In M. Reite and T. Field (Eds.), Psychobiology of attachment. New York: Academic Press. Field, T. (1996). The effects of mother’s physical and emotional un- availability on emotion regulation. The Development of Emotion Regulation: Biologi cal and Behavioral Considerations, 59, 208-227. Field, T. (2009). Complementary and alternative therapies research. Washington, DC: Amer ic an Psychological Association. doi:10.1037/11859-000 Field, T., Diego, M., Pelaez ,M., Deeds, O., & Delgado, J. (2009). Breakup distress in university students. Adolescence, 44, 705-727. Fisher, H. E. (2004). Why we love: The nature and chemistry of roman- tic love. New York: Holt Paperbacks. Fisher, H. E., Aron, A., & Brown, L. L. (2006). Romantic love: A mammalian brain system for mate choice. Philosophical Transac- tions of the Royal Society, 3 6 1 , 2173-2186. doi:10.1098/rstb.2006.1938 Frazier, T. W., Strauss, M. E., & Steinhauer, S. R. (2004). Respiratory sinus arrhythmia as an index of emotional response in young adults. Psychophysiology, 41, 75-83. doi:10.1046/j.1469-8986.2003.00131.x Freyd, J. J., Klest, B., & Allard, C. B. (2005). Betrayal trauma: Rela- tionship to physical health, psychological distress, and a written dis- closure intervention. Journal of Trauma Dissociation, 6, 83-104. doi:10.1300/J229v06n03_04 Gerra, G., Monti, D., Panerai, A. E., Sacerdote, P., Anderlini, R., Avanzini, P. et al. (2003). Long-term immune-endocrine effects of bereavement: Relationships with anxiety levels and mood, Psychia- try Research, 121, 145-158. doi:10.1016/S0165-1781(03)00255-5  T. FIELD 387 Griffin M. G., & Taylor, G. T. (1995). Norephinephrine modulation of social memory: Evidence for a time-dependent functional recovery of behavior. Behavioral Neuroscience, 109, 466-538. doi:10.1037/0735-7044.109.3.466 Gündel, H., O’Connor, M. F., Littrell, L., Fort, C., & Lane, R. (2003). Functional neuroanatomy o f grief: An fMRI study. American Journal of Psychiatry, 160, 1946-1953. doi:10.1176/appi.ajp.160.11.1946 Hardison, H. G., Neimeyer, R. A., & Lichstein, K. L. (2005). Insomnia and complicated grief symptoms in bereaved college students. Be- havioral Sleep Medicine, 3, 99-111. doi:10.1207/s15402010bsm0302_4 Hofer, M. A. (1984). Relationships as regulators: A psychobiologic perspective on bereav ement. Psychosomatic Me d i c ine, 46, 183-197. Hofer, M. A. (1996). On the nature and consequences of early loss. Review. Psychosomatic Medicine, 58, 570-581. Johnson, A. K., & Grippo, A. J. (2006). Sadness and broken hearts: Neurohumoral mechanisms and co-morbidity of ischemic heart dis- ease and psychological depression. Journal of Physiology and Pharmacology, 57, 529- 534. Kawai, S., Suzuki, H., Yamaguchi, H., Tanaka, K., Sawada, H., Aizawa, T. et al. (2000). Ampulla cardiomyopathy (Takotsubo’ cardio- myopathy)—reversible left ventricular dysfunction: With ST seg- ment elevation. Japan Circulation Journal, 64, 156- 159. doi:10.1253/jcj.64.156 Kiecolt-Glaser, J. K., McGuire, L., Robles, T. F., & Glaser, R. (2002). Emotions, morbidity, and mortality: New perspectives from psycho- neuroimmunology. Annual R e vi ew o f Psych ol og y , 53, 83-107. doi:10.1146/annurev.psych.53.100901.135217 Kleinman, A., & Good, B. (1985). Introduction: Culture and depression. In A. Kleinman and B. Good (Eds.), Culture and depression: Studies in the anthropology and cross-cultural psychiatry of affect and dis- order. Berkeley and Los Angeles: University of Cali fornia Press. Kurisu, S., Sato, H ., Kawag oe, T., M asaharu , I., Yuji, S ., Ken ji, N. et al . (2002). Tako-Tsubo-like left ventricular dysfunction with ST-seg- ment elevation: A novel cardiac syndrome mimicking acute myocar- dial infarction. American He ar t Jo urn al , 143, 448-455. doi:10.1067/mhj.2002.120403 Leonard, B. (2006). HPA and immune axes in stress: involvement of the serotonergic system. Neuroimmunomodulation, 13, 268-276. doi:10.1159/000104854 Luciana, M., Collins, P. F., & Depue, R. A. (1998). Opposing roles for dopamine and serotonin in the modulation of human spatial working memory functions. Cerebral Cortex, 8, 218- 244. doi:10.1093/cercor/8.3.218 McDermott, O. D., Prigerson, H. G., Reynolds, C. F. III, Houck, P. R., Dew, M. A., Hall, M. et al. (1997). Sleep in the wake of complicated grief symptoms: An exploratory study. Biological Psychiatry, 41, 710-716. doi:10.1016/S0006-3223(96)00118-7 Monk, T. H., Houck, P. R., & Shear, M. K. (2006). The daily life of complicated grief patients-what gets missed, what gets added? Death Studies, 30, 77-85. doi:10.1080/07481180500348860 Najib, A., Lorberbaum, J. P., Kose, S., Bohning, D. E., & George, M. S. (2004). Regional brain activity in women grieving a romantic rela- tionship breakup. Am e r i c an J o u r n a l o f Psychiatry, 161, 2245- 2256. doi:10.1176/appi.ajp.161.12.2245 Nelson, J., & Harvey, A. G. (2002). The differential functions of im- agery and verbal thought in insomnia. Journal of Abnormal Psy- chology, 111, 665-673. doi:10.1037/0021-843X.111.4.665 Nelson, J., & Harvey, A. G. (2003). Pre-sleep imagery under the mi- croscope: A comparison of patients with insomnia and good sleepers. Behaviour Research and Therapy, 41, 273-356. doi:10.1016/S0005-7967(02)00010-4 Nyui, N., Yamanaka, O., Nakayama, R., Sawano, M., & Kawai, S. (2000). Takotsubo’ transient ventricular dysfunction: A case report. Japanese Circulation Journal, 64 , 715-723. doi:10.1253/jcj.64.715 O’Connor, M. F., Wellisch, D. K., Stanton, A. L., Eisenberger, N. I., Irwin, M. R., & Lieberman, M. D. (2008). Craving love? Enduring grief activates brain reward center. Neuroimage, 42, 969-972. doi:10.1016/j.neuroimage.2008.04.256 Powell, L. H., Lovallo, W. R., Matth ews, K. A., Meyer , P., Midg ley, A. R., Baum, A., et al. (2002). Physiologic markers of chronic stress in premenopausal, middle-aged women. Psychosomatic Medicine, 64, 502-509. Prigerson, H., & Jacobs, S. (2001). Traumatic grief as a distinct disor- der: A rationale, consensus criteria, and a preliminary empirical test. In M. S. Stroebe, R. O. Hansson, W. Stroebe and H. Schut, (Eds.), Handbook of bereavement research: consequences, coping, and care. Washington, DC: American Psychological Association, 613-645. doi:10.1037/10436-026 Raphael, B., Minkov, C., & Dobson, M. (2001). Psychotherapeutic and pharmacological intervention for bereaved persons. In M. S. Stroebe, R. O. Hansson, W. Stroebe and H. Schut, (Eds.), Handbook of be- reavement research: Consequences, coping, and care. Washington, DC: American Psychological Associatio n, 587-612. doi:10.1037/10436-025 Reynolds, C. F., Hoch, C. C., Buysse, D. J., Houck, P. R., Schlernit- zauer, M., Frank, E. et al. (1992). Electroencephalographic sleep in spousal bereavement and bereavement-related depression of late life. Biological Psychiatry, 31, 69-82. doi:10.1016/0006-3223(92)90007-M Sbarra, D. A., & Hazan, C. (2008). Coregulation, dysregulation, self regulation: An integrative analysis and empirical agenda for under- standing adult attachment, separation, loss, and recovery. Personality and Social Psychology Review, 12, 141-167. doi:10.1177/1088868308315702 Simon, G., VonKorff, M., Piccinelli M., Fullerton, C., & Ormei J. (1999). An international study of the relation between somatic symptoms and depression. The New England Journal of Medicine, 341, 1329-1334. doi:10.1056/NEJM199910283411801 Taylor, F., & Bryant, R. A. (2007). The tendency to suppress inhibiting thoughts, and dream rebound. Behavior Research and Therapy, 45, 163-168. doi:10.1016/j.brat.2006.01.005 Uchino, B. N., Kiecolt-Glaser, J. K., & Glaser, R. (2000). Psychologi- cal modulation of cellular immunity. In J. T. Cacioppo, L. G. Tassi- nary and G. G. Berntson (Eds.), Handbook of psychophysiology (2nd ed., pp. 397-424). New York: Cambridge University P re ss . Uvnäs-Moberg, K. (1998). Oxytocin may mediate the benefits of posi- tive social interaction and emotions. Psychoneuroendocrinology, 23, 819-835. doi:10.1016/S0306-4530(98)00056-0 Villareal, R. P., Achari, A., Wilansky, S., & Wilson, J. M. (2001). Anteroapical stunning and left ventricular outflow tract obstruction. Mayo Clinic Proceedings, 76, 79-83. doi:10.4065/76.1.79 Vrana, S. R., Cuthbert, B. N., & Lang, P. J. (1986). Fear imagery and text processing. Psychophysiology, 23, 247-253. doi:10.1111/j.1469-8986.1986.tb00626.x Wegner, D. M., Wenzlaff, R. M., & Kozak, M. (2004). Dream rebound: The retuned of suppressed/thoughts in dreams. Psychological Sci- ence, 15, 232-236. doi:10.1111/j.0963-7214.2004.00657.x Wikan, U. (1988). Bereavement and loss in two Muslin communities: Egypt and Bali. Social S cience and Medicine, 27, 451-460. doi:10.1016/0277-9536(88)90368-1 Wittstein, L. S., Thiemann, D. R., Lima, J. A. C., Baughman, K. T., Schulman, S. P., Gerstenblith, G. et al. (2005). Neurohumoral fea- tures of myocardial stunning due to sudden emotional stress. The New England Journal of Medicine, 352, 539-548. doi:10.1056/NEJMoa043046
|