 Vol.3, No.7, 517-529 (2011) Natural Science http://dx.doi.org/10.4236/ns.2011.37073 Copyright © 2011 SciRes. OPEN ACCESS The relationship of mineral and geochemical composition to artificial radionuclide partitioning in Yenisei river sediments dow nstream from mining-and-chemical combine Rosatom Bondareva Lydia1, Artamonova Svetlana2 1Institute of Nonferrous Metals and Materials, Siberian Federal University, Krasnoyarsk, Russia; *Corresponding Author: lydiabondareva@gmail.com 2Institute of Geology and Mineralogy SB RAS, Novosibirsk, Russia. Received 9 December 2010; revised 20 February 2011; accepted 4 March 2011. ABSTRACT Discharges from the Mining-and-Chemical Com- bine (MCC) of Rosatom, downstream from Kra- snoyarsk-26, near of the Krasnoyarsk resulted in radioactive contamination of sediments of the River Yenisei. The concentration of artificial gamma-emitting radionuclides (137Cs, 60Co, 152Eu, and 241Am) was determined to analyze the mi- gration processes leading to the transport of these radionuclides. The concentration of artifi- cial radionuclides in the surface layers of the studied area varied in wide ranges: 137Cs – 318 - 1800 Bq/kg, 60Co – 87 - 720 Bq/kg, 152Eu – 12 - 287 Bq/kg and 241Am – 6 - 76 Bq/kg. There was a sequence of migration of radionuclides inves- tigated in the surface layer of sediments that were collected in the near zone of influence of the MCC: 241Am ≈ 152Eu > 60Co > 137Cs. Radionu- clide species have been found to be directly related to sediment structure and composition. Keywords: Geochemical Properties; Sediments; Radionuclides; Correlation; the Yenisei River 1. INTRODUCTION Earth’s ecosystems, including aquatic ones, have ac- cumulated large amounts of artificial radionuclides. Ra- dioactive contamination of river ecosystems presents a challenge to many European countries. The Yenisei Riv- er is one of the environments affected by the conti- nu- ous radioactive contamination. The floodplain soils, sediments, aquatic plants, and water of the Yenisei River have been found to contain various artificial radionu- clides, both activation products and ones produced at the radiochemical plant of the Mining-and-Chemical Com- bine (MCC) of Rosatom [1-3]. There is a Mining and Chemical Combine of Rosatom (MCC) on the left bank of the Yenisei, 50 km downstream from Krasnoyarsk. MCC includes radiochemical facilities and nuclear reac- tors. The production facilities of the plant are located on both banks of the River Yenisei and connected by a tun- nel under the river bed. Since 1958 MCC has used water for cooling industrial reactors to produce weapon-grade - 238Pu. The river water after passing through the cooling system of the reactors has been brought back to the Riv- er Yenisei. Two once-through reactors were taken out of service in 1992, therefore, the reactivity level in the ef- fluents discharged from the MCC area decreased. The last reactor with a closed circuit which is to be stopped in 2010 has been operating till now. The composition of soils and bottom sediments is quite complex. Mineral particles of the bottom sediments are formed in the following way: a positively charged surface of a mineral particle is surrounded by negatively charged particles of iron hydroxide, aluminum hydrox- ide etc, where negatively charged silicic acid and organic colloids are further adsorbed. Besides particles of dif- ferent minerals (carbonates, silicates, nitrates, chlorides etc.) the bottom sediments include the organic constitu- ent: humic and fulvic acids, individual organic sub- stances (aminoacids, polysaccharose etc.), metal-organic and organic-mineral complexes [4]. The moiety of or- ganic substance dissolving in alkali, but deposited by acids (рН = 1), was named humic acid (its content in the soil being up to 70%). The moiety dissoluble both in acids and alkalies – is fulvic acid; and the one insoluble both in acids and alkalies is humic acid. Humus sub- stances are amorphous polyelectrolytes with a wide range of molecular weights―up to 1000 for the fulvic acids, 100 - 5*105 for the humic acids (according to some evidence, up to 106). It has been shown that these fractions include both aromatic and aliphatic compo-  B. Lydia et al. / Natural Science 3 (2011) 517-529 Copyright © 2011 SciRes. OPEN ACCESS 518 nents; their main building blocks are complex phenolic and benzene-carboxyl structures. These acids are closely interconnected and can transfer into each other. They are complex-formers and, consequently, metals in soils and bottom sediments can be in the form of soluble or in- soluble complexes with humic and fulvic acids [4]. It is known that radionuclides are associated with bottom sediments. The mechanisms of the radionuclide fixation in the mineral phase of the soil may be different: reversible physical absorption, chemosorption (reversi- ble and irreversible), introduction into the crystal lattice of the minerals or exchange for the cations located on the surface of the mineral particle. Here, radioisotopes may be: 1) in pore solutions of the bottom sediments in the form of ions, organic and inorganic complexes, 2) on the particles of the bottom sediments in cation-exchange form; also they may be more tightly connected due to the sorption, co-deposited with the oxides of other elements (for example, with Fe, Mn oxides), included in the bio- mass of dead plants and microorganisms, as well as in- cluded in the structures of the primary and secondary minerals of the bottom sediments. In spite of the variety of the existence forms all the substances present in the bottom sediment mass participate to a greater or lesser extent in the migration processes including also dissolv- ing-sedimentation, absorption-desorption, diffusion of a chemical gradient and diffusion into the micropores of the mineral particles. Consequently, the migration prob- lem of the physical and chemical forms of substances in the bottom sediments is important for understanding how mineral and geochemical peculiarities of the bottom sediments influence the geochemical cycles of a water reservoir. The diversity of migration flows and different forms of substance transport can be characterized by two main vectors: biogenic and abiotic. The abiotic flow is differ- entiated into vertical descending, vertical ascending, inter-horizontal (diffuse), lateral, and surface (erosive) sub-flows, and the sub-flow inside the sediment layer. The contribution of a sub-flow into the total migration flow is determined by the degree of heterogeneity and polydispersity of the sediment. Thus, description of the migration should be based on studies of both substance transfer (percolation, diffusion, dispersion, etc.) and ac- cumulation (particle sedimentation, mineral formation, sorption, etc.) [5]. To investigate the forms of existence and migration ability of radionuclides as well as that of metals different methods of the sequential chemical fractionation are used. According to the recommendations of the Interna- tional Union of Pure and Applied Chemistry (IUPAC), sequential extraction of the element forms from the soils, silts and bottom sediments is attributed to the methods of the element fractionation [6]. The main principle of such fractionation implies that every sequential leaching re- agent must be either stronger by its chemical action than the previous one or it must be of a different nature. It is possible to distinguish at least 8 different fractions of heavy metals, including radionuclides. It is worth noting that the fractionation is not associated with the extraction of individual chemical compounds but with the extrac- tion of their assembly. Each fraction extracted under the action of a particular leaching reagent, as a rule, turns out to include a group of compounds possessing similar properties (namely, physical and chemical mobility and bioavailability). In general the results of the fractionation of the element forms depend on the reagents used, con- tact time of the liquid and solid phases, intensity of shaking the mixture, ratio of the sample weight to re- agent amount and peculiarities of the soils and bottom sediments under investigation. Washing the sample with water and solution of some electrolyte is aimed at establishing the content of readily soluble and exchange forms of radionuclides in the sam- ple. However, after several tens of years of their entering the atmosphere, radionuclides could have coupled stro- ngly in partially soluble and insoluble forms. Exchange and readily soluble forms of radionuclide existence are of interest since, firstly, some of them may be destroyed by plant roots, resulting in penetration into the food chains; secondly, during spring floods and other natural events the bottom sediments can move down the river stream, leading to the contamination of areas located down the river as far as the estuary of the river and the ocean. The most frequently mentioned chemical compounds being present in the soils and bottom sediments are car- bonates, sesquioxides and hydroxides, organic substances and clay minerals. Here, carbonates can be destroyed by an acid solution, however, this may be accompanied by the destruction of other fractions, therefore, at present carbonates are destroyed using subacid solution of so- dium or ammonium acetate (рН = 5, which is achieved using nitric or acetic acid) [7-9]. Sesquioxides and hy- droxides are destroyed when interacting with acid solu- tions, though quite diluted ones, since using concen- trated acids may result in solving other fractions. For рН of the solution to change only slightly in the interaction process, the salt of this acid is added into the acid to cre- ate the buffer effect. Tamm’s solution has long been used which is an ethanediodic acid solution with ammonium oxalate [10,11]. At present the solution of hydroxyl- amine chloride in acetic acid is also used [6-8,12]. To destroy the organic substance use is usually made of the following oxidizing components: hydrogen peroxide [11,12], alkali pyrophosphates [8,12,13]. Amorphous  B. Lydia et al. / Natural Science 3 (2011) 517-529 Copyright © 2011 SciRes. OPEN ACCESS 519 silicates are destroyed by the solution of potassium or sodium hydroxide solutions [11]. When comparing the results obtained in the analysis using most of the techniques it is possible to partly de- scribe the type of the compounds formed and only with great approximation. Since a particular fraction may imply quite a wide range of compounds (movable or soluble in acids [10]) or a reagent is chosen which has an application limited by the frame of the objects under investigation (sodium pyrophosphate is convenient for studying light soils with a small amount of sand or for studying suspensions) [12]. The method of sequential chemical fractionation has the following shortcomings: 1) insufficient flexibility, low selectivity of the reagents used relative to the ex- tracted forms of the elements (soluble mineral phases of the sample) and, consequently, poor reproducibility of the results obtained [14]; 2) it takes several days of rou- tine work since the extraction of the element forms from the solid samples in static conditions (without renewing the reagent solutions) is slow. Consequently, estimating the flexibility and bioavailability of the element forms may not always be correct because the processes in nat- ural conditions always occur in dynamics, i.e. with con- stant renewing of the reaction soil solution. In spite of the shortcomings of the chemical fractiona- tion method it is frequently used, since being rather sim- ple it enables one to obtain important information. And the results obtained using sequential chemical fractiona- tion allows one to make preliminary forecasts on the possible migration of the radionuclides. The purpose of this study was to determine the rela- tionship between the mobility of artificial radionuclides and the mineral and geochemical properties of the Yeni- sei River sediments in the areas immediately affected by the operation of the MCC. 2. MATERIAL AND METHODS 2.1. Study Area. Material A great part of the population of the Krasnoyarsk Re- gion lives on the banks of the River Yenisei. The Yenisei is one of the biggest rivers in the world: its longitude from the conflux of the Big Yenisei and Little Yenisei to the Kara Sea is 3487 km (from the headstreams of the Little Yenisei—4287 km, from the headstreams of the Big Yenisei—4123 km). The conflux of the Big and Lit- tle Yenisei, near the town Kyzyl is considered to be the geographical center of Asia. Starting in the south of in the mountain deserts of Mongolia the River Yenisei over almost 3000 km flows to the north across different lati- tudinal geographical zones and inflows into the Arctic Ocean, forming an estuary up to 30 km wide. The length is bigger than that of the rivers Danube (2857 km); Mississippi (3770 km); Indus (3180 km). The Yenisei is the most full-flowing river of Russia with an annual wa- tershed yield 624 km3. The average water discharge in the estuary is 19800 m³/sec, the maximal one up to - 190000 m³/sec. As to the basin area (2580 thousand km2) the Yenisei is the second river in Russia (after the river Ob) and the seventh in the world. The River Yenisei is the nominal boundary between the West and East Siberia. There are three hydroelectric power stations on the Ye- nisei and tributaries. The river water is highly transpar- ent (up to 3 meters) and has low mineralization (the mean value is 54 mg/l) as well as high oxygen concen- tration. The flow speed and the river width vary signifi- cantly: from 1.5 to 12 - 15 km/h and from 0.2 - 0.5 km to 3 - 5 km. In the upper reaches of the river bed there are glacial boulder beds and cobble deposits which in the middle reach change into sandy gravel and sandy-clayish ones in the lower current of the river near the inflow into the Arctic Ocean. The operation of the greatest hydropower stations re- sults in constant mixing of water layers. Due to this, at a big distance down from the hydropower station the water temperature almost doesn’t change with the depth of the water flow. In early July the water temperature in the area near the Krasnoyarsk city and 100 - 150 km down- stream is ~10℃, and in the late July-August 15 - 17℃. The river ecosystem is an oligotrophic one with rich river fauna, there being more than 500 species of algae and diatoms [15]. Sediments were collected in the area immediately af- fected by the MCC operation, and samples of their up- permost layers (0 - 10 cm) were used in the study (Sam- ples 1 - 5) (Figure 1). 2.2. Sample Preparation 2.2.1. Geochemical Examination The ground sample was pressed into tablets with di- ameter 6 mm, mass 30 mg and subjected to synchrotron radiation X-ray fluorescence analysis (SXRF). Sediment texture analysis was performed using a hydrometer me- thod [16] after the destruction of organic matter with hydrogen peroxide (35%). The particle size analysis of silty sand is based on the fact that particles of different sizes have different free-fall velocities and, thus, they settle onto the bottom within different time periods. The suspension with the yet unsettled particles was repeat- edly decanted off at certain time intervals to separate fractions of <0.05-mm and <0.01-mm particles. The <0.05-mm fraction was separated by decanting off 6 cm of the suspension in 30 sec after the mixture was pre- pared and the <0.01-mm fraction by decanting off 2 cm  B. Lydia et al. / Natural Science 3 (2011) 517-529 Copyright © 2011 SciRes. OPEN ACCESS 520 Figure 1. Sketch-map of the south of the Krasnoyarsk Ter- ritory near the Mining-and Chemical Combine (MCC) of the Rosatom (Zheleznogorsk). “0 km”—place of industrial effluent water MCC; “15 km”—distant frontier region dis- card samples. of the suspension in 4 min. The separated suspensions were dried at 105˚C. The heavy mineral fraction was recovered from the sand fraction using tribrommethane (СНВr3) of the density 2.899 g/cm3 at 15˚C: quartz of the density 2.651 - 2.68 g/cm3 and feldspars of the den- sity 2.54 - 2.75 g/cm3 floated to its surface. 2.2.2. Differential Thermogravimetric Analysis For determining some components of the bottom sedi- ments (pore water, organic substances, carbonates, water incorporated into the layers of the clay minerals) the me- thod of the differential thermogravimetric analysis was used [17]. The portions of the naturally wet sediment samples under study (1 g) were placed into a porcelain crucible. Previously, the weight of the crucible had reached the constant value after burning in a muffle oven at 500˚C, for 3 days. The crucible with the bottom sediment sam- ple was placed into the muffle oven. At 105˚C the cruci- ble weight with the sample portion was also brought to a constant value during 48 h. In such a case there appeared water which could be attributed to pore (adsorption) wa- ter. After determining the adsorption water content in the sample the crucible with the portion was put into the muffle oven where it was heated at 350˚C, for 16 hours. In this case СО2 was released which had formed at the decomposition of the organic substances present in the bottom sediment samples. The water included into the interlayer space of the clay minerals and clays (incorpo- rated H2O), was determined at the subsequent sample heating at 600˚C, for 8 - 9 hours. When increasing the burning temperature up to 850˚C the sample weight loss was caused by the release of СО2, formed in the process of the thermal decomposition of the carbonates. In all the cases the samples were weighed only after cooling down to room temperature the crucible with the bottom sedi- ment sample in an exsiccator. The error of the differen- tial thermogravimetric method was less than 10%. The calculation of the content of organic substances in the bottom sediment samples was made using formula [18]: 0.458 0.4yx where y is the amount of the organic substance, mg; х is the weight decrease after burning at 350˚C, mg. 2.2.3. Sequential Chemical Extraction The total weight of the samples taken was 5 kg. Im- mediately after sampling the whole amount of the sub- stance was sieved with the mesh diameter being 0.5 μm and divided into several portions with the weight ap- proximately 1 kg. Each portion was packed into a plastic bag and placed into a fridge. Just before carrying out sequential chemical fractionation the bottom sediments from the bags were thoroughly mixed. From the whole amount the portions having 80 g of wet weight were taken. For each fractionation scheme 3 portions were selected. The literature data analysis has shown that the tech- nique of fractional extraction of the radionuclides from the soil includes several variants. All the existing tech- niques are based on the destruction of the constituents of the soils with the subsequent extraction of the radionu- clides included into a particular form. Two of the most widely spread schemes of sequential chemical fractionation were used in the work. This is fractionation developed on the basis of the Tessier tech- nique [11,19,20] and a scheme developed by the Com- munity Bureau of Reference (BCR) [21,22]. Table 1 presents both schemes used in our investigations of the existence forms of artificial radionuclides in the bottom sediment samples of the River Yenisei (Table 1). The remaining residue mainly consisted of clay min- erals, feldspars and quartz. The percentage of extraction in each step was determined as the ratio of the nuclide activity in the extract and its initial activity in the sedi- ment sample. In some cases the radionuclide activities in the extract were below the minimum detectable activity (MDA). For the calculation of the non-extracted residual fraction we have used (MDA/2 ± MDA/2)% as a, ficti- tious” value (recommendation by ISO Working Group TC85/WG17). 2.3. Measurement Procedures The silicate composition of sediment samples was de- 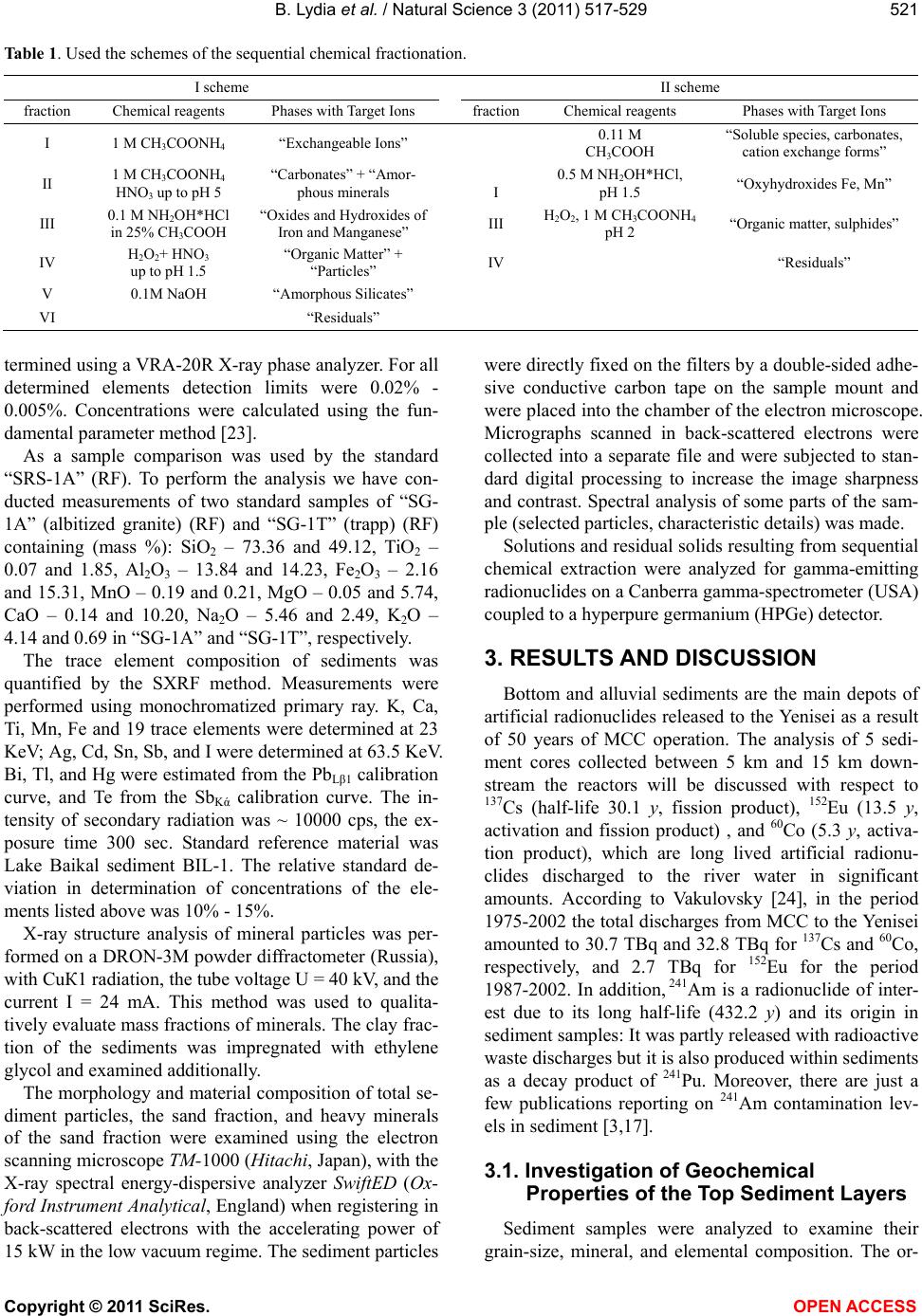 B. Lydia et al. / Natural Science 3 (2011) 517-529 Copyright © 2011 SciRes. OPEN ACCESS 521 Table 1. Used the schemes of the sequential chemical fractionation. I scheme II scheme fraction Chemical reagents Phases with Target Ions fractionChemical reagents Phases with Target Ions I 1 M CH3COONH4 “Exchangeable Ions” 0.11 М CH3COOH “Soluble species, carbonates, cation exchange forms” II 1 М CH3COONH4 HNO3 up to рН 5 “Carbonates” + “Amor- phous minerals I 0.5 M NH2OH*HCl, рН 1.5 “Oxyhydroxides Fe, Mn” III 0.1 M NH2OH*HCl in 25% CH3COOH “Oxides and Hydroxides of Iron and Manganese” III H2O2, 1 M CH3COONH4 pH 2 “Organic matter, sulphides” IV H2O2+ HNO3 up to рН 1.5 “Organic Matter” + “Particles” IV “Residuals” V 0.1М NaOH “Amorphous Silicates” VI “Residuals” termined using a VRA-20R X-ray phase analyzer. For all determined elements detection limits were 0.02% - 0.005%. Concentrations were calculated using the fun- damental parameter method [23]. As a sample comparison was used by the standard “SRS-1A” (RF). To perform the analysis we have con- ducted measurements of two standard samples of “SG- 1A” (albitized granite) (RF) and “SG-1T” (trapp) (RF) containing (mass %): SiO2 – 73.36 and 49.12, TiO2 – 0.07 and 1.85, Al2O3 – 13.84 and 14.23, Fe2O3 – 2.16 and 15.31, MnO – 0.19 and 0.21, MgO – 0.05 and 5.74, CaO – 0.14 and 10.20, Na2O – 5.46 and 2.49, K2O – 4.14 and 0.69 in “SG-1A” and “SG-1T”, respectively. The trace element composition of sediments was quantified by the SXRF method. Measurements were performed using monochromatized primary ray. K, Ca, Ti, Mn, Fe and 19 trace elements were determined at 23 KeV; Ag, Cd, Sn, Sb, and I were determined at 63.5 KeV. Bi, Tl, and Hg were estimated from the PbLβ1 calibration curve, and Te from the SbKά calibration curve. The in- tensity of secondary radiation was ~ 10000 cps, the ex- posure time 300 sec. Standard reference material was Lake Baikal sediment BIL-1. The relative standard de- viation in determination of concentrations of the ele- ments listed above was 10% - 15%. X-ray structure analysis of mineral particles was per- formed on a DRON-3M powder diffractometer (Russia), with СuК1 radiation, the tube voltage U = 40 kV, and the current I = 24 mA. This method was used to qualita- tively evaluate mass fractions of minerals. The clay frac- tion of the sediments was impregnated with ethylene glycol and examined additionally. The morphology and material composition of total se- diment particles, the sand fraction, and heavy minerals of the sand fraction were examined using the electron scanning microscope ТМ-1000 (Hitachi, Japan), with the X-ray spectral energy-dispersive analyzer SwiftED (Ox- ford Instrument Analytical, England) when registering in back-scattered electrons with the accelerating power of 15 kW in the low vacuum regime. The sediment particles were directly fixed on the filters by a double-sided adhe- sive conductive carbon tape on the sample mount and were placed into the chamber of the electron microscope. Micrographs scanned in back-scattered electrons were collected into a separate file and were subjected to stan- dard digital processing to increase the image sharpness and contrast. Spectral analysis of some parts of the sam- ple (selected particles, characteristic details) was made. Solutions and residual solids resulting from sequential chemical extraction were analyzed for gamma-emitting radionuclides on a Canberra gamma-spectrometer (USA) coupled to a hyperpure germanium (HPGe) detector. 3. RESULTS AND DISCUSSION Bottom and alluvial sediments are the main depots of artificial radionuclides released to the Yenisei as a result of 50 years of MCC operation. The analysis of 5 sedi- ment cores collected between 5 km and 15 km down- stream the reactors will be discussed with respect to 137Cs (half-life 30.1 y, fission product), 152Eu (13.5 y, activation and fission product) , and 60Co (5.3 y, activa- tion product), which are long lived artificial radionu- clides discharged to the river water in significant amounts. According to Vakulovsky [24], in the period 1975-2002 the total discharges from MCC to the Yenisei amounted to 30.7 TBq and 32.8 TBq for 137Cs and 60Co, respectively, and 2.7 TBq for 152Eu for the period 1987-2002. In addition, 241Am is a radionuclide of inter- est due to its long half-life (432.2 y) and its origin in sediment samples: It was partly released with radioactive waste discharges but it is also produced within sediments as a decay product of 241Pu. Moreover, there are just a few publications reporting on 241Am contamination lev- els in sediment [3,17]. 3.1. Investigation of Geochemical Properties of the Top Sediment Layers Sediment samples were analyzed to examine their grain-size, mineral, and elemental composition. The or- 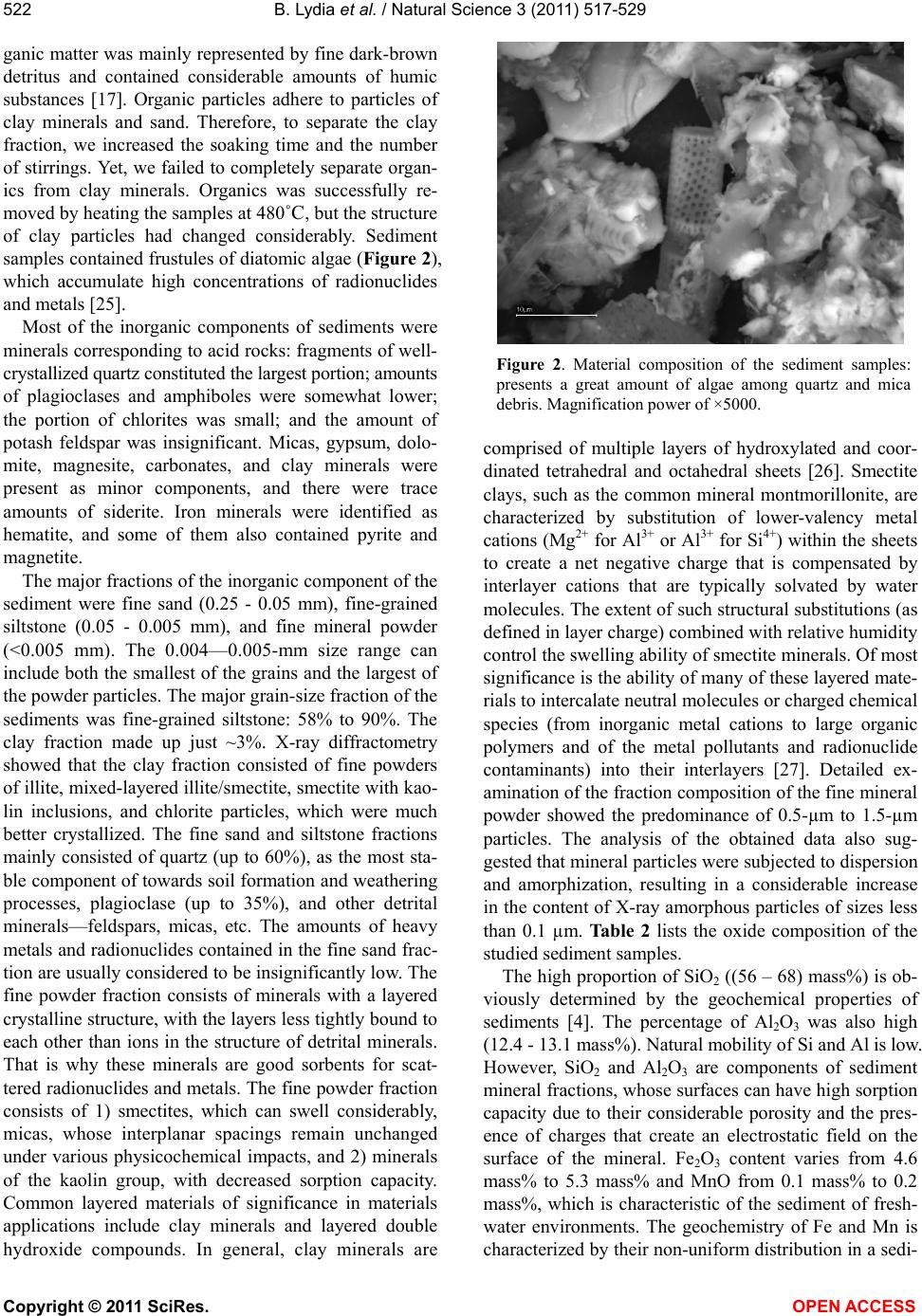 B. Lydia et al. / Natural Science 3 (2011) 517-529 Copyright © 2011 SciRes. OPEN ACCESS 522 ganic matter was mainly represented by fine dark-brown detritus and contained considerable amounts of humic substances [17]. Organic particles adhere to particles of clay minerals and sand. Therefore, to separate the clay fraction, we increased the soaking time and the number of stirrings. Yet, we failed to completely separate organ- ics from clay minerals. Organics was successfully re- moved by heating the samples at 480˚C, but the structure of clay particles had changed considerably. Sediment samples contained frustules of diatomic algae (Figure 2), which accumulate high concentrations of radionuclides and metals [25]. Most of the inorganic components of sediments were minerals corresponding to acid rocks: fragments of well- crystallized quartz constituted the largest portion; amounts of plagioclases and amphiboles were somewhat lower; the portion of chlorites was small; and the amount of potash feldspar was insignificant. Micas, gypsum, dolo- mite, magnesite, carbonates, and clay minerals were present as minor components, and there were trace amounts of siderite. Iron minerals were identified as hematite, and some of them also contained pyrite and magnetite. The major fractions of the inorganic component of the sediment were fine sand (0.25 - 0.05 mm), fine-grained siltstone (0.05 - 0.005 mm), and fine mineral powder (<0.005 mm). The 0.004—0.005-mm size range can include both the smallest of the grains and the largest of the powder particles. The major grain-size fraction of the sediments was fine-grained siltstone: 58% to 90%. The clay fraction made up just ~3%. X-ray diffractometry showed that the clay fraction consisted of fine powders of illite, mixed-layered illite/smectite, smectite with kao- lin inclusions, and chlorite particles, which were much better crystallized. The fine sand and siltstone fractions mainly consisted of quartz (up to 60%), as the most sta- ble component of towards soil formation and weathering processes, plagioclase (up to 35%), and other detrital minerals—feldspars, micas, etc. The amounts of heavy metals and radionuclides contained in the fine sand frac- tion are usually considered to be insignificantly low. The fine powder fraction consists of minerals with a layered crystalline structure, with the layers less tightly bound to each other than ions in the structure of detrital minerals. That is why these minerals are good sorbents for scat- tered radionuclides and metals. The fine powder fraction consists of 1) smectites, which can swell considerably, micas, whose interplanar spacings remain unchanged under various physicochemical impacts, and 2) minerals of the kaolin group, with decreased sorption capacity. Common layered materials of significance in materials applications include clay minerals and layered double hydroxide compounds. In general, clay minerals are Figure 2. Material composition of the sediment samples: presents a great amount of algae among quartz and mica debris. Magnification power of ×5000. comprised of multiple layers of hydroxylated and coor- dinated tetrahedral and octahedral sheets [26]. Smectite clays, such as the common mineral montmorillonite, are characterized by substitution of lower-valency metal cations (Mg2+ for Al3+ or Al3+ for Si4+) within the sheets to create a net negative charge that is compensated by interlayer cations that are typically solvated by water molecules. The extent of such structural substitutions (as defined in layer charge) combined with relative humidity control the swelling ability of smectite minerals. Of most significance is the ability of many of these layered mate- rials to intercalate neutral molecules or charged chemical species (from inorganic metal cations to large organic polymers and of the metal pollutants and radionuclide contaminants) into their interlayers [27]. Detailed ex- amination of the fraction composition of the fine mineral powder showed the predominance of 0.5-µm to 1.5-µm particles. The analysis of the obtained data also sug- gested that mineral particles were subjected to dispersion and amorphization, resulting in a considerable increase in the content of X-ray amorphous particles of sizes less than 0.1 µm. Table 2 lists the oxide composition of the studied sediment samples. The high proportion of SiO2 ((56 – 68) mass%) is ob- viously determined by the geochemical properties of sediments [4]. The percentage of Al2O3 was also high (12.4 - 13.1 mass%). Natural mobility of Si and Al is low. However, SiO2 and Al2O3 are components of sediment mineral fractions, whose surfaces can have high sorption capacity due to their considerable porosity and the pres- ence of charges that create an electrostatic field on the surface of the mineral. Fe2O3 content varies from 4.6 mass% to 5.3 mass% and MnO from 0.1 mass% to 0.2 mass%, which is characteristic of the sediment of fresh- water environments. The geochemistry of Fe and Mn is characterized by their non-uniform distribution in a sedi-  B. Lydia et al. / Natural Science 3 (2011) 517-529 Copyright © 2011 SciRes. OPEN ACCESS 523 ment layer, which is mainly caused by the ability of iron and manganese to change their valence and be then transformed to a soluble state. This creates migration fluxes, which involve other elements associated with Fe and Mn and their oxides. Figure 3 shows results of elemental analysis of sedi- ment sample particles. The distribution of the elements (Fe, Ca, Mg, Ti, Al, etc.) in sediment particles is not uniform and differs de- pending on the origin of the analyzed fragment. This me- thod is not sensitive enough to determine submicron quantities of artificial radionuclides. It is known that as a result of the chemical sorption (including the mechanisms of chemisorption, ion-exchange and co-deposition) there occurs the formation of chemi- cal associates between the surface of a bottom sediment particle and the ions of radionuclides and (or) their com- pounds in water. The content of some components of the bottom sediment absorbing complex with considerable reactivity can be one of the main indicators of the accu- mulation capacity of the samples under study with re- gard to artificial radionuclides. Such components are clay minerals and clays, specific and non-specific organic substances, carbonates. Most widely spread is the method of differential ther- mogravimetric analysis to determine the main compo- nents in the absorbing complex of the bottom sediment according to the sample weight loss when heated at dif- ferent temperatures. The temperatures used correspond to the decomposition of one or another bottom sediment component with the release of volatile products of the thermal reaction. The results of the differential thermogavimetry showed the following. The polar molecular structure of water contributes to its concentration on the surfaces of Fe- and Mn-hydroxides, organic substances and both on the outer surface and in the interlayer space of the clay min- erals [28]. The water loss upon heating at 105˚C is an indicator of humidity and is quantitatively connected with the relatively large surface areas of the compounds constituting the absorbing complex of the bottom sedi- ments. As is seen from the given results, the bottom se- diment sample under study contains about 38 wt% of adsorption water. This is characteristic of the surface layer of the bottom sediment (0 - 10 cm) of river systems with a high rate of water exchange. The amount of СО2 released at the destruction is equal to ~8.6 wt%. It cor- responds to ~16 mg of the organic substance in 1 g of the sample. The content of water incorporated in the clay minerals does not exceed 1 wt%. The weight loss at 8500С caused by the release of СО2 at the decomposition of the carbonates is equal to about 0.6 wt%. 3.2. Investigation of the Distribution of Artificial Radionuclides in Sediments In this study, extraction experiments of the following artificial radionuclides are discussed: 137Cs, 60Co, 152Eu, and 241Am. In the study area (from the site drains to the MCC the boundaries of our study—15 km downstream), the contents of the radionuclides varied in wide ranges: 137Cs—318-1800 Bq/kg, 60Co—87-720 Bq/kg, 152Eu— 12 - 287 Bq/kg and 241Am—6-76 Bq/kg. When using scheme 1 it was obtained that the migrat- ing form (associated with the exchange fraction and carbonates) was represented by (%): 60Co—4.3-6.9, 137Cs—3.3-3.8, 152Eu—8.2-9.7, 241Am—19.7-29.5. The content of the radionuclides bound with sesquioxides of Fe and Mn, as well as with the organic substance of the bottom sediment under study was determined, these ra- dionuclides being in a potentially migrating form. The following radionuclides were extracted (%): 60Co—12.6 - 13.6, 137Cs—2.1 - 5, 152Eu—38 - 51.8, 241Am—13.9 - 43.9. In the undecomposed residue, i.e. in the non-mi- grating form in the samples studied the content of the radionuclides corresponds to their physical and chemical peculiarities and to the type of association with the min- eral fragments of the bottom sediments. The following radionuclides were determined (%): 60Co—79.5 - 83.1, 137Cs—9.1 - 94.1, 152Eu—38.5-53.6, 241Am—44.7 - 66.4 (Table 2). When using scheme 2 in the first fraction radionu- clides having high migration ability and potentially bio- available radionuclides were extracted which are poten- tially dangerous for the environment. The radionuclides extracted were (%): 60Co—2.3 - 5.5, 137Cs—0.2 - 0.5, 152Eu—0.2 - 0.7. At the destruction of fraction II the following was extracted (%): 60Co—4.9 - 11.6, 137Cs— 5.8 - 8.1, 152Eu-45.8—55.7, 241Am—33 - 36.7. Fe/Mn oxides are known to exist as inclusions, concretions, binding agent between the particles or as covering layers on the particles. They are perfect radionuclide accumu- lators. At the destruction of this component under certain conditions the radionuclide content can considerably increase both in the pore space and in the mineral resi- due due to the formation of new compounds different in solubility and stability (Table 3). At the destruction of fraction III the following was extracted (%): 60Co—1.4 - 4.6, 137Cs—7.9 - 8.8, 152Eu— 17 - 27.6, 241Am—31.2 - 49.6. It is believed that under certain conditions (e.g. with the change of the content of organic compounds in the aquatic medium [28], the ra- dionuclides present in the oxidized fraction can again be involved into the local migration. Consequently, the mo- iety of the radionuclides present in this fraction indicates the contribution into the further migratory ability of the technogenic radionuclides in an aquatic ecosystem. 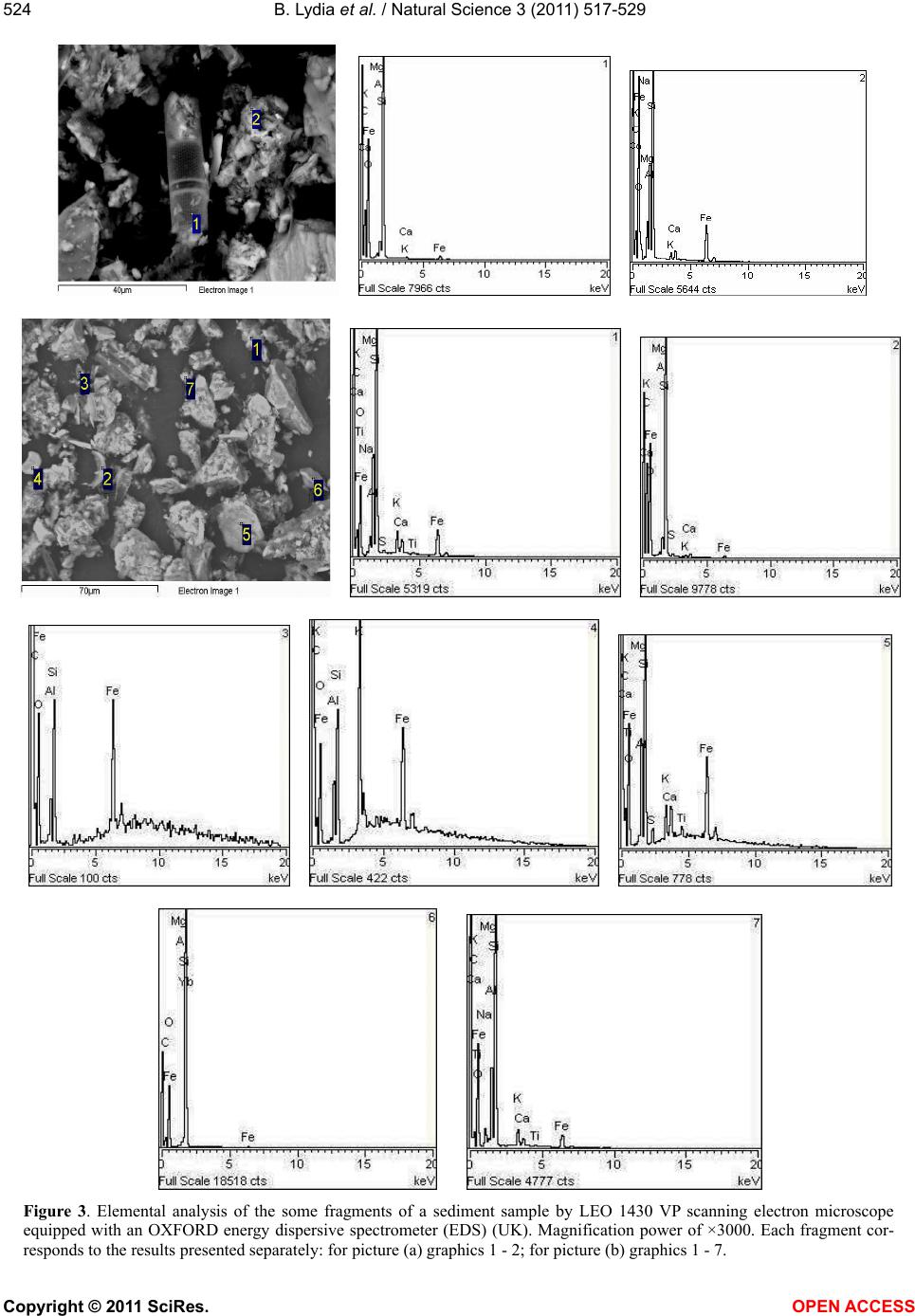 B. Lydia et al. / Natural Science 3 (2011) 517-529 Copyright © 2011 SciRes. OPEN ACCESS 524 Figure 3. Elemental analysis of the some fragments of a sediment sample by LEO 1430 VP scanning electron microscope equipped with an OXFORD energy dispersive spectrometer (EDS) (UK). Magnification power of ×3000. Each fragment cor- responds to the results presented separately: for picture (a) graphics 1 - 2; for picture (b) graphics 1 - 7.  B. Lydia et al. / Natural Science 3 (2011) 517-529 Copyright © 2011 SciRes. OPEN ACCESS 525 Table 2. (a) Sediment oxide composition, mass %; (b) Averaged results of sequential fractionation of sediment samples (scheme 1). (a) Oxide compositions Mean Minimum Maximum SiO2 63 56 68 Ti O2 0.76 0.7 0.8 Al2O3 12.8 12.4 13.1 Fe2O3 4.9 4.6 5.3 MnO 0.14 0.1 0.2 MgO 2.1 1.9 2.3 CaO 2.92 2.9 3.1 Na2O 2.46 2.0 2.9 K2O 1.88 1.7 2.0 (b) 60Co 137Cs 152Eu 241Am 1 2 3 1 2 3 1 2 3 1 2 3 I, % 0.9 2.9 3 2.8 3 0.9 2.1 18.5 2.6 II, % 4.3 6 4.9 0.4 0.8 0.6 5.8 7.3 7.6 11 17.1 III, % 8 8.6 7.2 0.6 0.7 0.8 22.519.8 21 21.8 19.9 IV, % 4.6 5 5 2.3 1.4 4.2 21.818.2 30.8 4 13.9 24 V, % 0.1 0.8 0.5 0.2 10.5 6.1 VI, % 83.1 79.5 82.9 93.7 94.1 90.8 46.453.4 38.5 34.2 60.3 56.1 Table 3. Averaged results of sequential fractionation of sediment samples (scheme 3). 60Co 137Cs 152Eu 241Am 1 2 3 1 2 3 1 2 3 1 2 3 I, % 5.5 2.5 2.3 0.2 0.5 0.7 0.6 0.2 II, % 11.6 5.3 4.9 7.5 8.1 5.8 54.755.7 46.8 36.7 33.0 III, % 4.6 1.4 1.5 8.1 7.9 8.8 23.817 27.6 44 31.2 49.6 IV, % 78.3 90.8 91.3 84.2 83.8 84.9 20.826.7 25.4 19.3 35.8 50.4 The radionuclide content detected at the destruction of the reducing fraction (scheme 2, fraction II) is higher than at the destruction of fraction III (scheme 1) similar in the main components. It is due to the fact that along with the sesquioxides, iron is also reduced which is bound with some organic substances, for example, with fulvic acids, and, therefore, radionuclides are released forming mixed complexes with these iron compounds [14]. Probably for this reason the radionuclide redistri- bution between fractions 2 and 3 is observed according to scheme 2 as compared to the radionuclide redistribu- tion between fraction 3 and 4 according to scheme 1. Moreover, the mobility of the radionuclides associated with the fraction being reduced is considered to be high- er than the mobility in the fraction being oxidized. The radionuclide content present in the non-migratory form was found in the undecomposed residue after the sequential extraction according to scheme 2: 60Co— 78.3% - 91.3%, 137Cs—83.8% - 84.9%, 152Eu—20.8% - 26.7%, 241Am—19.3% - 50.4% which was also different from the radionuclide content in fraction VI according to scheme 1. This is, probably, due to the fact that at the previous stages of chemical fractionation the compounds present in the reduced or oxidized form were destroyed, e.g. iron compounds [14]. Therefore, a greater number of radionuclides were extracted associated with these iron forms. The high percentages of 152Eu and 241Am in the frac- tion V+VI (scheme 1) can be accounted for by the pres- ence of microparticles containing significant radionu- clide concentrations. The occurrence and the properties of such particles were described by Bolsunovsky and his co-authors [29]. A significant part of the radionuclides released during various nuclear events has been associ-  B. Lydia et al. / Natural Science 3 (2011) 517-529 Copyright © 2011 SciRes. OPEN ACCESS 526 ated with particles. Radioactive or hot particles were released into the environment as a result of nuclear weapon tests, fallouts from accidents at nuclear reactors, discharges from reprocessing plants and associated ra- dioactive wastes. Therefore, releases of radioactive par- ticles have occurred more frequently than perhaps usu- ally anticipated [30]. Most of those particles were ura- nium containing graphite particles of relatively large size (300 to 700 μm); practically in all particles 137Cs was the dominating radionuclide [31,32]. At the same time active particles with much lower activities (n·101 to n·103 Bq per particle) were spread more abundantly and could be demonstrated only in laboratory conditions using re- peated quartering and high precision gamma-spectros- copy [32,33]. Their contribution to the total contamina- tion was significant and reached up to 25% - 100% for different isotopes in flood land soils [33]. The composi- tion and size of those particles were not completely clear. Gritchenko et al. [33] estimated their size to be in the order of 10 to100 μm. Sukhorukov et al. [32] catego- rized the active particles into polyisotopic (containing 152Eu, 154Eu, 155Eu, 137Cs, 60Со and 241Am) and monoiso- topic (60Со or 137Сs) particles. 3.3. Determination of the Relationship between the Mobility of Artificial Radionuclides and Mineral and Geochemical Properties of Sediments Relationships, including correlations, between the properties of objects of study and certain parameters of these objects are investigated using statistical methods or mathematical models. In this study, correlations between the species of artificial radionuclides and mineral and geological parameters of the examined sediments were obtained using the statistical package of Microsoft Of- fice Exсel 2003. 60Co interacts, via an ion exchange mechanism, with compounds present on the surface of clay minerals (r2 = 0.63) and sand (r2 = 0.5). 60Co concentration depends on the levels of Al2O3 (r2 = 0.75) and K2O (r2 = 0.74), which are incorporated in crystal structures of sediment parti- cles. On the other hand, surface phenomena at the inter- action interface of the two compartments (mineral- aqueous medium) are known to be caused by the water molecule adsorption and formation of a hydrate aqua film on the surface. The hydration degree of the clay particles is mainly determined by the influence of ca- tions-compensators (for example, potassium) which are located on the surface of the solid particles. The cations – compensators actively attach water molecules to their surface. This results in weakening the bonds between the cations and the surface in the aqueous medium, thus, a number of the cations is removed from the solid particles. The cations remaining on the surface are considered to form an adsorption layer and the removed cations create a diffusion layer. In this connection, the negatively charged surface of the solid particles and the adsorption and diffusion cation layers compensating its charge form a double electric layer of the particle. The double electric layer structure is not constant but changes with time un- der the influence of environment conditions [28]. Additionally, mobile species are formed as a result of 60Co interaction with the readily soluble mixed carbon- ates, which are associated with the surface of the miner- als containing Al2O3 (r2 = 0.8) and CaO (r2 = 0.94). Par- ticles of clay minerals and sand are covered with a Fe2O3 polymer film with a high sorption capacity, which inter- acts with metals that are transported to sediments [34]. Potentially mobile 60Co species may be formed as a re- sult of 60Co interaction with Fe2O3 (r2 = 0.57), where they can correlate with MgO (r2 = 0.83). As certain or- ganic substances (such as lignin) are poorly soluble, the immobile 60Co may also correlate with the organic con- tent (r2 = 0.53). Similarly to 60Co, 152Eu and 241Am interact with fine carbonate powder, forming mobile species, which mainly occur on the surface of the sand (r2 = 0.65 and r2 = 0.98 for 152Eu and 241Am, respectively) containing Al2O3 (r2 = 0.87 and r2 = 0.99 for 152Eu and 241Am, respectively). 241Am was found to be related to MgO (r2 = 0.94). As stated above, the potentially mobile 152Eu and 241Am react mostly with organic components of sediments (r2 = 0.64 and r2 = 0.66 for 152Eu and 241Am, respectively). The data on the geochemical composition of sediment samples showed that a certain portion of the organic component is firmly bound to the surface of the particles, mostly clay minerals. Hence, levels of 152Eu and 241Am in the organic fraction are related to the amount of clay minerals (r2 = 0.93 and r2 = 0.69 for 152Eu and 241Am, respectively). The proportion of 241Am associated with the organic component of the sediment is related to Al2O3 concentration (r2 = 0.96), whereas 152Eu is related to Fe2O3 (r2 = 0.78), MnO (r2 = 0.97), and TiO2 (r2 = 0.77). Much of the potentially mobile portion of 152Eu and 241Am is formed by the interaction of the radionu- clides with Fe and Mn oxides, which belong to the frac- tion “sesquioxides and hydroxides” (III): r2 = 0.85 for 241Am-Fe2O3 and r2 = 0.92 for 152Eu-MnO. In the same fraction, 241Am concentration is related to the levels of CaO (r2 = 0.89), Na2O (r2 = 0.81), and K2O (r2 = 0.93). As assumed previously [3,17], the immobile 152Eu and 241Am were mainly bound to clay minerals (r2 = 0.91 and r2 = 0. 79 for 152Eu and 241Am, respectively). Analysis of the relationships for the chemical fractions of 152Eu and 241Am showed that the radionuclide amounts extracted  B. Lydia et al. / Natural Science 3 (2011) 517-529 Copyright © 2011 SciRes. OPEN ACCESS 527 from the organic fraction correlated with their concen- trations in the residual solids (r2 = 0.82 and r2 = 0.97 for 152Eu and 241Am, respectively). This can indicate that under different conditions, radionuclides can be con- verted from the potentially mobile form to the immobile one. The conditions can be changed if, for instance, sedi- ment humic substances are humified, forming poorly so- luble humus. On the other hand, the immobile form can become more readily soluble and, thus, more mobile. A mobile species of 137Cs is formed when the ra- dionuclide interacts, via an ion exchange mechanism, with Na2O (r2 = 0.98) and K2O (r2 = 0.78), which are present in the diffuse layer on the surface of sediment particles, including the sand (r2 = 0.62). The amount of potentially mobile 137Cs is mainly related to concentra- tions of Fe2O3 (r2 = 0.78) and MnO (r2 = 0.57) in the fraction “sesquioxides and hydroxides” and to MnO concentration (r2 = 0.63) in the organic fraction of the sediment. 137Cs, which can be incorporated into crystal- line structures of primary and secondary minerals, be- comes firmly bound to particles of sand (r2 = 0.62) and clay minerals (r2 = 0.93), in which it resides in the im- mobile form. Correlation analysis of 137Cs concentra- tions in the chemical fractions indicated a relationship between the mobile 137Cs concentration (“carbonates”) and the potentially mobile 137Cs concentration (“organ- ics”) (r2 = 0.91). So, a change in geochemical conditions, resulting in various transformations in sediments, can cause a reversible redistribution of 137Cs between less mobile and more mobile forms. Thus, we can make the following assumptions. The mobility of artificial radionuclides is directly related to the mineral and geochemical properties of the sediments they interact with: the structure and the composition of the minerals determine the type of sedimentary struc- tures that form on their surface. The surface can be composed of fine-crystalline or amorphous deposits, or a mixture of both. In addition to this, the surface can be positively or negatively charged. These factors affect possible mechanisms of the uptake of metals and ra- dionuclides from the liquid layer that contacts the sedi- ments. The presence and the degree of the physico- chemical affinity between the radionuclides and the ele- ments comprising the sediment mineral component de- termine the stability or instability of the relationships between them. For instance, the interaction between ra- dionuclides and the exchangeable fraction of sediments, via an ion exchange, results in the formation of unstable associates. As the exchangeable fraction is usually char- acterized as a diffuse layer on the surface of the particles, the associates are easily destroyed to form soluble com- pounds. 137Cs, e.g., correlates well with Na and K com- pounds in the exchangeable fraction because these ele- ments are alkali metals and have similar physicochemi- cal properties (Table 2). Sediments contain various poorly soluble carbonate compounds (up to 3.3 mass%). If, however, the condi- tional equilibrium in a sediment layer is somewhat shifted (e.g., if the sediment is acidulated due to humifi- cation processes), this can result in the formation of hy- drogen carbonates or their complete transformation. Thus, radionuclides bound to the chemical fraction “carbon- ates” are converted into other species, which have dif- ferent solubility properties and, hence, different mobility. This can be confirmed by the positive correlations ob- tained for 60Co- CaO , 241Am-MgO and 137Cs associated with the fractions “carbonates” and “organics” (Table 2). Fe and Mn sesquioxides and hydroxides, forming po- lymer film on the surface of mineral particles, bind con- siderable amounts of radionuclides, which change their valence and, hence, are able to form redox pairs with the corresponding potential, such as 60Co- Fe2O3, 152Eu- -Fe2O3-MnO. Moreover, the film formed by sesquiox- ides and hydroxides has charged sites, which bind ra- dionuclide compounds with a corresponding opposite charge, e.g., 137Cs-Fe2O3-MnO, 241Am-Fe2O3. Iron, how- ever, can be a component of both silicate and non-sili- cate compounds [17]. Thus, radionuclides with similar physicochemical properties can form such compounds with Fe, e.g., 60Co (fractions V+VI)-Fe2O3. The organic constituent of sediments consists mainly of humic substances: humic acids, fulvic acids, and humin [4]. The proportions of these components deter- mine the percentage of radionuclides bound to the or- ganic fraction [14,17]. As organic substances rather firmly adhere to a large part of the surface of sediment particles, their reactivity also depends on the reactivity of humic substance components. The percentages of 152Eu and 241Am associated with organics, particularly with low-molecular-weight and free fulvic acids, were the highest among the radionuclides examined in this study [17]. The total amount of organics correlates with clay minerals: their layered structure makes them pene- trable by organic substances and their complexes with, e.g., radionuclides. On the other hand, certain compo- nents of the organics contained in sediments are resistant to chemical reagents (such as lignin). This portion of organics, together with associated radionuclides, stays in the residual solids, i.e. is considered to be firmly bound to the mineral structures of sediments; for instance, 152Eu and 241Am concentrations associated with the organics correlate with the residual solids. Geochemically speaking, residual solids are a coarse fraction of sediments, consisting of insoluble crystalline structures. Residual solids also contain clay minerals deformed by chemical fractionation and an insignificant  B. Lydia et al. / Natural Science 3 (2011) 517-529 Copyright © 2011 SciRes. OPEN ACCESS 528 portion of insoluble organics. Hence, radionuclides as- sociated with residual solids are bound to them either via the mechanism of isomorphic incorporation in the crys- tal lattices of silicate structures (e.g., 137Cs concentration correlates with the portions of sand and clay minerals) or as a result of compound formation in interlayer spaces of clay minerals (e.g., interaction of 152Eu and 241Am with clay minerals). 4. CONCLUSIONS The results of this study suggest the following conclu- sions: 1) The fractionation according to scheme 2 is mainly based on the physical-chemical properties of the ele- ments included into the mineral geochemical compo- nents of the bottom sediments, e.g. iron compounds. In this connection there is some overlapping between the main fractions and, thus, the redistribution between the forms of existence, whereas fractionation according to scheme 1 is based on the properties of the mineral com- positions of the bottom sediments. However, when using both fractionation schemes it is possible to compare the obtained results and use them for a more complete de- scription of the radionuclide behavior in the bottom se- diments. 2) The identified correlations can indicate that under different conditions, radionuclides can be converted from the potentially mobile form to the immobile one. As changing conditions can be considered, for instance, sediment humic substances are humified, forming poorly soluble humus. But on the other hand, the immobile form can become more readily soluble and, thus, more mobile. In the latter case, it formed flows of secondary contamination of the Yenisei River floodplain radionu- clides. 5. ACKNOWLEDGEMENTS The authors would like to express their gratitude to Razvorotneva L.I., Ph.D., and Armancheva T.A. and Dubova V.P., technicians, at the Institute of Geology and Mineralogy SB RAS, and to Kolmogorov Y.P., a technician of the Elemental Analysis Station at the VEPP-3 of the Institute of Nuclear Physics SB RAS. REFERENCES [1] Bolsunovsky, A.Ya., Ermakov, A.I., Myasoedov, B.F., Novikov, A.P. and Sobolev, A.I. (2000) New data on the content of transuranic elements in bottom sediments of the Yenisei River. Doklady Earth Sciences, 386, 971-974. [2] Bolsunovsky, A. (2004) Artificial radionuclides in aqua- tic plants of the Yenisei River in the area affected by ef- fluents of a Russian plutonium complex. Aquatic Ecol- ogy, 38, 57-62. [3] Bolsunovsky, A. and Bondareva, L. (2007) Actinides and other radionuclides in sediments and submerged plants of the Yenisei River. Journal of Alloys and Compounds, 444-445, 495-499. [4] Orlov, D.S., Sadovnikova, L.K. and Sukhanova, N.I. (2005) Khimiya pochv (soil chemistry). Vysshaya Shkola, Moscow. [5] Yashin, I.M., Shishov, L.L. and Raskatov, V.A. (2001) Metodologiya i opyt izucheniya migratsii veshchestv (a methodology of and experience in studying material mi- gration). MSkhA Publishers, Moscow. [6] VcLean, J.E. and Bledsoe, B.E. (1992) Behavior of metal in soils. Report EPA USA. 25. [7] Schultz, M., Burnett, B., Kenneth, G.W., et al. (1995) Development of a sequential extraction technique to define the fractionation of radioactive elements in NIST natural matrix standards. 41st Conference on Bioassay, Analytical, and Environmental Radiochemistry, Boston, 12-16 November 1995, 707-715. [8] Howard, J.L. and Vanderbrink, W.J. (1999) Sequential extraction analysis of heavy metals in sediments of vari- able composition using nitrilotriacetic acid to counteract resorption. Environmental Pollution, 106, 285-292. [9] Wang, Z., Shan, X.-Q. and Zhang, S. (2002) Com- parison between fractionation and bioavailability of trace elements in rhizosphere and bulk soils. Chemosphere, 46, 1163-1171. [10] Goryachenkova, T.A., Pavlotskaya, F.I. and Bin, N.T. (1990) Forms of the existence of plutonium in soils. Radiochemistry, 2, 47-54. [11] Putyrskaya, V., Klemt, E. and Röllin, S. (2009) Migration of 137Cs in tributaries, lake water and sediment of Lago Maggiore (Italy, Switzerland)—Analysis and comparison with Lago di Lugano and other lakes. Journal of Envi- ronmental Radioactivity, 100, 35-48. [12] Gasco, C., Anton, M.-P. and Gonzales, A.M. (1999) Geochemical association of transuranics in svalbard se- diments. 4th International Conference on Environmental Radioactivity in the Arctic. Edinburgh, 20-23 September 1999, 216-218. [13] Sowder, A.G., Bertch, P.M. (1998) Speciation of uranium and nickel in aged-contaminated sediments: Coupling spatially resolved SXRT and XANES with chemical ex- tractions/Savannah River ecology laboratory. The Uni- versity of Georgia, Aiken. [14] Fedotov, P.S. and Spivakov, B.Y. (2008) Statistical and dynamic methods of fractionation of element forms in soils, silts and bottom sediments. Success in Chemistry, 7. 690-703. [15] Gitelson, I.I., Abrosov, N.S. and Gladyshev, M.I. (1988) The main hydrological and hydrobiological characteris- tics of the Yenisei River. Transport of Carbon and Min- erals in Major World Rivers, Lakes and Estuaries, Ge- ologisch-Palaeontologisches Institut, Hamburg. [16] Gee, G.W. and Bauder, J.W. (1986). Particle-size analysis. In: Klute, A. Ed., Methods of Soil Analysis, Annual Meetings of the American Society of Agronomy and Crop Science Society of America, Madison, 383-411. [17] Bondareva, L.G. and Bolsunovsky, A.Ya. (2008) Speci- ation of artificial radionuclides 60Co, 137Cs, 152Eu and 241Am in bottom sediments of the Yenisei River, Radio- chemistry, 50, 547-552. [18] Alvarado, J.S., Neal, T.J., Smith, L.L. and Erickson, M.D.  B. Lydia et al. / Natural Science 3 (2011) 517-529 Copyright © 2011 SciRes. OPEN ACCESS 529 (1996) Microwave dissolution of plant tissue and the subsequent determination of trace lanthanide and actinide elements by inductively coupled plasma-mass spec- trometry. Analytical Chemica Acta, 322, 11-20. [19] Kaminski, S., Konoplev, A., Lindner, G. and Schröder, H.G. (1998) The fate of artificial caesium radionuclides in Lake Constance. Archive fur Hydrobiologie Special Issues Advanced Limnology, 53, 369-409. [20] Klemt, E., Kaminski, S., Miller, R., Zibold, G., Astner, M., Burger, M. and Schmid, E. (2000) Normierung von extraktionsexperimenten zur bestimmung der bindung von radiocaesium an sedimente des luganersees. Um- weltradioaktivität und Strahlendosen in der Schweiz 1999. Bundesamt für Gesundheit. B. 4.4.1-5. [21] Kartal, Ş., Aydın, Z. and Tokalıoğlu, Ş. (2006) Fractiona- tion of metals in street sediment samples by using the BCR sequential extraction procedure and multivariate statistical elucidation of the data. Journal of Hazardous Materials, 132, 80-89. [22] Rauret, G., López-Sanchez, J., Sauquillo, et al. (1999) Improvement of the BCR three step sequential extraction procedure prior to certification of new soil and sediment reference materials. Journal of Environmental Monito- ring, 1, 57-60. [23] Afonin, V.P., Gunicheva, T.N. and Piskunova, L.F. (1984) Rentgenofluorestsentnyi silikatnyi analiz (X-ray fluore- scence silicate analysis). Nauka, Novosibirsk. [24] Vakulovsky, S.M. (Ed.) (2003). Estimation and predi- ction of the consequences for the environment and popu- lation of radioactive contamination of the river Yenisei by discharges of the Krasnoyarsk mining and chemical industrial complex. Final Project Technical Report of In- ternational Science and Technology Centre Project 1404, SPA-Typhoon, Obninsk. [25] Zotina, T.A., Bolsunovsky, A.Ya. and Bondareva, L.G. (2010) Accumulation of 241Am by suspended matter, di- atoms and aquatic weeds of the Yenisei River. Journal of Environmental Radioactivity, 101, 148-152. [26] Grim R.E. (1968) Mineralogy, second edn., McGraw-Hill, New York. [27] Cygan R.T., Greathouse J.A., Heinz H., Kalinichev A.G., (2009). Molecular model and simulations of layered ma- terials. Journal of Materials Chemistry, 19, 2470-2481. [28] Shvanov, V.N., Frolov, V.T., Sergeyeva, E.I., et al. (1998) Systematika i klassifikacia ocadochnih porod i ih analo- gov. S-Pb., Nedra. [29] Bolsunovsky, A.Ya. and Tcherkezian, V.O. (2001) Hot particles of the Yenisei River flood plain, Russia. Journal of Environmental Monitoring, 57,167-174. [30] Salbu, B. (2001) Hot particles—A challenge within ra- dioecology. Journal of Environmental Radioactivity, 53, 267-268. [31] Bolsunovsky, A.Ya., Goryachenkova, T.A., Tcherkezian, V.O. and Myasoedov, B.F. (1998) Hot particles in Kras- noyarsk Region. Radiochemistry, 40, 278-281. [32] Sukhorukov, F.V., Melgunov, M.S. and Chuguevsky, A.V. (2009) “Hot” and active particles in alluvial soils and se- diments of the Yenisei River: Radioisotope composi- tion. Radioprotection, 44, 227-231. [33] Gritchenko, Z.G., Kuznetsov, Yu.V., Legin, V.K., Strukov, V.N., Myasoedov, B.F., Novikov, A.P., Shishlov, A.E. and Savitskii, Y.V. (2001) Hot particles of the second kind in flood lands of the Yenisey River. Radiochemistry, 43, 639-642. [34] Pavlotskaya, F.I. (1974) Migratsiya radioaktivnykh pro- duktov globalnykh vypadenii v pochvakh (migration of radioactive products of global fallouts in the soils). At- omizdat, Moscow.
|