 World Journal of Engineering and Technology, 2015, 3, 48-54 Published Online December 2015 in SciRes. http://www.scirp.org/journal/wjet http://dx.doi.org/10.4236/wjet.2015.34C006 How to cite this paper: Sugiani, D., Lusiastuti, A.M., Taukhid and Purwaningsih, U. (2015) Treatments for Temnocephalids Ectosymbiont Craspedella sp. on Cherax quadricarinatus and Cherax albertisii “Papua Freshwater Lobster”. World Journal of Engineering and Technology, 3, 48-54. http://dx.doi.org/10.4236/wjet.2015.34C006 Treatments for Temnocephalids Ectosymbiont Craspedella sp. on Cherax quadricarinatus and Cherax albertisii “Papua Freshwater Lobster” Desy Sugiani, Angela Mariana Lusiastuti, Taukhid, Uni Purwaningsih Research Institute for Freshwater Aquaculture, Ministry of Marine Affairs and Fisheries, Bogor, Indonesia Email: desysu giani@yah oo.co .id Received 9 November 2015; accepted 13 December 2015; published 17 December 2015 Abstract There were two species of crayfish “red-claw” (Cherax quadricarinatus) and “blue-huna” (Cherax albertisii) for their aquaculture potential. Crayfish were susceptible to fungal (crawfish plague), parasitic (protozoa and nematodes), and bacterial pathogen. A number of ectosymbiont Cras p ed ell a sp. have been observed on red-claw and blue-huna. The flatworms were commonly found almost in the whole body, on the upper exoskeleton behind the head, in the gill cavity and on the claws and underside of crayfish. Although their number sometimes was very high, they didn’t cause any problems especially for the new molting crayfish. Micro organisms living on the crayfish surface body and worms didn’t cause any pathological changes. Adults Cras pe del la sp. can be eliminated by a short bath in salt water or formaldehyde 37% solutions for several hours. This treatment didn’t kill worm eggs, so it needs to be repeated every one week. Moreover, hyposalinity or OST (Osmotic Shock Therapy) is one of the most effective therapi es for ectoparasites on Cra s pe d ell a sp. with dose of bath treatment 15 grams per litre of salt (15 ppt) for more than 3 hours, and dipped in salt water at 30 ppt (or 3.0 ppm, seawater salinity) for 15 - 20 minutes. Keywords Crasp ed el la sp., Cherax quadricarinatus, Cherax albertisii, Tre atmen t 1. Introduction Red claws have b een gro wn succ essfull y in po nds on a resea rch scale for t he last four ye ars. In a dditio n, man y pri vate compa nies have been aggr essively e ngaged i n the deve lopment of red c law prod uction in Ind onesia and abroad for several years. Kurniasih (2008) [1] has evaluated growth performance of freshwater cryfish by Chironomus sp. and artificial feed in Irian (C. albertisii) an d Australian ( C. quadricarinatus). The red claw may reach a total length of about 250 mm and weigh up to 600 grams. This is a non-burrowing species that is tolerant of a wide variety of habitats. Found in tropical regions throughout the world, including its native range of  D. Sugiani et al. Northern Australia and Papua New Guinea, as well as introductions in Jamaica, parts of South America, and Puerto Rico, the red claw thrives in temperatures between 15 and 30 degrees Celsius (U.S. Fish & Wildlife Service, 2012) [2]. Cherax quadricarinatus can tolerate intensive culture conditions without affecting their growth and survival and has ability to maintain a low routine metabolic rate, is highly tolerant to limited dissolved oxygen conditions and thus uses energy efficently, cryfish survives in earthen ponds under ambient winter temperatures (Carreno-Leon et al., 2014; Karplus et al., 1998) [3] [4]. This species has been reported to be a carrier of a number of pathogens, including viruses, bacteria, fungi, protozoan and metazoan parasites (Austin et al., 2009) [5]. Diseases such as the European Crayfish Plague (Aphanomyces) have devastated European stocks and reached British native stocks in 1986. Australia is the only continent to be Crayfish Plague free. Porcelain disease (or White Tail disease) can also occur in the yabby, caused by a single celled microscopic animal, the microsporidian Thelophania. The disease can easily be detected in its late stages when the u nde rsid e of t he tai l tur ns white a nd t he wal kin g leg s ofte n be come r igid and splayed. It is invariab ly fatal and seems to be transmitted by cannibalism of dead or dying yabbies that have the disease. This disease is not harmful to humans, and is present in possibly 5% of natural crayfish populations. Temnocephalid flatworms are 1 to 110 mm long leech-like animals, and their eggs are laid on the soft under surfaces of the crayfish. These worms are not true parasites but commensals because they feed upon particles of food scattered in the water while the crayfish is eating. They are not actively harmful to the crayfish (Australian Blue Yabby Aquaculture, 2010) [6]. Craspedellas occur as a result of poor water quality. They may be considered as commensals, however, with stress, overcrowding, low oxygen levels, improper water parameters, changes in temperature or poor diet, the parasites can multiply and the crayfish may then develop signs of systemic disease. Most parasitic organisms are opportunistic and may be present all the time in the tank or on the crayfish in low numbers, and only cause disea ses whe n the crayfish is stressed. The amount of fish present in a tank, pH, lighting, the water temperature, type of filtratio n s ystem and water che mistries all influences the health of the fis h. There are several options of drugs or chemicals placed in the water, they are commonly referred to as bath treatments. Drugs delivered orally are generally mixed in the food, and are meant to deliver systemic effects. Injections may be utilized in cases where small numbers of fish are involved. Bath treatments are the most variable, in that a specific concentration of the chemical is placed in the water for a specific length of time. As a rule, lower concentrations are used for longer periods of time, and vice versa. This research is a case study on the identification of parasites Craspedella sp. o n crayfis h “re d-claw” (Cherax quadricarinatus) and “blue-huna” (Cherax albertisii) and how to control them with the option to follow the rules of fish health management practices using OST (Osmotic Shock Therapy) and parasiticide. 2. Materials and Methods 2.1. Experimental Specimens and Identification Specimens of ectosymbiont Craspedella were collected from the commercial rearing unit of redclaw Cherax quadricarinatus and blue-huna Ch erax alb ertisii and were observed for 1) number of individual infestat ion and 2) experimentation on treatment. To obtain live Craspedella sp., gills and wet mount from whole body of crayfish were placed in a shallow glass vessel containing clean water for scanning microscopy examination. For the identification of Craspedella a temnocephalid tubellarian of Foundations of Parasitology by Schmidt and Roberts were used. Craspedella sp. was collected from the commercial redclaw Cherax quadricarinatus and blue-huna Cherax albertisii in two cr ayfish re aring tanks fro m West Java, Banten, Jakarta, and Central Java [7]. 2.2. Study Design and Treatment Conditions This trial examined formaldehyde and hypo salinity treatment on crayfish after exposure to Craspedella sp. Totally, 150 crayfish (6.7 ± 0.6 cm) were used in this trial, with 15 crayfish tank−1 and 2 tanks per treatment. Success treatment was indicate signifcantly effects with all crayfish from each treatment has negative for Craspedella sp. Parasite loads were evaluated base on severity level of infection, light (<50 parasites fish−1), medium (50 - 100 parasites fish−1), and heavy infection (>100 parasites fish−1). The following treatments were conducted: 1) prolonged immersion wit h 0 .1 mL for maldehyde per 10 liter water, 2) bath treat me nt with 0.2 mL fo rmaldehyde 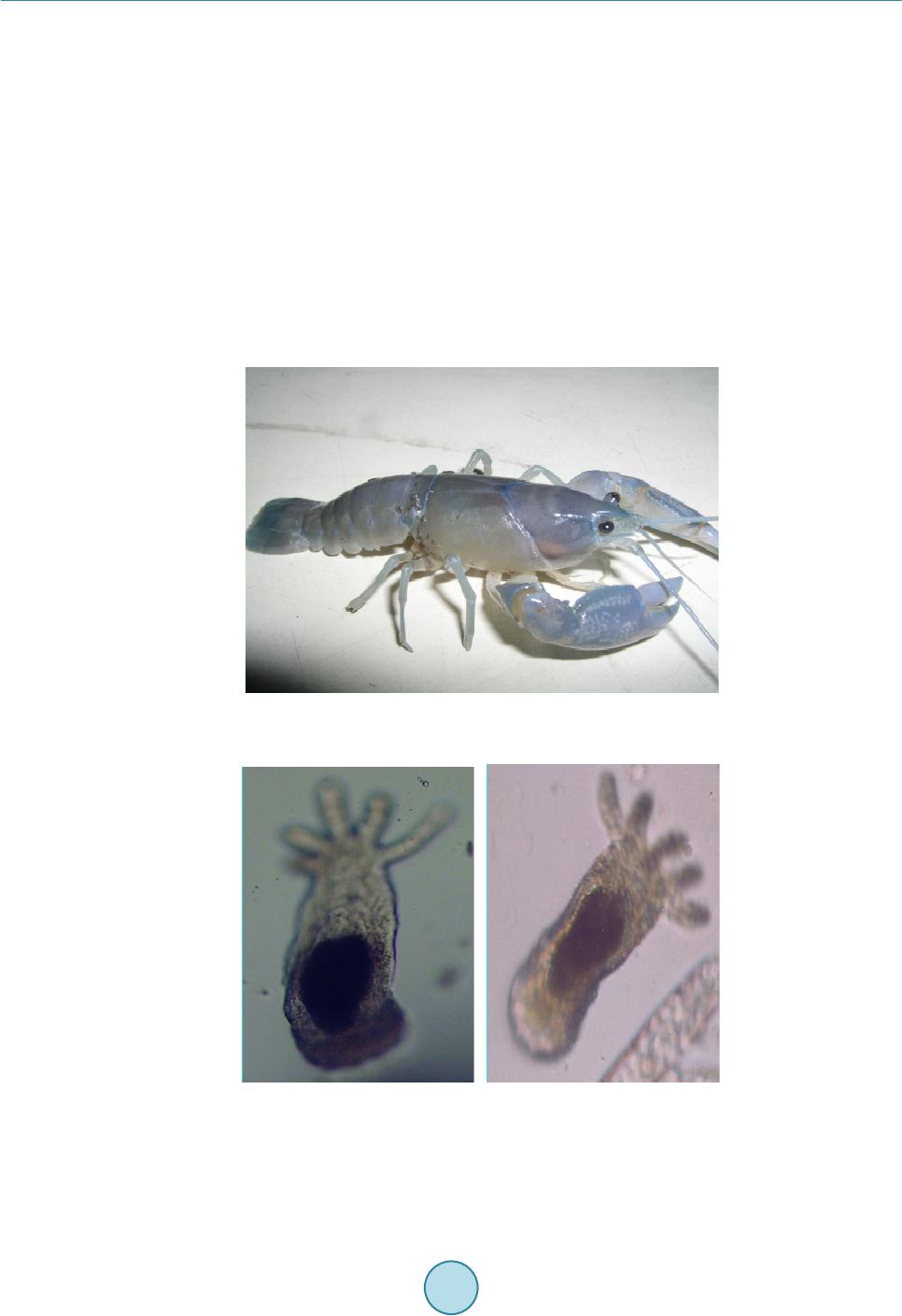 D. Sugiani et al. per 10 liter water, 3) prolonged immersion used 3 ppt salt water, 4) bath treatment used 15 ppt salt water, 5) dipped in 30 ppt salt water, and 6) control. 3. Result Craspedella was found on 120 crayfish (out of 150) in two crayfish rearing tanks when water temperature was varied from 24˚C - 32˚C. Craspedella sp. prevalence which observed on carapace, abdomen, walking legs, uropod, antennae, and antennulae of the crayfishwas 40% - 60% (Figure 1). Ultrastructural study of Craspedella sp. who attached to the gills showed that Craspedella sp. d id not damage the gill epicutic le of crayfish (Figure 2). Therefore, it can be concluded that although Craspedella sp. did not have harmful effects on the respiration system o f crayfis h, they coul d have an adverse effect on their marketability. The anatomy of Craspedella sp. a temnocephalid turbellarian showed in Figure 3. Temnocephalids are small and flattened, with tentacles at the anterior end and a weak. Adhesive sucker at the posterior end. They have leech-like move ments, alternately attaching with the tentacles and posterior sucker. Rhabdites are located only at the anterior end, and mucous glands are mainly around the posterior sucker. Figure 1. Craspedella sp. on the carapace and abdomen of red claw Cherax quadricarinatus. (a) (b) Figure 2 . (a) micro scopic vie w of Craspedella sp. from the carapa ce and abdomen of redclaw C. quadricarinatus; (b) microscopic view of Craspedella sp. from the gill of blue-huna C. albertisii Papua fresh- water lo bster.  D. Sugiani et al. Crayfish “red-claw” (Cherax quadricarinatus) and “blue-huna” (Cherax albertisii) are susceptible to ectosymbiont Craspedella sp. These Craspedella sp. can be treated with bath treatment used 15 grams per 10 litre of salt (15 ppt) for up to 3 hours or it can be treated with short dipped (15 - 20 minutes) in 30 ppt per 10 litre of salt. Formaldehyde 37% were used as a medicated treatments for Craspedella sp., using prolonged immersions for 24 hours, dosed at 0.1 mL per litre and bath treatments for 6 hours with 0.2 mL per litre of water (Table 1). Figure 3 . The anato my of Craspedella sp. a temnocephalid turbellarian from C. albertisii. (1) tentacle, ( 2) excreto ry ampul la, (3) pharynx, (4) intestine, (5) papillate band succer. Table 1. Evaluation of assay treatments in cryfish. Experiment Level of infection Pre treatment Post treatment Doses (L-1) Treatment time Water changes Day 0 Day 1 Day 3 Day 7 1 0.1 mL formaldehyde prolonged immersion (24 h) No Heavy Medium Light No infection 2 0.2 mL formaldehyde bath treatment (6 h) Yes (70% - 80% day−1) Heavy Light No infection No infection 3 3 ppt salt water prolonged immersion (24 h) No Heavy Medium Medium light infectio n 4 15 ppt salt water bath treatment (3 h) Yes (70% - 80% day−1) Heavy Light Light No infection 5 30 ppt salt water dipped in (15 - 20 minutes) Yes (100 % da y−1) Heavy No infection No infection No infection 6 con trol No treatment No Heavy Hea vy Medium Heavy  D. Sugiani et al. 4. Discussion Sewell & Cannon (1995) [8] examined Craspedella sp. from the branchial chamber of redcla w cryfish, Cherax quadricarinatus from Queensland Australia. Rhabdites were observed to discharge from ducts opening mainly in small distal region of the ventral epidermis of the three central (of five) tentacles. The regions, devoid of ciliated sensory papilae, serve to adhere the anterior end of the worms during locomotion. Craspedella spenceri (Haswell, 1893 [9]) is the only described species from the gills cavity of Cherax sp., the other undescribed species of Craspedella are known to live in the branchial chamber of cra yfish. Rhabdites were found in discharge at open ducts mainly in a small distal re gion of the ventral ep idermis of the three central (of five) tentacles (Hawking e t al., 2006; Sewell & Whittin gto n, 1995) [10] [11]. Craspe della has a non -cil iated e pid ermis wi th nuclei lo cated in the ep ider mis and with shor t microvi lli. T here is a thin basal la mina and thic k underlying fibrous matrix. Rhabd ites are secreted through ducts lined by micro- tubules. Multiciliate sense receptors consist of bundles of dendrites in a depression of the epidermis. Each dendrite has a cilium with a cross-striated rootlet; there are no electron-dense collars. Spermatozoa have pe- ripheral microtubules which in cross-section are arranged in a ring-like or spiral fashion, numerous electron- dense granules, mitochondria and a nucleus; axonemes of the 9 + “1” type are free for most of their length. Centrioles occur in some nerve fibres. In Didymorchis parts of the epidermis are ciliated and epidermal perikarya are “insunk”, connected to the surface part of the epidermis by a single cytoplasmic process. Epidermal cilia has c ross-striated vertical and horizontal rootlets. In the ciliary tips a short electron-dense rod along the central pair of tubules extends to the tip, where it widens to become a terminal plate; peripheral doublets are gradually disappeared by losing their microtubules. Receptors observed are uniciliate. Spermatozoa are as in Craspedella. Ultrastructural evidence indicates that Craspedella and Didymorchis are closely related and belong to the Rhabdocoela (Rohde & Watson, 1995; Rohde, 2005) [12] [13] . The five anterior tentacles and the single posterior attached to organ of the temnocephalan flatworm Craspe della sp. performed important roles in locomotion and attachment. Craspedella sp. moved using a low “looping” motion by alternately attaching the ventro-distal regions of the central three tentacles and the ventral surface of the posterior attachment organ. Temnocephalid flat worms are very mobile, moving with a leech-like loo p ing mot io n over t he s ur fa c e o f t he hos t crust ac ea n. Ind ivid ua l s ar e he r maphr od i tes an d self ferti lizatio n may be possible, but cross fertilization is typical. Eggs are enclosed in a leathery capsule then attached to the surface of the ho st b y a sho r t sta l k. Yo un g te mno c ep ha lid fla t wor ms hatc h as mini a tur e forms o f the ad ults ( Ha wki ng et al., 2006; Hartenstein & Hartenstein, 2001) [10] [14]. Temnocephalidae species are ectocommensal on freshwater crustaceans, crayfish in particular, so that they are always there on the host. They live attached to the outside body surface of the host or within the branchial chamber. The host crustacean is not harmed by the temnocephalid flat worm. Temnocephalid flat worms are predators feeding on small crustaceans, rotifers, insect larvae and nematodes. Prey is captured using tentacles. They may also be cannibalistic and at least one species, Craspedella spenceri, is primarily an algal feeder. Temnocephalidae species can live for long periods without food. Formaldehyde 37% was u sed as a medicated bath as a treatment for Craspedella sp., for 24 hours, and dosed at 1 mL per li tre and 2 mL per litre o f water. T his is repeat ed for t hree treat ments ev er y day. P artial water c hanges (70% - 80%) should be performed between treatments. Formaldehyde added to inside fish tank; however, formaldehyde is toxic, it is preferable to use it in a treatment tank. This treatment is effective for removing Craspe della sp. f rom the whole body. Jung et al. (2001) [15] describes a scientific relevant database suitable for determination of an appropriate withdrawal time and environmental risk assessment for therapeutic use of formaldehyde in the aquaculture industry of marine finfish. Formaldehyde concentrations in muscle were determined in cultured olive flounder (Paralichthys olivaceus) and black rockfish (Sebastes schlegeli) after bath treatment of formaldehyde 37% at 100, 300 and 500 mg/L for 1 h. Loss of formaldehyde from aerated seawater by air blower and non-aerated seawater containing 25, 50, 100, 150 and 200 mg/L formaldehyde was also determined. Formaldehyde concentrations in the muscle of fish treated with 500 mg/L of formaldehyde for 1 h were significantly higher (p—0.05) than the formaldehyde concentration control tissue when analyzed immediately after bath exposures 0 h withdrawal. However, there were no significant differences (p—0.05) between formaldehyde concentrations in control tissue and tissue from fish sampled 24, 48 and 72 h after the exposure. Formaldehyde in seawater  D. Sugiani et al. containing 25 - 200 mg/L formaldehyde reached detection limit concentration 0.05 mg/mL within 8 - 19 days, but its degradation was accelerated within 6 - 10 days by aeration. Formaldehyde is an excellent parasiticide for use in aquariums, however, special care should be taken when usin g. Formaldehyde chemically removes oxygen from the water, thus supplying vigorous aeration with an air stone or power head and air diffuser during treatment is always recommended. Formaldehyde is a solution of 37% formaldehyde gas dissolved in water. Formaldehyde is a potent germicide against parasitic organisms, and it has even shown certain properties to be considered an algaecide, fungicide, protozoacide and crustacide. Oxygen can eliminate formaldehyde from water, so it is important that the treated water should be well aerated. Formaldehyde is a carcinogenic so anyone who handling this chemical must be educated about its safety handling. Weekly treatments with formaldehyde (25 mg/L) are reasonable and likely to avert serious illness in many cases. Chemicals placed in the water are commonly referred to as “bath” treatments (Jung et al., 2001) [15]. Anothe r treat ment fo r Craspedella sp. should be dipped in salt water at 30 ppt (3 ppm, seawater salinity) for up to several minutes, o r until the fis h rolls on its side and use tank water and dissolve 30 teaspoons of salt per gallon, then place the fish into this water, monitor it the enti re time. After dip ping, fish should be placed back in normal salinity water. This treatment repeated daily for three days, with a 30% - 70% tank water change between treatments. T his treatment is d ifferent from other s , it is used to tre at organi sms i n fish s kin, and i t’s not for free -livi ng sta ge s in fish ta nk. Lower d ose s may be use d as a cont inuo us bat h in fish ta nk. At do se 5 - 10 ppt (0.5 - 1.0 ppm), crayfish can survive fo r several hours to several days, and this will effectively kil l the organisms. Diagnosis and treatment should be decide as soon as organisms founded, as prompt treatment is important to controlling th is o rganism. Interestingly, salt (sodium chloride), which is often ignored as a drugs and chemicals in fact is one safer and more effective method of treatin g Craspedella sp. and other external parasites than forma ldehyde that are often thought to be better and more powerful. Salt is the best chemical to manage fish health. For most purposes, uniodized table salt is adequate, although it’s justified to use aquarium salt specifically sold for that purpose, such as sea salt, solar salt, salt for livestock feed and Kosher salt. As an aid to osmoregulation, salt may be added in a concentration of 2 - 3 ppt (or 0.02 - 0.03 parts per million: ppm). This concentration is safe for freshwater fish, it’s approximately one tenth the strength of seawater and protozoa cannot live on this concentration level. Prolonged immersion used 1 - 3 grams of salt per litre (1 - 3 ppt—0.5%). The lower dosage is recommended for crayfish ponds as a prophylactic treatment over rainy seasons or on a permanent basis. A slightly higher dose of 5 grams per litre should be used for treating bacterial ulcers which appear as secunder infection of Craspedella. Adult red claws appear to be more tolerant of saline waters than juveniles, adult C. quadricarinatus responses to salinitie s r a nged fro m 0 to 3 5 g∙L−1 (Saoud & Ghanawi, 2013) [1 6]. Hyposalinity or OST (Osmotic Shock Therapy) is one of the most effective, non-chemical ways to control parasitic diseases. We should learn all about how to effectively and safely use this treatment method, bath treatment uses 10 - 3 0 grams per litre of salt (10 - 30 ppt or 1% - 3% ) fo r more tha n 3 0 mi nute s . The hi gher d os e may only be tolerated for a few minutes. The selected dosage if needed a higher dose is 20 grams per litre for 20 minutes. Salt has long been used as a treatment in fish. Salt works well in some conditions, makes sure it is fully dissolved to prevent “sal t bur ns”, aer ate water in short-term b aths, re move fi sh i f the y “go o ver ” as weaker ones may not stand t he full treatment time, u se cookin g salt. It is safer than many pond treatments a nd will not have adversely affect. It is generally used at fairly high rates in short-term baths or dips, but it can be used as a long- term suppo rtive treatment i n ponds. Do not us e sa lt co ntaining anti-caking agents such as sodium ferrocyanide. Acknowledgements Autho r s a re gra tef ul to P r o f. D r. Su ha rso no , Ind o ne si an I ns tit ute o f Sc iences, for p ro vi di n g t he ne cessa r y l ec t ure to carry out obove work. References [1] Kurniasih, T. (2008) Evaluasi pertumbuhan, sintasan, dan nisbah kelamin Huna Biru (Cherax alb ertisii) d an Red Claw (Cherax quadricarinatus) dengan p emberian pakan alami dan p akan buatan . Jurnal Ilmu-ilmu Pera iran dan Perik anan Indonesia, 15, 61-68. [2] U.S. Fish and Wildlife Service (2012) Australian Redclaw (Cherax quadricarinatus) Ecological Risk Screening Summary. Web Version. http://www.fws.gov/injuriouswildlife/pdf_files/Cherax_quadricarinatus_WEB_9-18-12.pdf 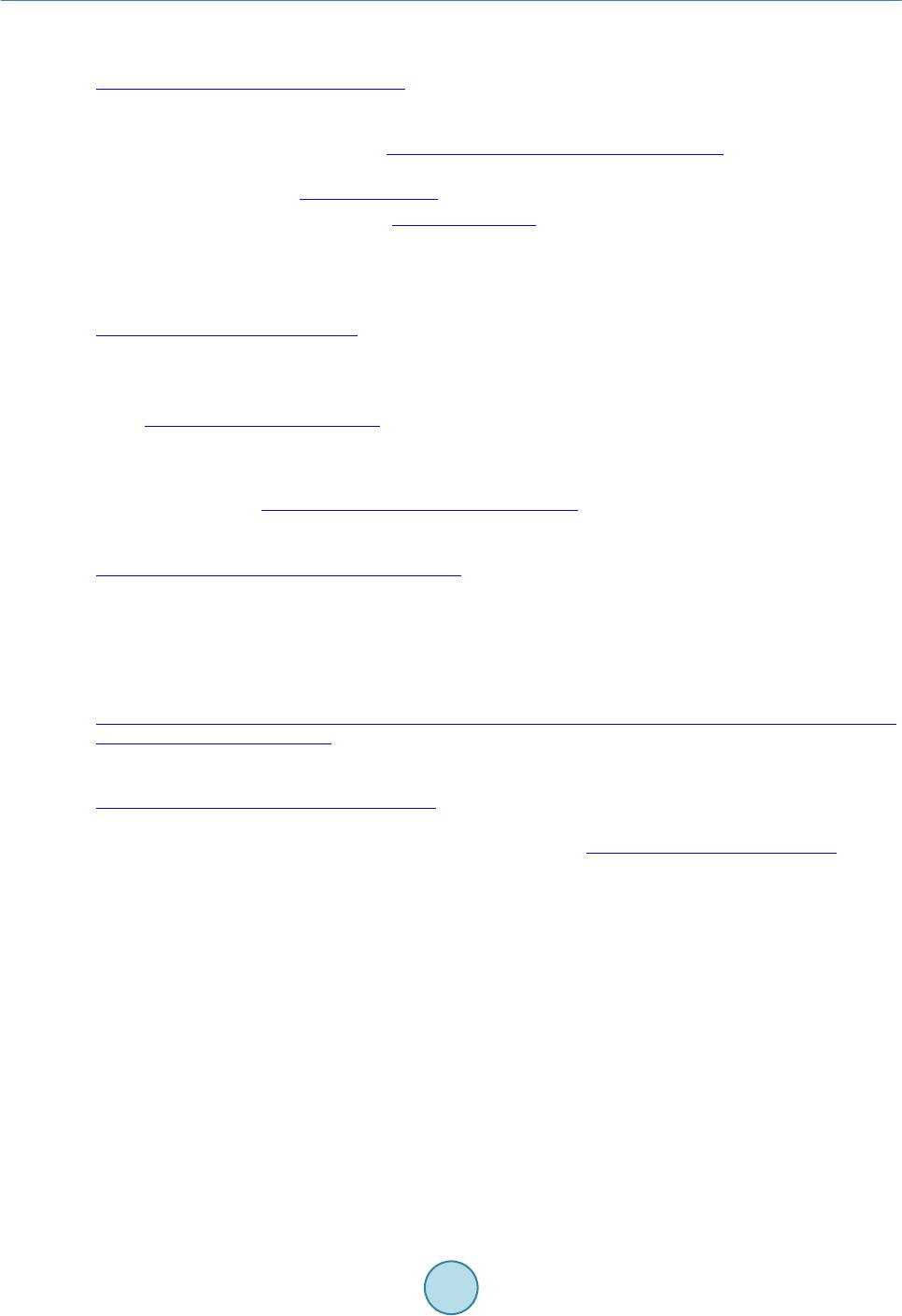 D. Sugiani et al. [3] Carreno-Leon, D., Racotta-Dimitrov, I., Casillas-Hernández, R., Monge-Quevedo, A., Ocampo-Victoria, L., et al. (2014) Growth, Metabolic and Physiological Response of Juvenile Cheraxquadricarinatus Fed Different Available Nutritional Substrates. Journal Aquaculture Research Development, 5, 7. http://dx.doi.org/10.4172/2155-9546.1000220 [4] Karplus, I., Zoran, M., Milstein, A., Harpaz, S., Eran, Y., Joseph, D. and Sagi, A. (1998) Culture of the Australian Red-Claw Crayfish Cherax quadricarinatus in Israel. III. Survival in Earthen Ponds under Ambient Winter Temperatures. Aquaculture, 166, 259-267. http://dx.doi.org/10.1016/s0044-8486(98)00290-7 [5] Austin, C.M., Jones, C. and Wingfield, M. (2009) Cherax quadricarinatus. In: IUCN 2011, IUCN Red List of Threat- ened S pecies Versi on 2011.1. www.iucnredlist.org [6] Au stralian Blue Yabby Aquaculture (2010) www.blueyabby.com [7] Schmidt, G.D. and Roberts, L.S. (1977) Foundations of Parasitology. The C.V. Mosby Company, Saint Louis, 190- 195. [8] Sewell, K.B. and Cannon, L.R.G. (1995) A Scanning Electron Microscope Study of Craspedella sp. from th e B ranchi al Chamber of Redclaw Cryfish, Cherax quadricarinatus, from Queensland, Australia. Hydrobiologia, 305 , 151-158. http://dx.doi.org/10.1007/BF00036378 [9] Haswell, W.A. (1893) A Monograph of the Temnocephaleae. In: Fletcher, J.J., Eds. , Proceedings of the Linnean Society of New South Wales, Sydney, 93-152. [10] Hawking, J.H., Smith, L.M. and Le Busque, K. (2006) Identification and Ecology of Australian Freshwater Inverte- brates. http://www.mdfrc.org.au/bugguide [11] Sewell, K.B. and Whittington, I.D. (1995) A Light Microscope Study of the Attachment Organs and Their Role in Locomotion of Craspedella sp. (Platyhelminthes: Rhabdocoela: Temnocephalidae), an Ectosymbiont from the Branch ial Chamber o f Cherax quadricarinatus (Crustacea: Parastaci dae) in Queensland , Australia. Journal of Natural History, 29, 1121-1141 . http://dx.doi.org/10.1080/00222939500770471 [12] Rohde, K. (2005) Ultrastructural Studies of Epidermis, Sense Receptors and Sperm of Craspedella sp. and Didymorchi s sp. (Platyhelminth es, Rhabdocoela) . Zoologica Scripta, 16, 289 -295. http://dx.doi.org/10.1111/j.1463-6409.1987.tb00075.x [13] Rohde, K. and Watson, N.A. (1995) Comparative Ultrastructural Study of Posterior Succer of Four Species of Symbiotic Platyhelminthes, Themnocephala sp. (Themnocephalida), Udonella caligorum (Udonellidae), Anoplodiscus cirrusspiralis (Monogenea: Monopisthocotylea), and Philophthalmus sp . (Trematod a: Digenea). Folia Parasitologica, 42, 11-28. [14] Hartenstein, Y.A. and Hartenstein, V. (2001) The Embryonic Development of the Temnocephalid Flatworms Craspedella pedum and Diceratocephala boschmai. Cell Tissue Res., 30 4, 295-310. http://www.pubfacts.com/detail/11396723/The-embryonic-development-of-the-temnocephalid-fl atw orms-Craspedella- pedum-and-Diceratocephala-bosch [15] Jung, S.H., Kim, J.W., Jeon, I.G. and Lee, Y.H. (2001) Formaldehyde Residues in Formaldehyde-Treated Olive Flounder (Paralichthys olivaceus), Black Rockfish (Sebastes schlegeli) , and Seawater. Aquaculture, 194, 253-262. http://dx.doi.org/10.1016/S0044-8486(00)00530-5 [16] Saoud, I.P. and Ghanawi, J. (2013) A Review of the Culture and Diseases of Redclaw Crayfish Cherax quadricarinatus (Von Martens 1868). Journal of The World Aquaculture Society, 44, 29. http://dx.doi.org/10.1111/jwas.12011
|