Paper Menu >>
Journal Menu >>
 Advances in Chemical Engi neering and Science , 2011, 1, 133-139 doi:10.4236/aces.2011.13020 Published Online July 2011 (http://www.SciRP.org/journal/aces) Copyright © 2011 SciRes. ACES Development of Siliconized Epoxy Resins and Their Application as Anticorrosive Coatings Prashant Gupta, Madhu Bajpai Department of Oil & Paint Technology, H. B. Technological Institute, Kanpur, India E-mail: prashant123hbti@gmail.com, madhubajpai_6@rediffmail.com Received March 31, 201 1; revised May 17, 2011; accepted July 3, 2011 Abstract The present work involves the development of siliconized epoxy resin to overcome the drawback of epoxy resin like poor impact strength, high rigidity and moisture absorbing nature because of which they are not applied as corrosion resistant coating. By embedding silicone into the back bone of polymeric resin the above drawback can be reduced to substantial level. For achieving this, siliconised epoxy resins were pre- pared by reacting amine terminated silicone resin with novolac epoxy resin and meta-phenylenediamine was used as curing agent. The applied films of coating were baked at 150˚C. Cured films were evaluated for their thermal, mechanical, chemical and corrosion resistance properties to ascertain the commercial utility of these eco-friendly resins for use in anti corrosive formulations. The siliconized epoxy resins system was found to exhibit good thermal and anticorrosive properties. Keywords: Epoxy Resin, Silicone Resin, Anticorrosive Coatings 1. Introduction A novel coating of high performance polymeric m at erial is a need of today. These polymeric materials have superior mechanical, thermal and anticorrosive characteristics ide- ally suitable for adverse environmental conditions [1]. Inorganic-organic hybrid resins have become a creative polymer with unique combination of distinctive properties of both constituents for applications in various industries. Epoxy resins are widely used in protective coatings, adhesives, sealant, fiber reinforced composites and elec- tronic industry due to their outstanding surface pro perties like low shrinkage, ease of cure and possessing good moisture, solvent and chemical resistance, and excellent adhesion performances [2,3]. They lack fracture resis- tance, impact strength, low thermal stability, low pigmen t holding ability, flexibility and poor hydrophobicity, which restrict their wide application in the field of coat- ings and paints [4,5]. To improve these properties the component like rubber, polyurethane silicone are added as modifier to the epoxy resins [6-8]. Silicones are used in coating materials because of the following properties: improved water repellency improved thermal stability; resistance to oxidation they retain physical properties over a wide range of temperature low toxicity they impart unique flexibility to the backbone chain and intrinsic surface active property. Silicone is suitable modifier for epoxy resins. The modified resin has superior thermal and thermo-oxida- tive stability, fracture energy, excellent moisture resis- tance, partial ionic nature, low surface energy and good hydrophobicity [9 ]. Aliphatic epoxy modified polysiloxane coatings can be prepared by the combination of aliphatic epoxy resins, polysiloxane, organo oxysilane and difunctional ami- nosilanes hardners which provide highly improved resis- tance to ultraviolet and weathering in sunlight and im- proved chemical and corrosion resistance. In practical applications, amine-based hardeners hold the largest share in epoxy hardener markets. Aromatic amine cured epoxy resin systems have excellent resistance to water, solvents an d alkaline solu tions as well as a range of good to excellent dilut e acid resistance [10,11] . Generally, the incorporation of siloxane into polymer has been carried out through physical blending methods [12,13]. The blending of siloxane and epoxy causes an increase in viscosity, phase separation and bleeding of siloxane component of the blended system. In the present work an attempt has been made to im-  P. GUPTA ET AL. Copyright © 2011 SciRes. ACES 134 prove the shortcomings of siloxane epoxy blended coat- ing. The properties of novolac epoxy resin are enhanced by embedding the amine terminated silicone into the backbone of polymeric resin. As silicone is chemically bonded to epoxy resin, the resulting materials have de- sired properties and with more consistent results. 2. Experimental 2.1. Materials Phenol novolac epoxy resin, Synthesized in laboratory, Epichlorohydrin, EMerck, Diethoxydimethyl silane, Al- drich -aminopropyl diethoxy methyl silane, Lancaster. 2.2. Methods 2.2.1. Synthesis of No vol ac R e si n Novolac Resin was prepared by condensation reaction between phenol and formaldehyde in acidic medium. Ini- tially, phenol (1 mole) with some quantity of water was taken in three neck flask. The pH was adjusted to 0 .5 with sulphuric acid (used as catalyst) and the contents were heated to 90˚C with constant stirring. The required amount (0.5 mole) of formaldehyde (37% formaline solution) was added over a period of 3 hours through a dropping funnel, and stirring was continued for an additional 30 minutes, water was then removed under vacuum. 2.2.2. Synthesis of Ep o xy N o vol ac Resi n Laboratory prepared Novolac Novolac Resin (1 mole) was reacted with Epichlorohydrin (10 mole) at 110˚C and 40% Sodium hydroxide solution was added gradu- ally to the reactants over a period of 3 hours through a dropping funnel. After completion of reaction, salt (NaCl) was removed by washing with hot water and then water was removed through vacuum distillation. 2.2.3. Determ ina tion of Epoxide Equivalent Weight (EEW) Epoxide equivalent weight was determined by standard BIS method. 2.2.4. Cohydrolysis of Diethox ydi methyl Silane and -Aminopropyldiethoxy Methyl Silane Calculated amount of Diethoxydimethyl silane and - aminopropyldiethoxy methyl silane in 97:3 ratio were taken in three-necked flask, 50 ml aq. alchohol (50:50) was added dropwise. The reaction was continued at 0˚C with constant stirring. After complete addition of aque- ous alcohol, the reaction was further continued for half an hour. The mix ture was sep arated and d istilled to make it solvent free. The resin was dried. 2.3. Characterization of Resins The characterization of synthesized resins like epoxide equivalent and texture appearance are shown in Table 1. 2.4. Coating Composition and Film Preparation The coating compositions were prepared by incorpora- tion of amino terminated silicone resin in the ratio of 1.5 to 7.5 with curing agent (conventional amine). Simultaneously a standard was also prepared by re- acting epoxy resin with conventional amine. The samples EA, EAS1, EAS2, EAS3, EAS4 and EAS5 of different molar ratio (as shown in Table 2) were ap- plied on steel and glass panels with the help of 100a film applicator. All efforts were made to maintain a uniform film thickness of 100 for the general mechanical and chemical resistance properties. The films were cured at 150˚C till becomes tack free. 2.5. Curing of Epoxy Resin The curing of the above prepared resin was carried out by using different curing ag ent under varying cond itions: a) Curing of epoxy resin with aromatic amine: The ep- oxy resin was melted and mixed with stoichiometric amount of curing agent, i.e. m-phenylenediamine ( M PDA) at a temperature 80˚C. b) Curing of epoxy resin with mpda and amino termi- nated silicone resin in different ratios at 150˚C. 3. Results and Discussion 3.1. Spectral Analysis Figure 1 shows the I.R. spectra of novolac epoxy resin. A prominent band is observed at 940 cm–1, which shows Synthesis of Novolac Resin OH Phenol +HCHO H2SO4 p H 0.5, 90˚C CH2 OH OH N ovolac Resin 2 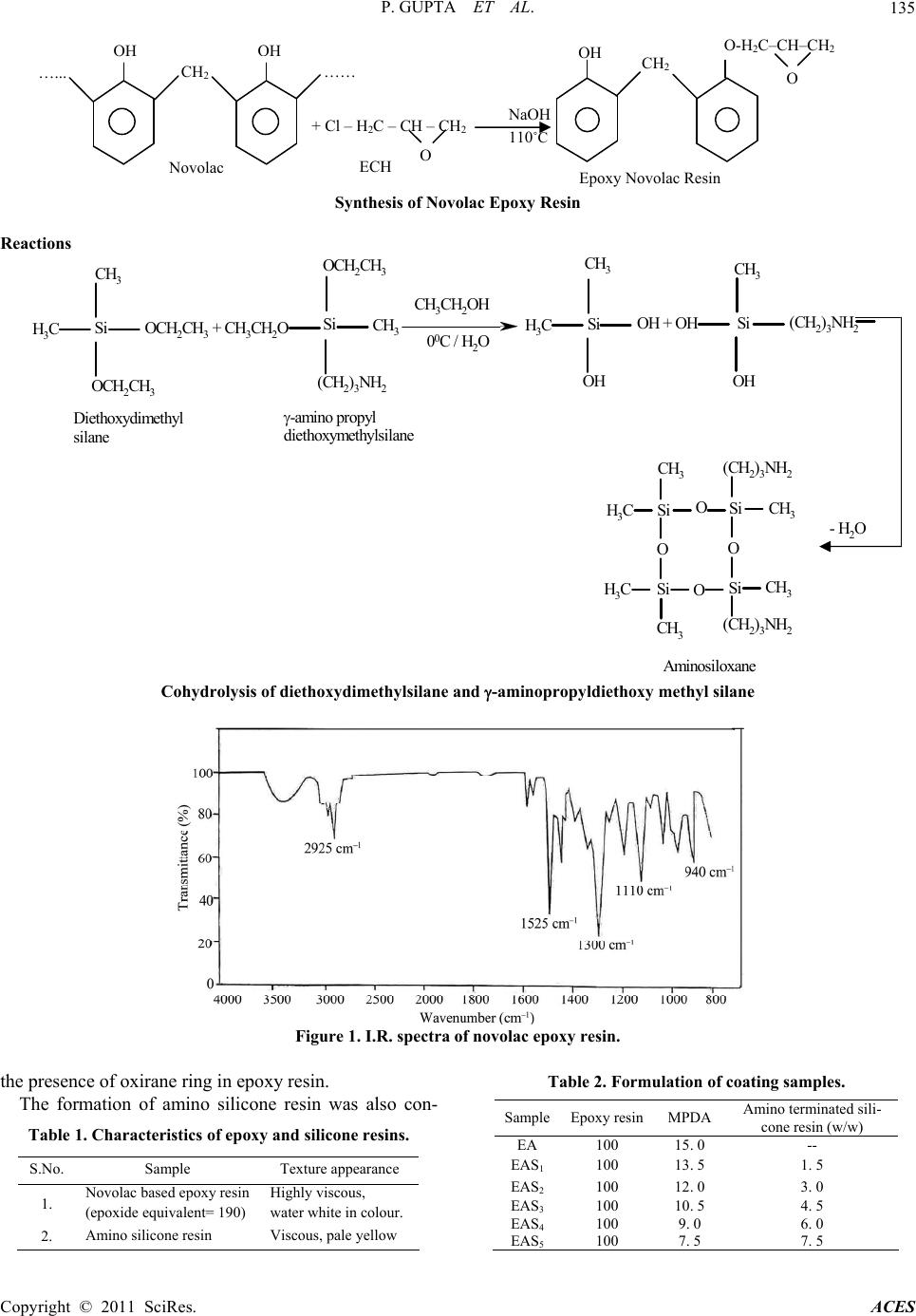 P. GUPTA ET AL. Copyright © 2011 SciRes. ACES 135 Synthesis of Novolac Epoxy Resin Reactions Si CH3 H3C OCH2CH3 OCH2CH3 + CH 3CH2OSi OCH2CH3 CH3 (CH2)3NH2 CH3CH2OH 00C / H2OSi CH3 H3C OH OH + OHSi CH3 OH (CH2)3NH2 Diethoxydimethyl silane -am ino propyl diethoxymethylsilane - H 2O Si CH3 O Si CH3 H3C H3C O O Si Si CH3 CH3 (CH2)3NH2 (CH2)3NH2 O Aminosiloxane Cohydrolysis of diet hoxydimethylsilane and -aminopropyldiethoxy methyl silane Figure 1. I.R. spectra of novolac epoxy resin. the presence of oxirane ring in epoxy resin. The formation of amino silicone resin was also con- Table 1. Characteristics of epoxy and silicone resins. S.No. Sample Texture appearance 1. Novolac based epoxy resin (epoxide equivalent= 190) Highly viscous, water white in colour. 2. Amino silicone resin Viscous, pale yellow Table 2. Formulation of coating samples. SampleEpoxy resinMPDA Amino terminated sili- cone resin (w/w) EA 100 15. 0 -- EAS1 100 13. 5 1. 5 EAS2 100 12. 0 3. 0 EAS3 100 10. 5 4. 5 EAS4 100 9. 0 6. 0 EAS5 100 7. 5 7. 5 O-H2C–CH–CH2 …... CH2 …… OH OH + Cl – H2C – CH – CH2 ECH Novolac NaOH 110 ˚C O OH CH2 O Epoxy Novolac Resin  P. GUPTA ET AL. Copyright © 2011 SciRes. ACES 136 firmed by the I.R. spectral analysis. A peak was observed at 1021 cm–1 referred as Si-O-Si linkage and a peak near 3369 cm–1 showed the presence of –NH2 group. The spectrum is presented in Figure 2. 3.2. Evaluation of Film Properties The cured films were evaluated for their optical, me- chanical, chemical resistance and anticorrosive properties as per the standard test methods viz. gloss at 60˚ (ASTM: D523-99), scratch hardness (ASTM: D 5178), pencil hard- ness (ASTM: D 3363-00), chemical resistance (ASTM:D 1308-87) and solvent resistance (ASTM:D 5402). 3.3. Mechanical Properties The results of evaluation of various mechanical proper- ties have been shown in Table 3. a) Scratch hardness: It was determined by using an automatic scratch hard- ness tester (Sheen U.K.). The scratch hardness varies from 1700 - 2000 g. It is clear from the data that as we increase the silicone percentage the scratch hardness of the film goes on increasing. b) Pencil hardness: It was measured by using pencil hardness tester (Sheen U.K.). The coating films showed that it varies from 3H- 5H. Higher pencil hardness is due to higher silicone con- tent. c) Cross-hatch adhesion: It was measured by using crosscut adhesion tester (Sheen U.K.). All the coating films demonstrated good cross-hatch adhesion. d) Flexibility: Flexibility was determined by using ¼ inch Mandrel Bend tester (Sheen U.K.). Films of all the coating com- positions passed ¼ in ch mandrel bend test. Based on th is qualitative measurement, it can be said that all the films had reasonably good flexibility. e) Gloss: It was measured by using triglossometer (Sheen U.K.). On watching the films at 60˚ angle, it was observed that all coating films had good gloss. 3.4. Chemical Resistance The cured films of coating samples were tested for their chemical resistance. The codified results of the chemical resistance have bee n presented in Table 4. a) Acid resistance: To examine the acid resistance, coated films were immersed in 1.0 N aqueous solution of sulfuric acid. Results showed that all th e samples exhibit from good to very good resistance against acid. b) Alkali resistance: To examine the alkali resistance, coated films were immersed in 1.0 N aqueous solution of sodium hydroxide. These films showed good to excellent resistance against alkali. It was observed that sample with higher ratio of silicone were found to be excellent. c) Water resistance: All the coated films exhibited very good to excellent resistance against distilled water when they were ex- posed to it. d) Solvents resistance: All the coated films exhibited very good to excellent solvent resistance when they were exposed to non polar solvents like xylene and mineral turpentine oil. Resis- tance against MEK was fair to good because MEK is very polar solvent. Samples with high silicone content showed better performance than those having low con- tent of silicone. 3.5. Salt-Spray Test Results No visible corrosion products were seen on the surface of the unscratched area of the coated panels at the end of the salt-spray test. Corrosion products were seen mainly on scratched area of the coated panels. Corrosion is lower in the case of silicon ized epoxy coated pan els than in epoxy. Siliconized epoxy coated panels show excel- lent corrosion resistance in salt-spray test. The superior corrosion resistance showed by coating systems may be due to the inherent water repelling nature of silicone. 3.6. TGA Analysis Figure 3 shows the TGA graph of the samples. Activa- tion energy (E) for the thermal decomposition of sili- conized epoxy has been evaluated from the dynamic thermograms. The fractional decomposition for the re- spective temperature has been evaluated from TGA graph. Higher value of activation energy (see Table 5) may be due to the presence of silicon in the resin. High activation energy for the decomposition of system leads to better thermal stability o f the compound [14,15]. 4. Conclusions Amino containing siliconised epoxy resin was synthe sized. The spectral analysis, thermal stability, mechanical and chemical resistance properties of siliconised epoxy resins were studied using FTIR, TGA and standard methods respectively. It was found that on increasing the ratio of silicone resin, the thermal stability was increased and the effect of amino silicone resin on epoxy resin im- 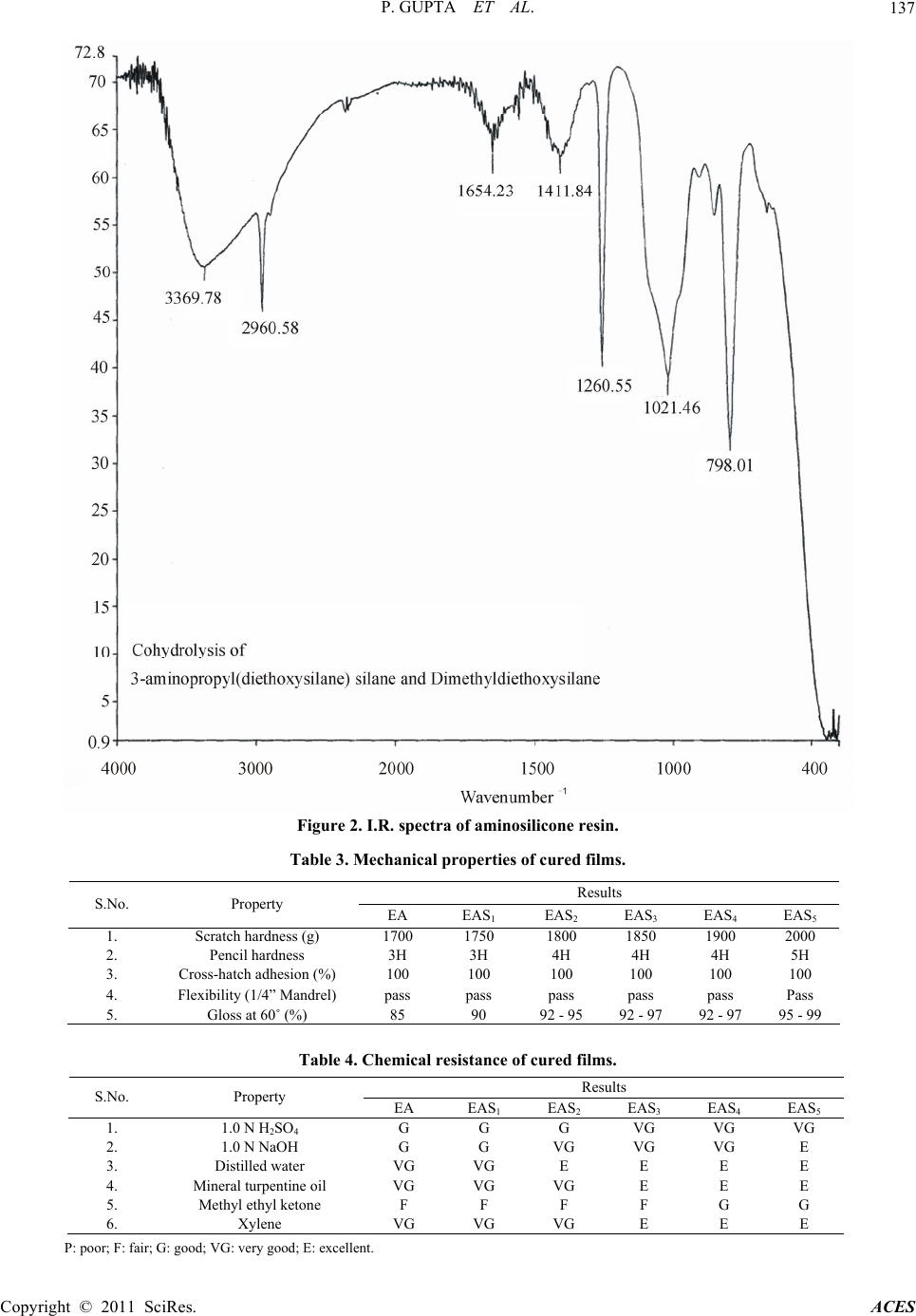 P. GUPTA ET AL. Copyright © 2011 SciRes. ACES 137 Figure 2. I.R. spectra of aminosilicone resin. Table 3. Mechanical properties of cured films. Results S.No. Property EA EAS1 EAS2 EAS3 EAS4 EAS5 1. Scratch hardness (g) 1700 1750 1800 1850 1900 2000 2. Pencil hardness 3H 3H 4H 4H 4H 5H 3. Cross-hatch adhesion (%) 100 100 100 100 100 100 4. Flexibility (1/4” Mandrel) pass pass pass pass pass Pass 5. Gloss at 60˚ (%) 85 90 92 - 95 92 - 97 92 - 97 95 - 99 Table 4. Chemical resistance of cured films. Results S.No. Property EA EAS1 EAS2 EAS3 EAS4 EAS5 1. 1.0 N H2SO4 G G G VG VG VG 2. 1.0 N NaOH G G VG VG VG E 3. Distilled water VG VG E E E E 4. Mineral turpentine oil VG VG VG E E E 5. Methyl ethyl ketone F F F F G G 6. Xylene VG VG VG E E E P: poor; F: fair; G: good; VG: very good; E: excellent. 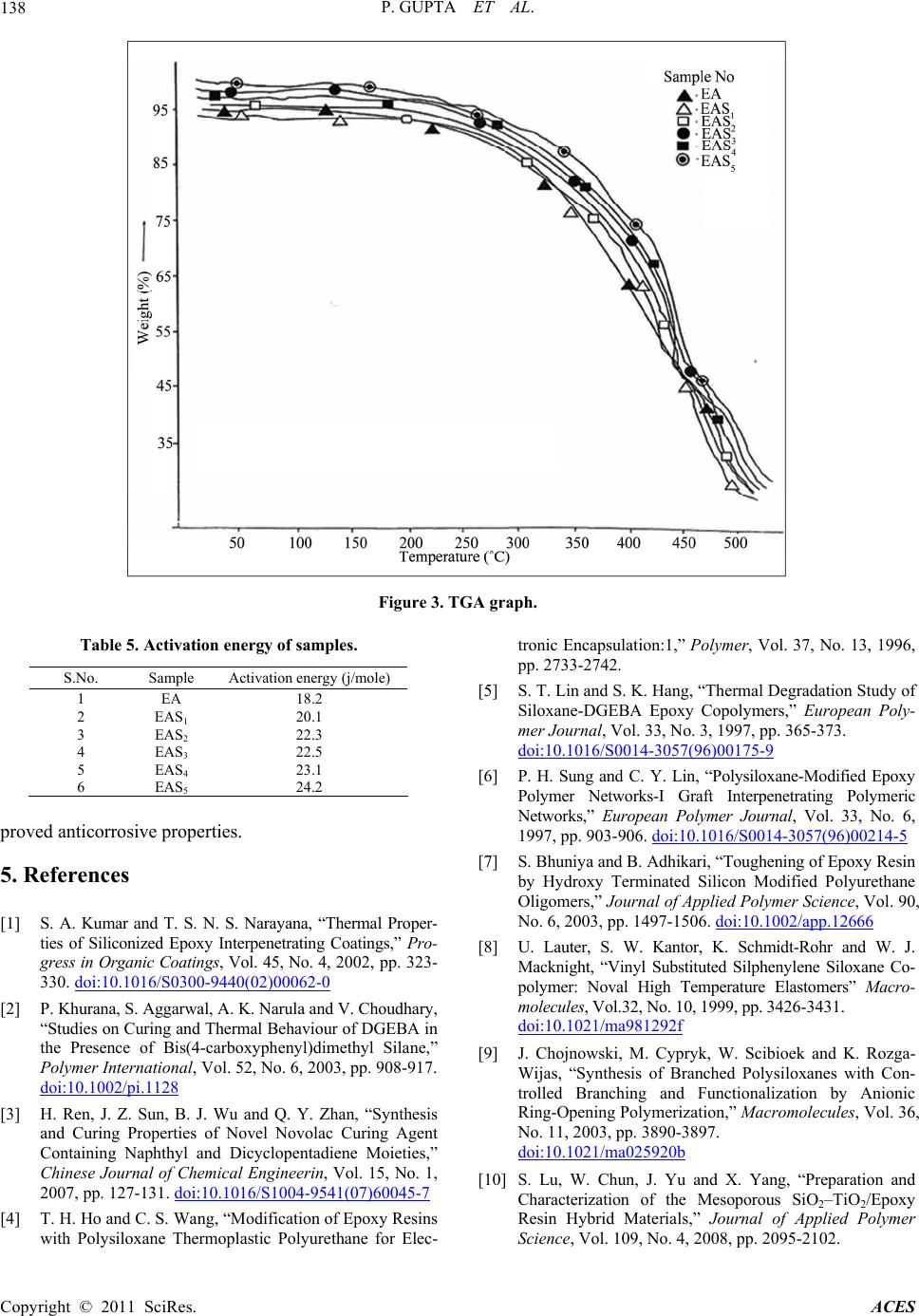 P. GUPTA ET AL. Copyright © 2011 SciRes. ACES 138 Figure 3. TGA graph. Table 5. Activation energy of samples. S.No. Sample Activation energy (j/mole) 1 EA 18.2 2 EAS1 20.1 3 EAS2 22.3 4 EAS3 22.5 5 EAS4 23.1 6 EAS5 24.2 proved anticorrosive properties. 5. References [1] S. A. Kumar and T. S. N. S. Narayana, “Thermal Proper- ties of Siliconized Epoxy Interpenetrating Coatings,” Pro- gress in Organic Coatings, Vol. 45, No. 4, 2002, pp. 323- 330. doi:10.1016/S0300-9440(02)00062-0 [2] P. Khurana, S. Aggarwal, A. K. Narula and V. Choudhary, “Studies on Curing and Thermal Behaviour of DGEBA in the Presence of Bis(4-carboxyphenyl)dimethyl Silane,” Polymer International, Vol. 52, No. 6, 2003, pp. 908-917. doi:10.1002/pi.1128 [3] H. Ren, J. Z. Sun, B. J. Wu and Q. Y. Zhan, “Synthesis and Curing Properties of Novel Novolac Curing Agent Containing Naphthyl and Dicyclopentadiene Moieties,” Chinese Journal of Chemical Engineerin, Vol. 15, No. 1, 2007, pp. 127-131. doi:10.1016/S1004-9541(07)60045-7 [4] T. H. Ho and C. S. Wang, “Modification of Epoxy Resins with Polysiloxane Thermoplastic Polyurethane for Elec- tronic Encapsulation:1,” Polymer, Vol. 37, No. 13, 1996, pp. 2733-2742. [5] S. T. Lin and S. K. Hang, “Thermal Degradation Study of Siloxane-DGEBA Epoxy Copolymers,” European Poly- mer Journal, Vol. 33, No. 3, 1997, pp. 365-373. doi:10.1016/S0014-3057(96)00175-9 [6] P. H. Sung and C. Y. Lin, “Polysiloxane-Modified Epoxy Polymer Networks-I Graft Interpenetrating Polymeric Networks,” European Polymer Journal, Vol. 33, No. 6, 1997, pp. 903-906. doi:10.1016/S0014-3057(96)00214-5 [7] S. Bhuniya and B. Adhikari, “Toughening of Epoxy Re sin by Hydroxy Terminated Silicon Modified Polyurethane Oligomers,” Journal of Applied Polymer Science, Vol. 90, No. 6, 2003, pp. 1497-1506. doi:10.1002/app.12666 [8] U. Lauter, S. W. Kantor, K. Schmidt-Rohr and W. J. Macknight, “Vinyl Substituted Silphenylene Siloxane Co- polymer: Noval High Temperature Elastomers” Macro- molecules, Vol.32, No. 10, 1999, pp. 3426-3431. doi:10.1021/ma981292f [9] J. Chojnowski, M. Cypryk, W. Scibioek and K. Rozga- Wijas, “Synthesis of Branched Polysiloxanes with Con- trolled Branching and Functionalization by Anionic Ring-Opening Polymerization,” Macromolecules, Vol. 36, No. 11, 2003, pp. 3890-3897. doi:10.1021/ma025920b [10] S. Lu, W. Chun, J. Yu and X. Yang, “Preparation and Characterization of the Mesoporous SiO2–TiO2/Epoxy Resin Hybrid Materials,” Journal of Applied Polymer Science, Vol. 109, No. 4, 2008, pp. 2095-2102.  P. GUPTA ET AL. Copyright © 2011 SciRes. ACES 139 doi:10.1002/app.27856 [11] P. F. Brunis, “Epoxy Resin Technology,” New York In- terscience Publishers, New York, 1968. [12] S. Ahmad, S. M. Ashraf and A. Hsanat, “Studies on Cor- rosion Protective Epoxidised Oil Modified DGEBA Ep- oxy Paint,” Paint India, Vol. 52, No. 1, 2002, pp. 47-51. [13] S. Ahmad, S. M. Ashraf, S. N. Nusrat and A. Nsanat, “Synthesis, Characterization and Performance Evaluation of Hard, Anticorrosive Coatings Materials Derived from Diglycidyl Ether of Bisphenol a Acrylate s and Meth acry- lates,” Journal of Applied Polymer Science, Vol. 95, No. 3, 2005, pp. 494-501. doi:10.1002/app.21202 [14] W. G. Potter, “Uses of Epoxy Resin,” Newnes-Butter- worths, London, 1975. [15] A. F. Yee and R. A. Pearson “Toughening Mechanism in Elastomer-Modified Epoxies Part-1 Mechanical Studies,” Journal of Material Science, Vol. 21, No. 7, 1986, pp. 2462-2474. |

