 Advances in Chemical Engineering and Science, 2011, 1, 163-168 doi:10.4236/aces.2011.13024 Published Online July 2011 (http://www.SciRP.org/journal/aces) Copyright © 2011 SciRes. ACES Determination of Sterane an d Tri terpane in the Tamsagbulag Oilfield Erdenee Enkhtset seg1, Batdelger Byambagar1, Dalantai Monkhoobor2, Budeebazar Avid3*, Avirmed Tuvshinjargal4 1Mongolian University of Science and Technololgy, Ulaanbaatar, Mongolia 2National University of Mongolia , Ulaanbaatar, Mongolia 3Mongoli an Academy of Sciences, Ulaanbaatar, Mongolia 4Petroleum authority of Mongolia, Ulaanbaatar, Mongolia E-mail: *avidmas@gmail.com Received March 9, 2011; revised April 15, 2011; accepted April 26, 2011 Abstract Crude oils representing four different holes from the Tamsagbulag oilfield were analyzed by gas chromato- graphy-mass spectrometry (GC/M S ) spectroscopy. 52 biomarker compounds, 34 pentacyclic triterpanes and 18 steranes were identified and semi-quantitatively determined by selective ion monitoring (SIM) chromatography. Depending on the oil source, the variation in the pentacyclic triterpanes distribution is more significant than the variation in the sterane distribution. It is said that pregnane and honopregnane originated during the period of very salty sedimentary accumulation in the condition of diagenesis. Keywords: Crude Oil, Biomarker, GC-MS, Tamsagbulag Oilfield 1. Introductio n Biomarkers are complex molecular fossils derived from once living organisms and it can provide information on the organic source materials, environmental conditions during its deposition, the thermal maturity experienced by a rock or oil, and the degree of biodegradation. Stu- dies on identification and quantification of biomarker compounds have obtained great importance for recogni- tion and classification of crude oil source. Sterane and hopane biomarkers have great potential in helping to identify and allocate hydrocarbon sources [1-9]. It is very important to study polyaromatic aliphatic hydrocarbons with carbon of C27 or higher hydrocarbon chain like steroid and triterpenoid for the geochemical investigation of crude oil and other caustobiolithes. The chemical type and relative content of biomarker com- pounds in specific oil is considered as its unique finger- print. Among the various types of biomarkers, triterpanes and steranes are the best target molecules for identifica- tion and quantification. These molecules, with a molecu- lar weight of about 250 - 400, possess a relative high concentration in crude oil and they are resistant to petr o- chemical and microbial degradation [4,5]. Therefore, they have found their use in tracing the oil source, its characteristics, degree of weathering and the fate of spilled oil in the environment [6,7]. Utilizing the GC and GC/MS technique made it possible to arrive at a clear characterization and classification of crude oils accord- ing to their sources [8]. Source specific parameters (viz. DBT/P, Pr/Ph, C29/C27 sterane and hopane/sterane ra- tios and tri- and tetra-cyclic terpane distributions) indi- cate that family oils derived from a marine shale depo- sited under sub-oxic conditions, whereas family oils de- rived from terrigenous source, contain n-alkanes that are less depleted in 13C, consistent with their derivation from a terrigenous source rock and their higher thermal matur- ity. The more negative n-alkane 13C profiles of the family oils reflect their marine source [9]. The research demonstrated that the biomarker distribution and carbon isotopic composition could be used to differentiate oils derived from various lacustrine environments [10,11]. Recently several researchers have paid more attention on biomarker and geochemical investigation of the Mongolian crude oil. Composition of the hydrocarbons and structure of the heteroatom compounds with high molecular weight from the Zuunbayan, Tsagaan els and 19-3, 19-12, 19-13, 19-14, 19-17 wells of Tamsagbulag oilfields have been studied by Khongorzul. Based on the results, the author has estimated product properties of 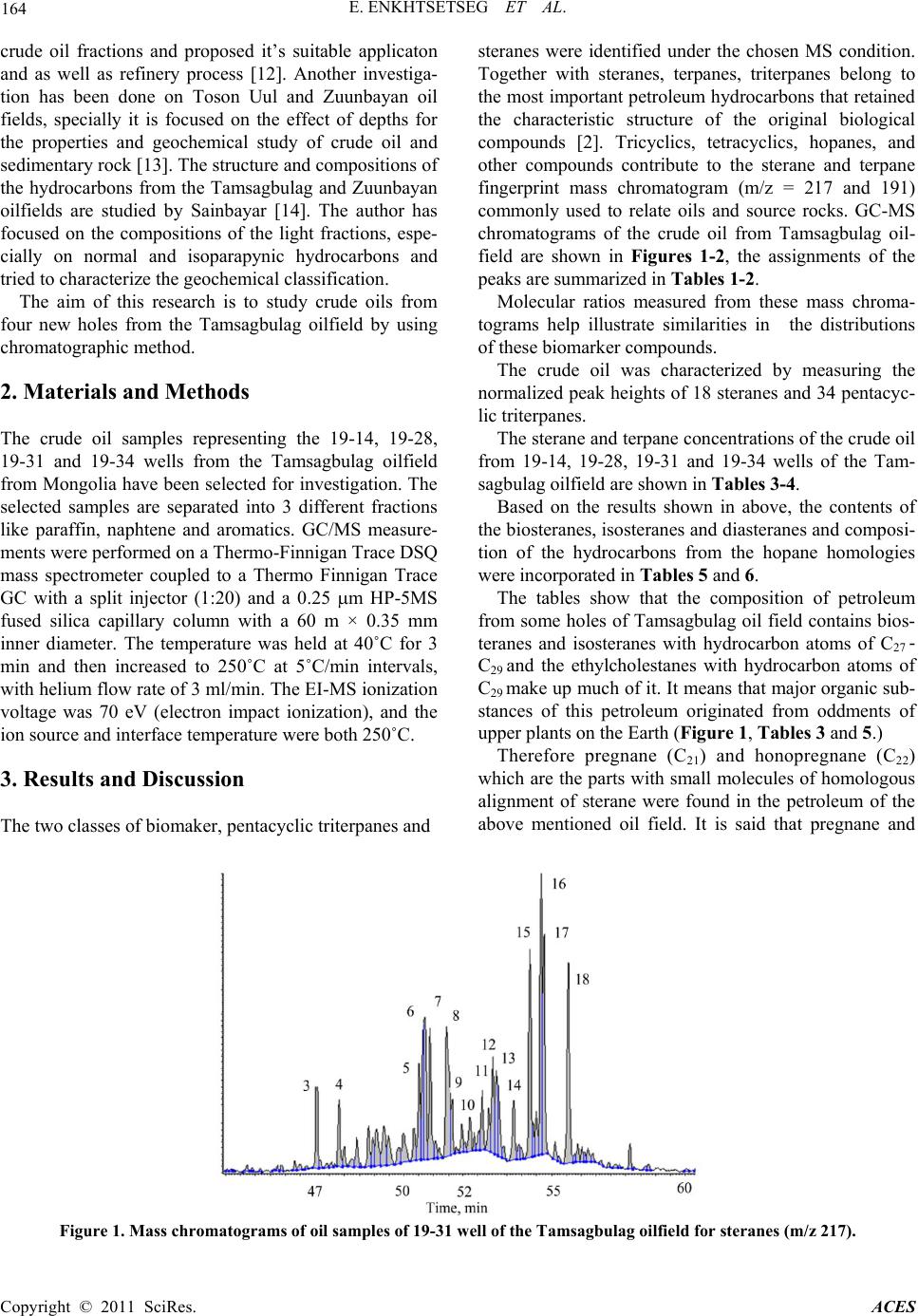 E. ENKHTSETSEG ET AL. Copyright © 2011 SciRes. ACES crude oil fractions and proposed it’s suitable applicaton and as well as refinery process [12]. Another investiga- tion has been done on Toson Uul and Zuunbayan oil fields, specially it is focused on the effect of depths for the properties and geochemical study of crude oil and sedimentary rock [13]. The structure and compositions of the hydrocarbons from the Tamsagbulag and Zuunbayan oilfields are studied by Sainbayar [14]. The author has focused on the compositions of the light fractions, espe- cially on normal and isoparapynic hydrocarbons and tried to characterize the geochemical classification. The aim of this research is to study crude oils from four new holes from the Tamsagbulag oilfield by using chromatographic method. 2. Materials and Methods The crude oil samples representing the 19-14, 19-28, 19-31 and 19-34 wells from the Tamsagbulag oilfield from Mongolia have been selected for investigation. The selected samples are separated into 3 different fractions like paraffin, naphtene and aromatics. GC/MS measure- ments were performed on a Thermo-Finnigan Trace DSQ mass spectrometer coupled to a Thermo Finnigan Trace GC with a split injector (1:20) and a 0.25 µm HP-5MS fused silica capillary column with a 60 m × 0.35 mm inner diameter. The temperature was held at 40˚C for 3 min and then increased to 250˚C at 5˚C/min intervals, with helium flow rate of 3 ml/min. The EI-MS ionization voltage was 70 eV (electron impact ionization), and the ion source and interface temperature were both 250˚C. 3. Results and Discussion The two classes of biomaker, pentacyclic triterpanes and steranes were identified under the chosen MS condition. Together with steranes, terpanes, triterpanes belong to the most important petroleum hydrocarbons that retained the characteristic structure of the original biological compounds [2]. Tricyclics, tetracyclics, hopanes, and other compounds contribute to the sterane and terpane fingerprint mass chromatogram (m/z = 217 and 191) commonly used to relate oils and source rocks. GC-MS chromatograms of the crude oil from Tamsagbulag oil- field are shown in Figures 1-2, the assignments of the peaks are summarized in Tables 1-2. Molecular ratios measured from these mass chroma- tograms help illustrate similarities in the distributions of these biomarker compounds. The crude oil was characterized by measuring the normalized peak heights of 18 steranes and 34 pentacyc- lic triterpanes. The sterane and terpane concentrations of the crude oil from 19-14, 19-28, 19-31 and 19-34 wells of the Tam- sagbulag oilfield are shown in Tables 3-4. Based on the results shown in above, the contents of the biosteranes, isosteranes and diasteranes and composi- tion of the hydrocarbons from the hopane homologies were incorporated in Tables 5 and 6. The tables show that the composition of petroleum from some holes of Tamsagbulag oil field contains bios- teranes and isosteranes with hydrocarbon atoms of C27 - C29 and the ethylcholestanes with hydrocarbon atoms of C29 make up much of it. It mean s that major organic sub- stances of this petroleum originated from oddments of upper pla nts on the Ea rth (Figure 1, Tables 3 and 5.) Therefore pregnane (C21) and honopregnane (C22) which are the parts with small molecules of homologous alignment of sterane were found in the petroleum of the above mentioned oil field. It is said that pregnane and Figure 1. Mass chromatograms of oil samples of 19-31 well of the Tamsagbulag oilfield for steranes (m/z 217). 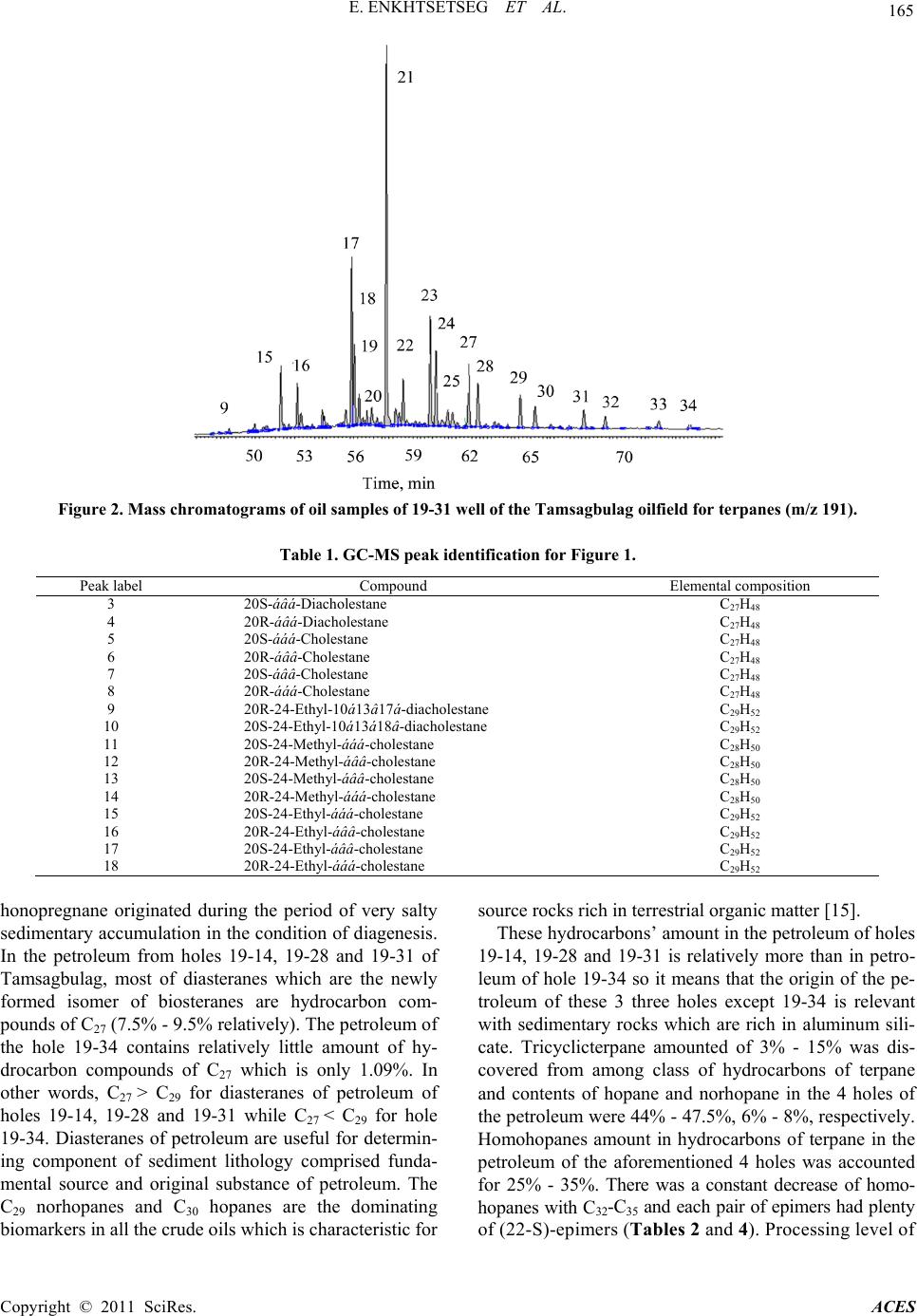 E. ENKHTSETSEG ET AL. Copyright © 2011 SciRes. ACES Figure 2. Mass chromatograms of oil samples of 19-31 well of the Tamsagbulag oilfield for terpanes (m/z 191). Table 1. GC-MS peak identification for Figure 1. 4 20R- -Diacholestane С27Н48 27 48 9 20R-24-Ethyl-10 13 17 -diacholestane С29Н52 20S-24-Ethyl-10á13á18â-diacholestane 29 52 20S-24-Methyl-ááá-cholestane 20R-24-Methyl-áââ-cholestane 20S-24-Methyl-áââ-cholestane 14 20R-24-Methyl- -cholestane С28Н50 20S-24-Ethyl-ááá-cholestane 29 52 20R-24-Ethyl-áââ-cholestane 20S-24-Ethyl-áââ-cholestane 20R-24-Ethyl-ááá-cholestane honopregnane originated during the period of very salty sedimentary accumulation in the condition of diagenesis. In the petroleum from holes 19-14, 19-28 and 19-31 of Tamsagbulag, most of diasteranes which are the newly formed isomer of biosteranes are hydrocarbon com- pounds of C27 (7.5% - 9.5% relatively). The petroleum of the hole 19-34 contains relatively little amount of hy- drocarbon compounds of C27 which is only 1.09%. In other words, C27 > C29 for diasteranes of petroleum of holes 19-14, 19-28 and 19-31 while C27 < C29 for hole 19-34. Diasteranes of petroleum are useful for determin- ing component of sediment lithology comprised funda- mental source and original substance of petroleum. The C29 norhopanes and C30 hopanes are the dominating biomarkers in all the crude oils which is characteristic for source rocks rich in terrestrial organic matter [15]. These hydrocarbons’ amount in the petroleum of holes 19-14, 19-28 and 19-31 is relatively more than in petro- leum of hole 19-34 so it means that the origin of the pe- troleum of these 3 three holes except 19-34 is relevant with sedimentary rocks which are rich in aluminum sili- cate. Tr icyclicterpane amounted of 3% - 15% was dis- covered from among class of hydrocarbons of terpane and contents of hopane and norhopane in the 4 holes of the petroleum were 44% - 47.5%, 6% - 8%, resp ectively. Homohopanes amount in hydrocarbons of terpane in the petroleum of the aforementioned 4 holes was accounted for 25% - 35%. There was a constant decrease of homo- hopanes with C32-C35 and each pair of epimers had plenty of (22-S)-epimer s ( Tab les 2 and 4). Processing level of  166 E. ENKHTSETSEG ET AL. Copyright © 2011 SciRes. ACES Table 2. GC-MS peak identification for Figure 2. 1 C19, 14â(methyl)-Tricyclicterpane C19H34 C20, 13â(H), 14á(H)-Tricyclicterpane C21, 13â(H), 14á(H)-Tricyclicterpane C22, 13â(H), 14á(H)- Tricyclicterpane C23,13â(H), 14á(H)-Tricyclicterpane 6 C24,13â(H), 14á(H)-Tricyclicterpane C24H44 25 , 13â(H), 14á(H)-Tricyclicterpane 25 46 11 C28, Tricyclicterpane C28H50 28 28 50 18á, -22, 29, 30-Trisnorhopane(Ts) 16 17á, -22, 29, 30-Trisnorhopane(Tm) C27H46 29 50 17â(H), 21á(H)-Normoretane 21 C3017á(H), 21â(H)-Hopane C30H52 30 52 22S-17á(H), 21â(H)-Homohopane C3122R-17á(H), 21â(H)-Homohopane 26 22R-17â(H), 21á(H)-Homomoretane C31H54 22S-17á(H), 21â(H)-Dihomohopane 32 56 22R-17á(H), 21â(H)-Dihomohopane 22S-17á(H), 21â(H)-Trihomohopane 22R-17á(H), 21â(H)-Trihomohopane 31 22S-17á(H), 21â(H)-Tetrahomohopane С34Н60 22R-17á(H), 21â(H)-Tetrahomohopane 34 60 22S-17á(H), 21â(H)-Pentahomohopane 22R-17á(H), 21â(H)-Pentahomohopane Table 3. Sterane concentration in the crude oil from Tamsagbulag oilfield, wt%. ID Compound Formula Molecular weight Oil wells 14 28 31 34 S1 5á(H), 14â(H)-Pregnane C21H36 288 2.30 2.80 2.45 1.62 S2 C22 Honopregnane C22H38 302 1.57 1.77 1.52 0.93 S3 20S-áâá-Diacholestane С27Н48 372 4.79 5.46 4.56 0.48 S4 20R-áâá-Diacholestane С27Н48 372 3.17 4.12 3.03 0.61 S11 20S-ááá-Cholestane С27Н48 372 5.05 4.48 4.69 6.64 S12 20R-áââ-Cholestane С27Н48 372 6.08 5.97 6.45 5.39 S13 20S-áââ-Cholestane С27Н48 372 6.28 7.07 6.07 5.49 S14 20R-ááá-Cholestane С27Н48 372 6.38 4.73 6.02 9.78 S15 20R-24-Ethyl-10á13â17á-diacholestane С29Н52 400 3.41 2.99 3.25 3.39 S16 20S-24-Ethyl-10á13á18â-diacholestane С29Н52 400 1.62 1.82 1.74 1.07 S17 20S-24-Methyl-ááá-cholestane С28Н50 386 2.88 3.71 3.12 2.56 S18 20R-24-Methyl-áââ-cholestane С28Н50 386 2.44 2.92 2.62 3.67 S19 20S-24-Methyl-áââ-cholestane С28Н50 386 4.83 4.99 4.96 5.96 S20 20R-24-Methyl-ááá-cholestane С28Н50 386 3.74 3.71 3.84 5.58 S21 20S-24-Ethyl-ááá-cholestane С29Н52 400 10.13 8.53 9.83 12.01 S22 20R-24-Ethyl-áââ-cholestane С29Н52 400 13.08 13.24 12.51 11.22 S23 20S-24-Ethyl-áââ-cholestane С29Н52 400 10.26 10.70 11.43 9.08 S24 20R-24-Ethyl-ááá-cholestane С29Н52 400 12.00 11.00 11.91 14.51  E. ENKHTSETSEG ET AL. Copyright © 2011 SciRes. ACES Table 4. Terpane c o ncentration in the crude oil from Tamsagbulag oilfield, wt%. ID Compound Formula Т1 C19, 14 (methyl)-Tricyclicterpane C19H34 262 0.55 0.70 0.57 0.38 2 C20, 13â(H), 14á(H)-Tricyclicterpane 20 36 C21, 13â(H), 14á(H)-Tricyclicterpane C22, 13â(H), 14á(H)-Tricyclicterpane C23, 13â(H), 14á(H)-Tricyclicterpane Т6 C24, 13â(H), 14á(H)-Tricyclicterpane C24H44 332 0.20 0.27 0.23 1.03 7 C25, 13â(H), 14á(H)-Tricyclicterpane 25 46 Т11 C28, Tricyclicterpane C28H50 388 - - - 1.17 12 28 50 18á, -22,29,30-Trisnorhopane(Ts) Т16 17 , -22,29,30-Trisnorhopane(Tm) C27H46 370 3.17 2.86 2.97 3.76 19 29 50 17â(H), 21á(H)-Normoretane Т23 17á(H), 21â(H)-Hopane C30H52 412 27.42 26.41 27.11 26.91 24 30 52 22S-17á(H), 21â(H)-Homohopane 22R-17á(H), 21â(H)-Homohopane Т28 22R-17 (H), 21 (H)-Homomoretane C31H54 426 1.22 1.14 1.63 2.15 29 22S-17á(H), 21â(H)-Dihomohopane 32 56 22R-17á(H), 21â(H)-Dihomohopane 22S-17á(H), 21â(H)-Trihomohopane 22R-17á(H), 21â(H)-Trihomohopane Т33 22S-17 (H), 21 (H)-Tetrahomohopane С34Н60 468 2.03 2.10 2.02 0.94 34 22R-17á(H), 21â(H)-Tetrahomohopane 34 60 22S-17á(H), 21â(H)-Pentahomohopane 22R-17á(H), 21â(H)-Pentahomohopane Table 5. The sterane concentration in the crude oil from some wells of the Tamsagbulag oilfield, wt%. Compound Oil wells 14 28 31 34 5á(H), 14â(H)-Pregnane 2.30 2.80 2.45 1.62 Total biosterane 40.18 36.16 39.41 51.08 27 Total normal sterane 83.15 81.05 83.45 91.89 27 the petroleum of these 4 holes was considered as low because none of diahopanes was found in their compo- nent. Some gammaceranes (1.5% - 3%) were found in the petroleum of holes 19-14, 19-28, 19-31 and 19-34 of Tamsagbulag oil field and this evidence shows characte- ristic of highly marine depositional conditions in the en- vironment of ocean, sea and marshy lake. 4. Conclusions 1) The composition of the sterane and terpane hyrdo- carbon derivatives of the Tamsagbulag crude oil well No. 14-19, 28, 31 and 34 has been determined by GC-MS. 2) Based on the contained biosteranes and isosteranes,  168 E. ENKHTSETSEG ET AL. Copyright © 2011 SciRes. ACES Table 6. The hydrocarbon compositions of hopane derivatives in the crude oil from some wells of the Tamsagbulag oilfield, wt%. Compound Hopane 47.43 45.64 46.58 43.99 the petroleum from some holes of Tamsagbulag oil field, originated from oddments of upper plants. The content of the gammacerane, pregnane and honopregnane found in the petroleum of the aforementioned oil field says that those are originated during the period of very salty sedi- mentary accumulation in the condition of diagenesis. 5. References [1] G. D. Hobson and W. Pohl, “Modern Petroleum Tech- nology,” Applied Science Publishers, London, 1973. [2] J. M. Hunt, “Petroleum Geochemistry and Geology,” Freeman Company, New York, 1979. [3] J. G. Speight, “The Chemistry and Technology of Petro- leum,” Marcel Dekker, Inc., New York, 1991. [4] D. R. Luellen and D. Shea, “Semipermeable Membrane Devices Accumulate Conserved Rations of Sterane and Hopane Petroleum Biomarkers,” Chemosphere, Vol. 53, No. 7, 2003, pp. 705-713. doi:10.1016/S0045-6535(03)00576-9 [5] A. Hauser, H. Dashti and Z. H. Khan, “Identification of Biomarker Compounds in Selected Kuwait Crude Oils,” Fuel, Vol. 78, No. 12, 1999, pp. 1483-1488. doi:10.1016/S0016-2361(99)00075-7 [6] J. Connan, J. Bouroullec, D. Dessort and P. Albrecht, “The Microbial Input in Carbonate-Anhydrite Facies of a Sabkha Palaeoenvironment from Guatemala: Molecular Approach,” Organic Geochemist ry, Vol. 10, No. 1-3, 1986, pp. 29-50. doi:10.1016/0146-6380(86)90007-0 [7] A. A. Petrov, “Hydrocarbons of the Crude Oil,” Nauka, Moscow, 1984. [8] M. I. Roushdy, M. M. El Nady, Y. M. Mostafa, et al., “Biomarkers Characteristics of Crude Oils from Some Oilfields in the Gulf of Suez, Egypt,” Journal of Ameri- can Science, Vol. 6, No. 11, 2010, pp. 911-925. [9] S. Aboglila, K. Grice, K. Trinajstic, et al., “Use of Biomarker Distributions and Compound Specific Isotopes of Carbon and Hydrogen to Delineate Hydrocarbon Cha- racteristics in the East Sirte Basin (Libya),” Organic Geochemistry, Vol. 41, No. 12, 2010, pp. 1249-1258. doi:10.1016/j.orggeochem.2010.05.011 [10] R. P. Philp, J. G. Li and C. A. Lewis, “An Organic Geo- chemical Investigation of Crude Oils from Shanganning, Jianghan, Chaidamu and Zhungeer Basins, China,” Or- ganic Geochemist ry , Vol. 14, No. 4, 1989, pp. 4 47-460. doi:10.1016/0146-6380(89)90010-7 [11] R. P. Philp, R. T. Simoneit and T. D. Gilbert, “Advances in Organic Geochemistry,” Wiley, Chichester, 1981, pp. 698-704. [12] B. Khongorzul, “Features of the Hydrocarbon Composi- tion and High Molecular Compounds of the High Paraf- finic Oil from Mongolia,” Ph.D. Thesis, Tomsk, 2008, pp. 67-77. [13] V. Alimaa, “Effect of the Depths on Crude Oil Properties,” Ph.D. Thesis, Mongolian University of Science and Tech- nology, UB, 2008, p. 118. [14] A. Sainbayar, “Study on Hydrocarbon’s Composition of Mongolian Petroleum,” Ph.D. Thesis, National University of Mongolia, Ulaanbaatar, 2006, p. 200. [15] G. Demaison, A. J. Holck, R. W. Jones and G. T. Moore, “Predictive Source Bed Strat igraphy: A Guide to Region- al Petroleum Occurrence,” Proceedings of the 11th World Petroleum Congress 2, PDI, Wiley, New York, 1984, pp. 17-29.
|