Sickle Cell Disease and Pregnancy: Does Outcome Depend on Genotype or Phenotype?
Copyright © 2011 SciRes. IJCM
317
[7] K. Hassell, “Pregnancy and Sickle Cell Disease,” Hema-
tology/Oncology Clinics of North America, Vol. 19, No. 5,
2005, pp. 903-916. doi:10.1016/j.hoc.2005.07.003
[8] W. D. Barfield, D. T. Barradas, S. E. Manning, et al.,
“Sickle Cell Disease and Pregnancy Outcomes: Women
of African Descent,” American Journal of Preventive
Medicine, Vol. 38, No. 4S, 2010, pp. S542-S549.
doi:10.1016/j.amepre.2009.12.020
[9] P. M. Sun, W. Wilburn, B. D. Raynor and D. Jamieson,
“Sickle Cell Disease in Pregnancy: Twenty Years of Ex-
perience at Grady Memorial Hospital, Atlanta, Georgia,”
Am Journal of Obstetrics and Gynecology, Vol. 184, No.
6, 2001, pp. 1127-1130. doi:10.1067/mob.2001.115477
[10] G. R. Serjeant, I. Hambleton and M. Thame, “Fecundity
and Pregnancy Outcome in a Cohort with Sickle
Cell-Haemoglobin C Disease Followed from Birth,” Brit-
ish Journal of Obstetrics and Gynaecology, Vol. 112, No.
9, 2005, pp. 1308-1314.
doi:10.1111/j.1471-0528.2005.00678.x
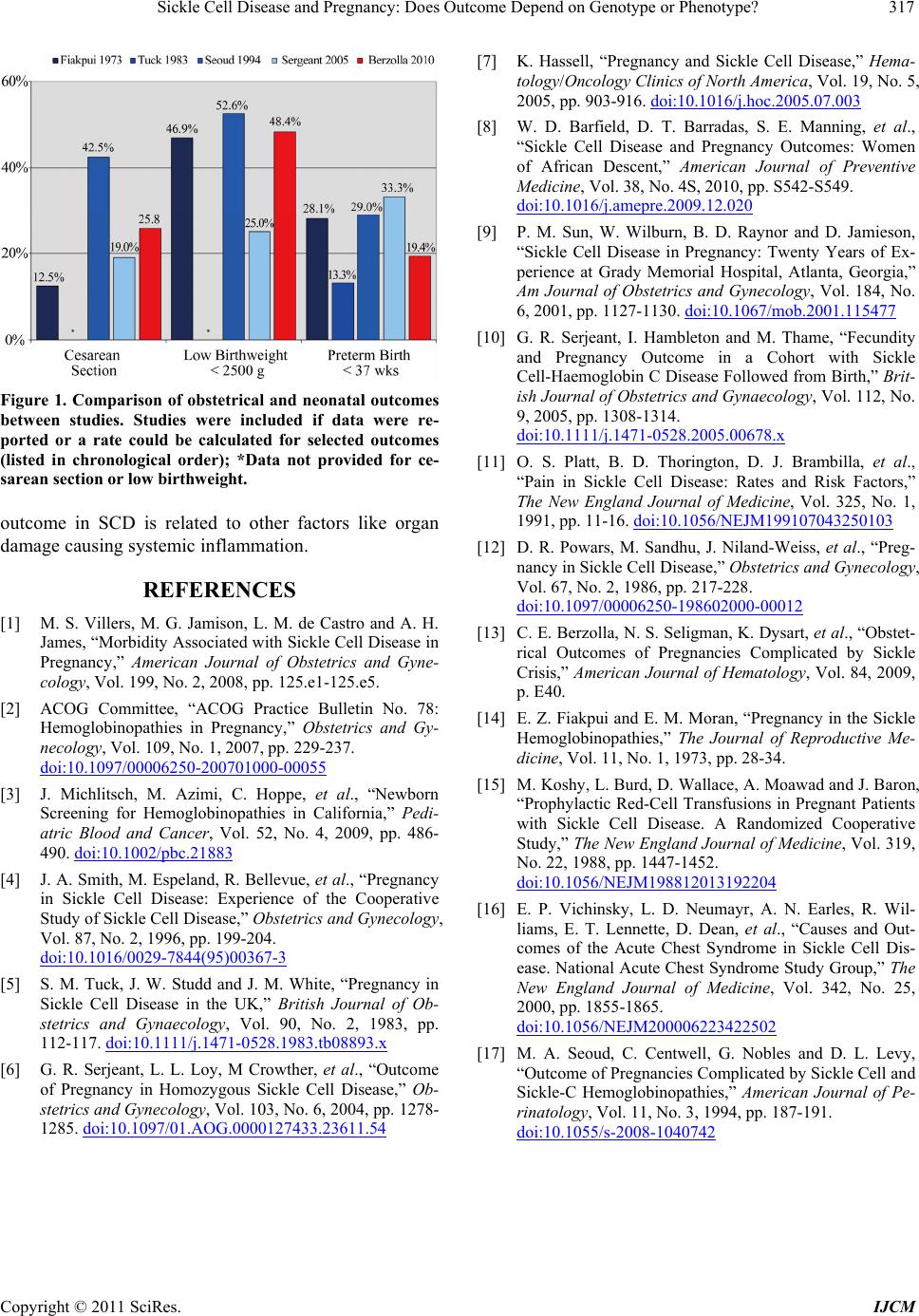
Figure 1. Comparison of obstetrical and neonatal outcomes
between studies. Studies were included if data were re-
ported or a rate could be calculated for selected outcomes
(listed in chronological order); *Data not provided for ce-
sarean section or low birthweight.
[11] O. S. Platt, B. D. Thorington, D. J. Brambilla, et al.,
“Pain in Sickle Cell Disease: Rates and Risk Factors,”
The New England Journal of Medicine, Vol. 325, No. 1,
1991, pp. 11-16. doi:10.1056/NEJM199107043250103
outcome in SCD is related to other factors like organ
damage causing systemic inflammation. [12] D. R. Powars, M. Sandhu, J. Niland-Weiss, et al., “Preg-
nancy in Sickle Cell Disease,” Obstetrics and Gynecology,
Vol. 67, No. 2, 1986, pp. 217-228.
doi:10.1097/00006250-198602000-00012
REFERENCES
[1] M. S. Villers, M. G. Jamison, L. M. de Castro and A. H.
James, “Morbidity Associated with Sickle Cell Disease in
Pregnancy,” American Journal of Obstetrics and Gyne-
cology, Vol. 199, No. 2, 2008, pp. 125.e1-125.e5.
[13] C. E. Berzolla, N. S. Seligman, K. Dysart, et al., “Obstet-
rical Outcomes of Pregnancies Complicated by Sickle
Crisis,” American Journal of Hematology, Vol. 84, 2009,
p. E40.
[2] ACOG Committee, “ACOG Practice Bulletin No. 78:
Hemoglobinopathies in Pregnancy,” Obstetrics and Gy-
necology, Vol. 109, No. 1, 2007, pp. 229-237.
doi:10.1097/00006250-200701000-00055
[14] E. Z. Fiakpui and E. M. Moran, “Pregnancy in the Sickle
Hemoglobinopathies,” The Journal of Reproductive Me-
dicine, Vol. 11, No. 1, 1973, pp. 28-34.
[15] M. Koshy, L. Burd, D. Wallace, A. Moawad and J. Baron,
“Prophylactic Red-Cell Transfusions in Pregnant Patients
with Sickle Cell Disease. A Randomized Cooperative
Study,” The New England Journal of Medicine, Vol. 319,
No. 22, 1988, pp. 1447-1452.
doi:10.1056/NEJM198812013192204
[3] J. Michlitsch, M. Azimi, C. Hoppe, et al., “Newborn
Screening for Hemoglobinopathies in California,” Pedi-
atric Blood and Cancer, Vol. 52, No. 4, 2009, pp. 486-
490. doi:10.1002/pbc.21883
[4] J. A. Smith, M. Espeland, R. Bellevue, et al., “Pregnancy
in Sickle Cell Disease: Experience of the Cooperative
Study of Sickle Cell Disease,” Obstetrics and Gynecology,
Vol. 87, No. 2, 1996, pp. 199-204.
doi:10.1016/0029-7844(95)00367-3
[16] E. P. Vichinsky, L. D. Neumayr, A. N. Earles, R. Wil-
liams, E. T. Lennette, D. Dean, et al., “Causes and Out-
comes of the Acute Chest Syndrome in Sickle Cell Dis-
ease. National Acute Chest Syndrome Study Group,” The
New England Journal of Medicine, Vol. 342, No. 25,
2000, pp. 1855-1865.
doi:10.1056/NEJM200006223422502
[5] S. M. Tuck, J. W. Studd and J. M. White, “Pregnancy in
Sickle Cell Disease in the UK,” British Journal of Ob-
stetrics and Gynaecology, Vol. 90, No. 2, 1983, pp.
112-117. doi:10.1111/j.1471-0528.1983.tb08893.x [17] M. A. Seoud, C. Centwell, G. Nobles and D. L. Levy,
“Outcome of Pregnancies Complicated by Sickle Cell and
Sickle-C Hemoglobinopathies,” American Journal of Pe-
rinatology, Vol. 11, No. 3, 1994, pp. 187-191.
doi:10.1055/s-2008-1040742
[6] G. R. Serjeant, L. L. Loy, M Crowther, et al., “Outcome
of Pregnancy in Homozygous Sickle Cell Disease,” Ob-
stetrics and Gynecology, Vol. 103, No. 6, 2004, pp. 1278-
1285. doi:10.1097/01.AOG.0000127433.23611.54