 Materials Sciences and Applications, 2015, 6, 1014-1021 Published Online November 2015 in SciRes. http://www.scirp.org/journal/msa http://dx.doi.org/10.4236/msa.2015.611101 How to cite this paper: Chouchene, W. and Bellakhal, N. (2015) Humid Air Plasma Treatment of Birnessite Surface: Applica- tion to the Removal of Cochineal Red. Materials Sciences and Applications, 6, 1014-1021. http://dx.doi.org/10.4236/msa.2015.611101 Humid Air Plasma Treatment of Birnessite Surface: Application to the Removal of Cochineal Red Wafa Chouchene, Nizar Bellakhal UR de Catalyse, Electrochimie de Nanomatériaux et Leurs Applications et de Didactique, Institut National des Sciences Appliquées et de Technologie (INSAT), Tunis, Tunisie Received 8 October 2015; accepted 17 November 2015; published 20 Nov emb er 2015 Copyright © 2015 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract The thin layers of birnessite (Mn7O13∙5H2O) are exposed to reactive species gliding arc plasma in humid air, which induces the treatment of the thin layers surface. Plasma treatment thin layer of birnessite was used for the degradation of Cochineal Red. The experimental results showed that 95% of the CR solution was completely decolorized by thin layer of birnessite treated by plasma compared to 80% of the same solution after interaction of thin layer of birnessite untreated. The decay kinetics always follows a pseudo-first order reaction. The application of the humid air plas- ma for the surface treatment of thin layers of birnessite improves the efficiency of treatment for Cochineal Red degradation. Keywords Humid Air Plasma, Surface Treatment, Thin Layers of Birnessite, Cochineal Red, Degradation 1. Introduction Dyes production industries which used dyes and pigments generated wastewater, characteristically high in color and o rgani c co nte nt [1]. Azo dyes represent the largest class of dyes, and a part of them are suspected to be car- cinogenic [2]-[4]. Environmental pollution by organic azo dyes presents a severe ecological problem that leads to the necessity of treatment by the fact that most of the se dyes are di fficult to d egrade b y traditio nal techniques [5]-[9]. But d eve lo p ing ne w e nvir onme nta l l y me t hod s t o de gr ad e o r gani c p o ll uta nt s i n wa stewate r se e ms a l wa ys inter est i n g. I n thi s a i m, we have s hown the s uc ce s s ful po ssibilit y to use bir nessite t hi n films to d ecolo rize d solu- tions c o nta i ni n g az o d yes [10]. T he birnessite (Mn7O13∙5H2O) wa s c o nsidered as the most int er e sti n g man ga ne se 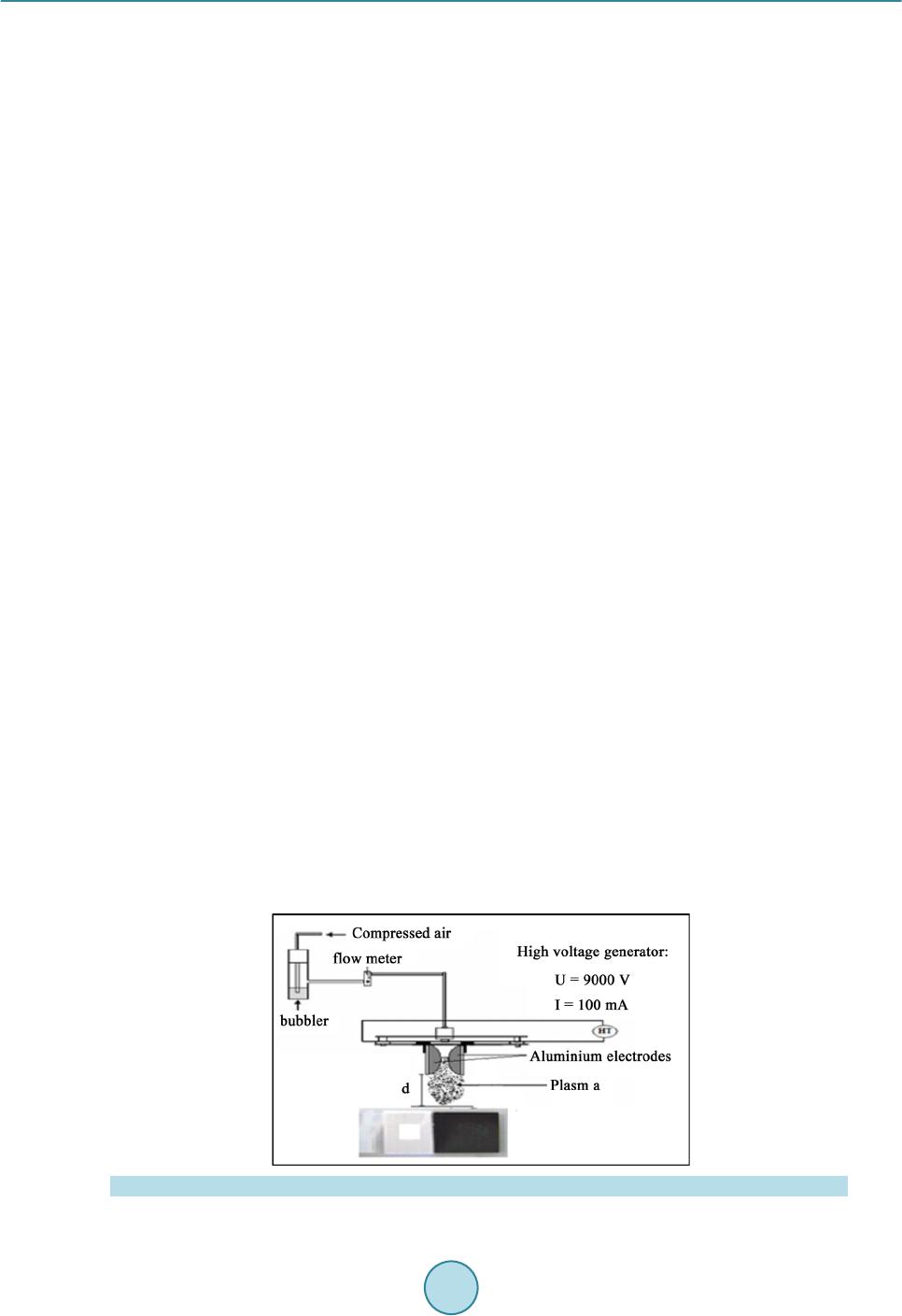 W. Chouchene, N. Bellakhal oxide because of its high absorption capacity and its redox properties [10]-[15]. These results encouraged us to investigate to use thin films of birnessite treated initialy by an humid air plasma generated by a gliding arc, in the degradation of Cochineal Red (2-hydroxy-1-(4-sulfonate-1-naphtylazo) -6,8-naphtalène disulfonate trisodium). A humid air plasma is characterized by the occurrence of a large number of excited species which confers on them an enhanced reactivity involved in numerous plasma treatments of materials. These chemical properties are classified into acid-base and oxidation-reduction properties. This work was performed with a humid air plasma gas produced by the gliding arc proposed by Lesueur et al. [16]. Several processes such as ionization, dissocia- tion by electron impact, and attachment phenomena take place in the plas ma [17]. The spectroscopic investiga- tion of the emission bands of gliding arc discharge in humid air in the 230 - 650 nm range showed the occur- rence of the radicals NO˙ and OH˙ as the main species present in the plasma [18]. T hese activated species show an enhanced chemical reactivity and particular some of them behave as strong oxidizing agents (OH˙, H2O2, O3, NOx, NO˙) [19]. This paper is devoted of degradation of Cochineal Red by thin layers of birnessite after chang- ing its oxidizing properties by humid air plasma. 2. Experimental 2.1. Plasma Device An electric arc was created between two diverging aluminium electrodes raised to a convenient voltage. The al- ternative current generator delivered a suitable energy (100 mA; 9000 V). A gas flow along the axis of the reac- tor blew the arc and made it glide along the electrodes before breaking. After breaking, a new arc formed and the cycle resumed. The air provided by an air compressor was saturated with water by bubbling in a Durand flask before entering the reactor through a nozzle (diameter 0.99 mm). The ar c is p ushed away fr o m the ig nitio n point by the feeding gas flow and sweeps along the maximum length of the electrode gap and forming a large plasma plu me, so that it lic ks the sur face of thi n layer of bi rne ssit e. T he t rea tment is d one in o pen syst em f ixi ng the functioning parameters. The gas flow is fixed at Q = 650 L∙h−1, the divergence between the electrodes e = 3.5 mm and the distance between the electrodes and the surface of thin layers of birnessite d = 5 cm. The brass fo ils were expos ed to the p l asma flux as shown in Figure 1. The OH˙ species is the main responsible for strong oxidizing character of the discharge [20] [21]. The hy- droxyl radical is a very reactive species having an oxidation potential of E˚(OH˙/H2O) = 2.85 V/NHE [22]. On the other hand, the NO˙ radical leads to the formation of NO2, nitrite a nd nitrate io ns acco rding to the follo wing overall reactions [23]: (1) (2) (3) (4) (5) Figure 1 . Schema of a gliding arc device: HT = 9000V; Q = 650 L∙h−1; d = 5 cm; e = 3.5 mm; φ = 0.99 mm. 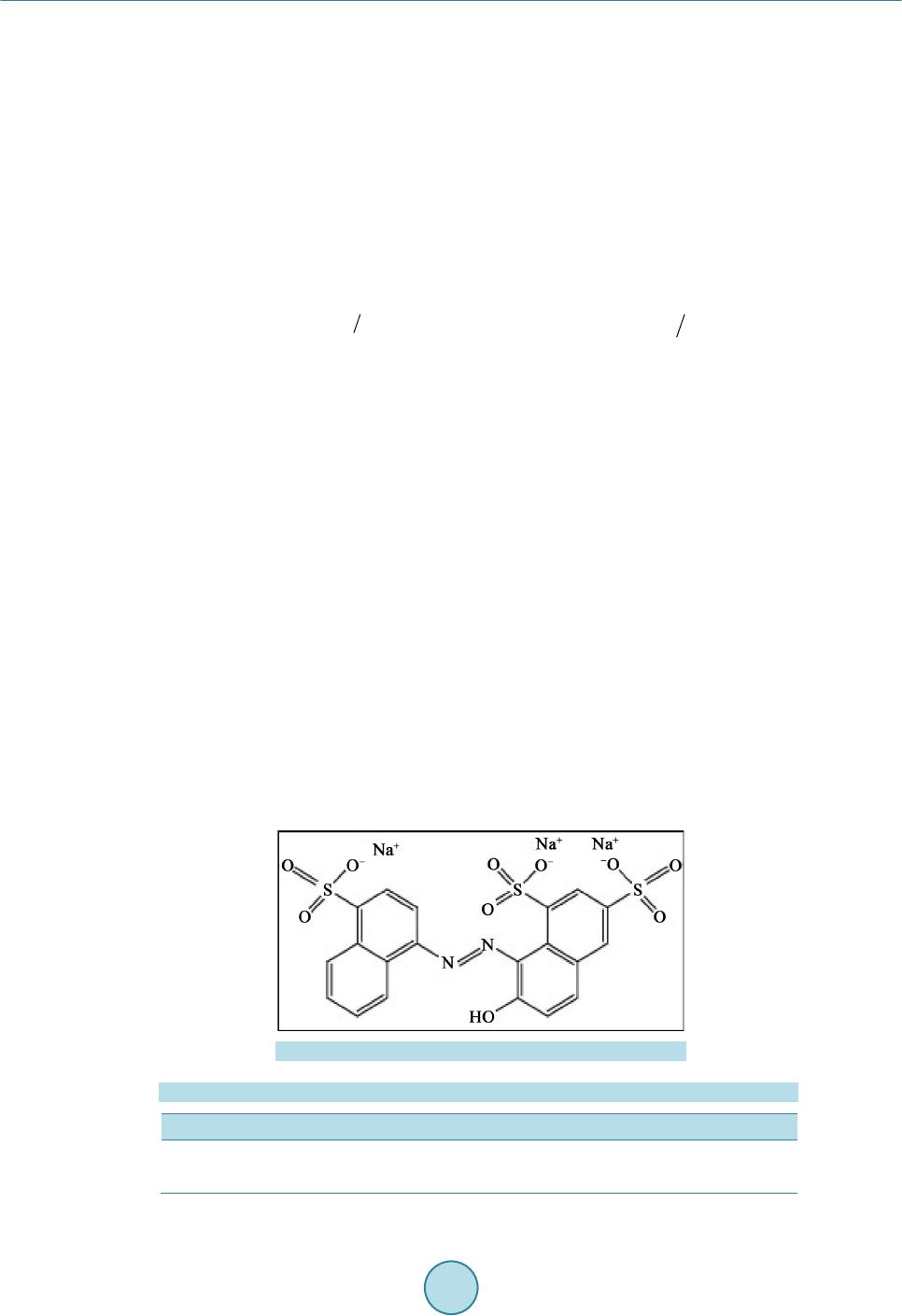 W. Chouchene, N. Bellakhal 2.2. Materials and Methods The azo dye Cochineal Red was obtained from Sigma-Aldri ch and has a pur it y of 99 %. T he molec ular str uct ure of Cochineal Red (C20H11N2Na3O10S3) was given in Figure 2. A classical electrochemical cell with three electrodes was used. The thin layers of birnessite were electrode- posited by chronoamperometry at E = 0.6 V/MSE, in neutral aerated solution containing sodium sulphate and manga ne se s ulp ha te [10]. The electro-oxidation of Mn2+ in neutral aerate d sulfate so lutio n leads to the for mation of birnessite ( M n7O13·5H2O) [ 10] [24]. The Cochineal Red concentrations in the solution were determinated by an UV-vis spectrophotometer (Backman). Chemical oxygen demand (COD) measurements were carried out using the French AFNOR norm. The organic matter was oxidized by potassium dichromate K2Cr2O7 under energetic conditions. The temperature of the solution was raised 170˚C over 2 h. The excess of potassium dichromate was measured out by Mohr salt titration. COD was calculated from the expression: ( ) ( )( ) ( ) ( ) 20 MS blankMS sampleMS CODmgOL8000VVNV=×− × where V(MS)blank a nd V(M S)sample are the volumes of standard Mohr salt solution used for the blank and the sample respectively, N(MS) is the normalit y of the Mo hr salt solution, and V0 is the volume of the sample. 3. Results and Discussions 3.1. Treatment of Birnessite Surface by Humid Air Plasma Like most manganese oxides, birnessite oxidizes organic pollutants via an electronic exchange that occurs at the surfa ce ( Shin B uz go et a l.) [25] [26]. Birnessite (Mn7O13∙5H2O) contains manganese with two degrees of oxida- tion (Mn4+ and M n3+) but M n4+ most oxidizing species whi ch is suppo sed to react preferentially with po llutants [10]. In general, organic pollutants react with the manganese oxides via Mn(IV) ions which are reduced to Mn(II) in one step [27]-[29] or via a compound Mn(III) [30]. The determination of the average oxidation degree of manganese allows as to evaluate the Mn(III)/Mn(VI ) of the differe nt for ms of manga nese e xisting i n the s heets of thin layers of birnessite. To determine the average oxidation degree of manganese, we use the titration poten- tiometry method allong with sodium pyrophosphate and Mohr’s salt [31]. The average oxidation degree of manganese of thin layers of birnessite untreated and treated by humid air plasma is set in Table 1. As sho wn in Ta ble 1, the sheets of thin layers of birnessite untreated are formed from a mixture of Mn4+ and Mn3+ while proportions are respectively 60% and 40% [10] [31]. However, the sheets of thin layers of birnessite treated by humid air plasma are more loaded with Mn4+ ions while proportions are 80% Mn4+ and 20% Mn3+. Therefore, thin layers of birnessite obtained under these conditions are more oxidizing and loaded with Mn4+ ions. This is in good agreement with improved degradation of dyes [31]. Figure 2 . Cochineal red structure. Table 1. Average degree of o xi dation of manganese of bi rnessite untreat ed and treat ed. Avera ge deg re e of oxidation %Mn3+ %Mn4+ Birnessite untreated 40% 60% Birnessite treated 20% 80% 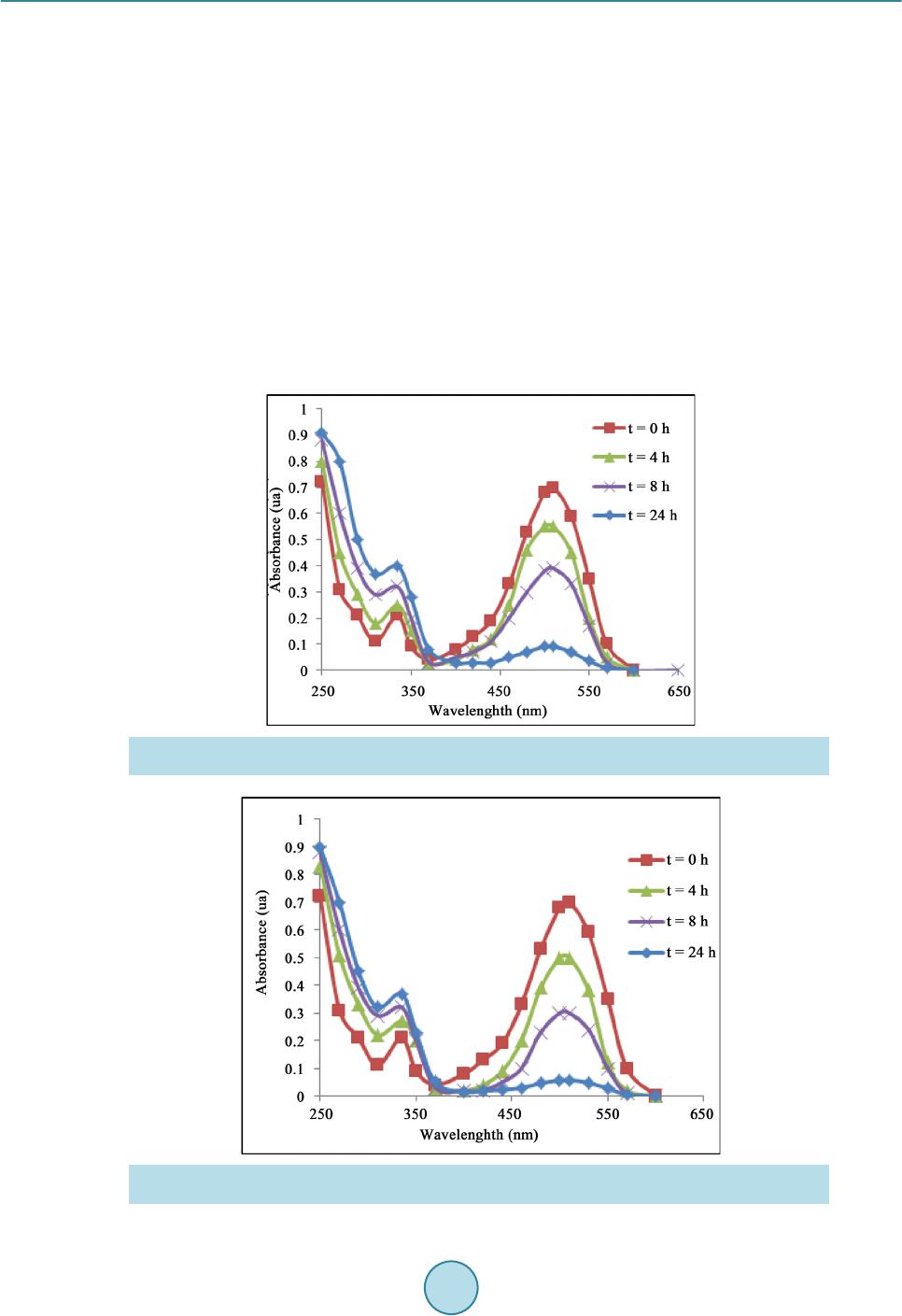 W. Chouchene, N. Bellakhal 3.2. Removal of Cochineal Red by Thin Layers of Birnessite Figure 3 shows the UV-Visible spectra obtained during interaction, between one thin layer of birnessite un- treated and a solution of CR during 24 h. The 512 nm peak of intensity of the CR solution decreases as treatment time increases implying that the ma- jority of Cochineal Red molecules are destroyed. After 24 h of interaction, the peak at 512 nm was decreased and the solution was totall y discolored 80%. These r esults pro ves the efficienc y of the thin layers of birnessite to the degradation of dyes [10] [32]-[34]. 3.3. Removal of Cochineal Red by Thin Layers of Birnessite Treated by Humid Air Plasma Figure 4 shows the UV-Vis s pectra obta ined after the interaction be tween CR solutio n and thin layer o f birnes- site that has been exposed firstly to the discharge of humid air plasma. The 512 nm peak of intensity of the treated solution decreases as treatment time increases implying that the majority of Cochineal Red molecules are destroyed. After 24 h of interaction, the peak at 512 nm was decreased and solution was totally discolored 95% with a thin layer of birnessite treated by humid air plasma compared to 80% with a thin layer of birnessite untreated. Figure 3 . UV-Visible spectra o f coch in eal red solution in interaction with a thin layer of birnessite un- treated (C = 8.25 × 10−6 mol∙L−1, V = 10 ml, pH = 3). Figure 4. UV-Visible spectra of cochineal red solution in interaction with thin layer of birnessite treated by humid air plasma (C = 8.25 × 10−6 mol∙L−1, V = 10 ml, pH = 3, Q = 650 L∙h−1; d = 5 cm). 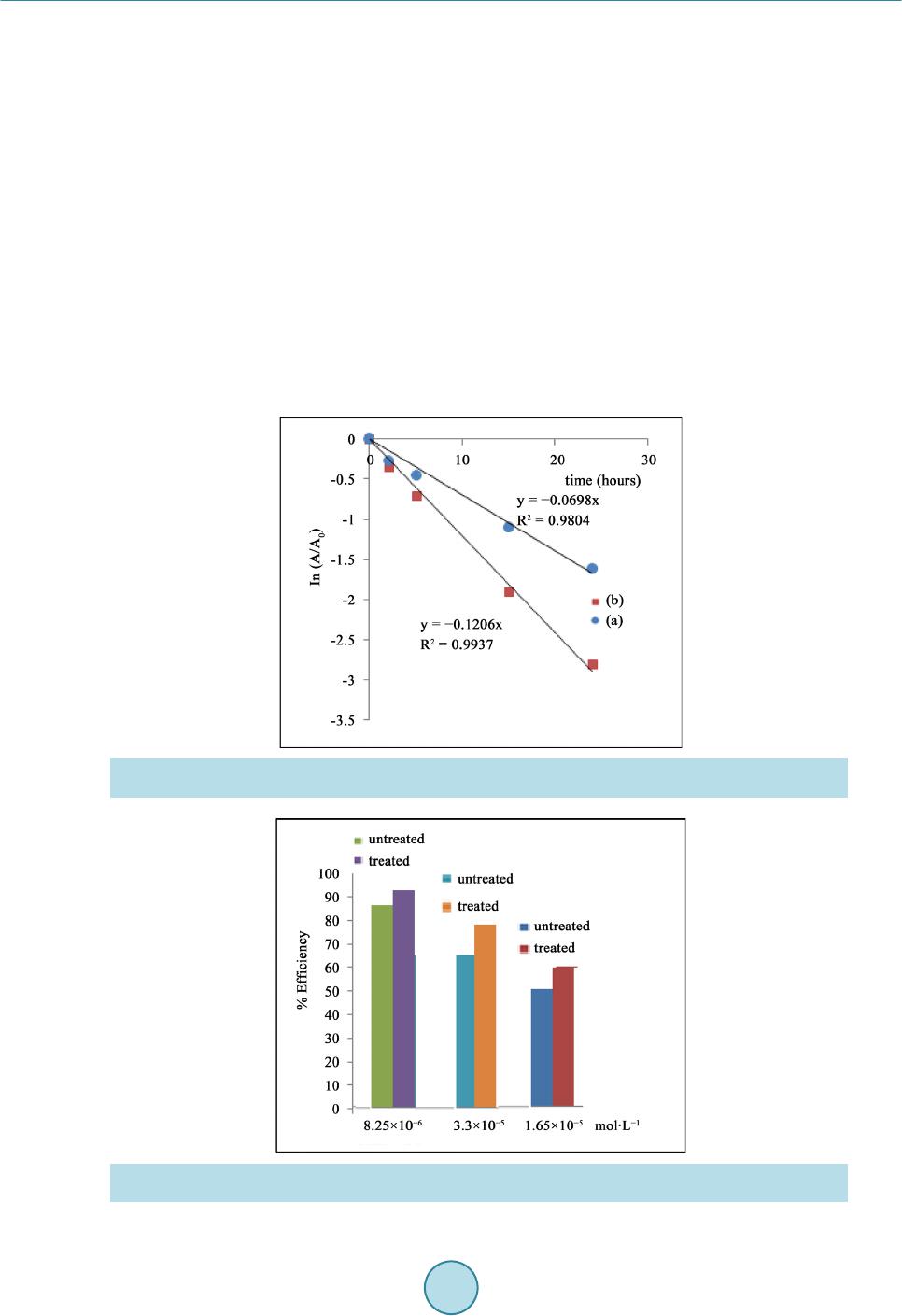 W. Chouchene, N. Bellakhal These results confirm the very important effect of thin layer of birnessite after c hanging its o xidizing prope rties by humid air plasma on the degradation of Cochineal Red. 3.4. Kinetic Studies of the Degradation of Cochineal Red Our interest is turned to study the kinetic order of the degradation of Cochineal Red. T he kinetic of CR disco lo- ration with birnessite thin layer was studied with concentration of CR 8.25 × 10 −6 mol∙L−1. Using t he dye con- centration-absorbance relationship (Beer-La mber t ’s law: A = Cεl) the pseudo-first order kinetic model can be written as: Ln (A0/A) = K app ∙t. As sho wn in Figure 5, the degra dation reaction follo ws pseudo-first ord er kinetic with correla tion coefficient R2 value of 0.993 - 0.980 in good agreement with kinetic degradations of organic pollutants by manganese oxides [10] [35]-[37]. The degradation r ates are high s howing the ver y import ant effec t of birne ssite on t he de- gradation of Cochineal Red especially with birnessite treated by humid air plasma. 3.5. Influence of Cochineal Red Conc entr ation The effect of CR concentration was evaluated in the range that varied between 8.25 × 10−6 mol∙L−1 and 3.3 × 10−5 mol∙L−1. Figure 6 shows the discoloration efficiency (%), calculated versus time for various concentrations Figure 5 . Kinetic o rder of the degradation of cochineal red with a thin layer of birnes site (a) Untreated and (b) Treated by humid air plasma. (C = 8.25 × 10−6 mol ∙L−1, V = 10 ml, pH = 3, Q = 650 L∙h−1; d = 5 cm). Figure 6. Influence of the concentration of CR solutions on the effectiveness of treatment with a thin layer of birnessite unt reated and t r eated by humid air plasma (V = 10 ml, pH = 3, Q = 650 L∙h−1; d = 5 cm). 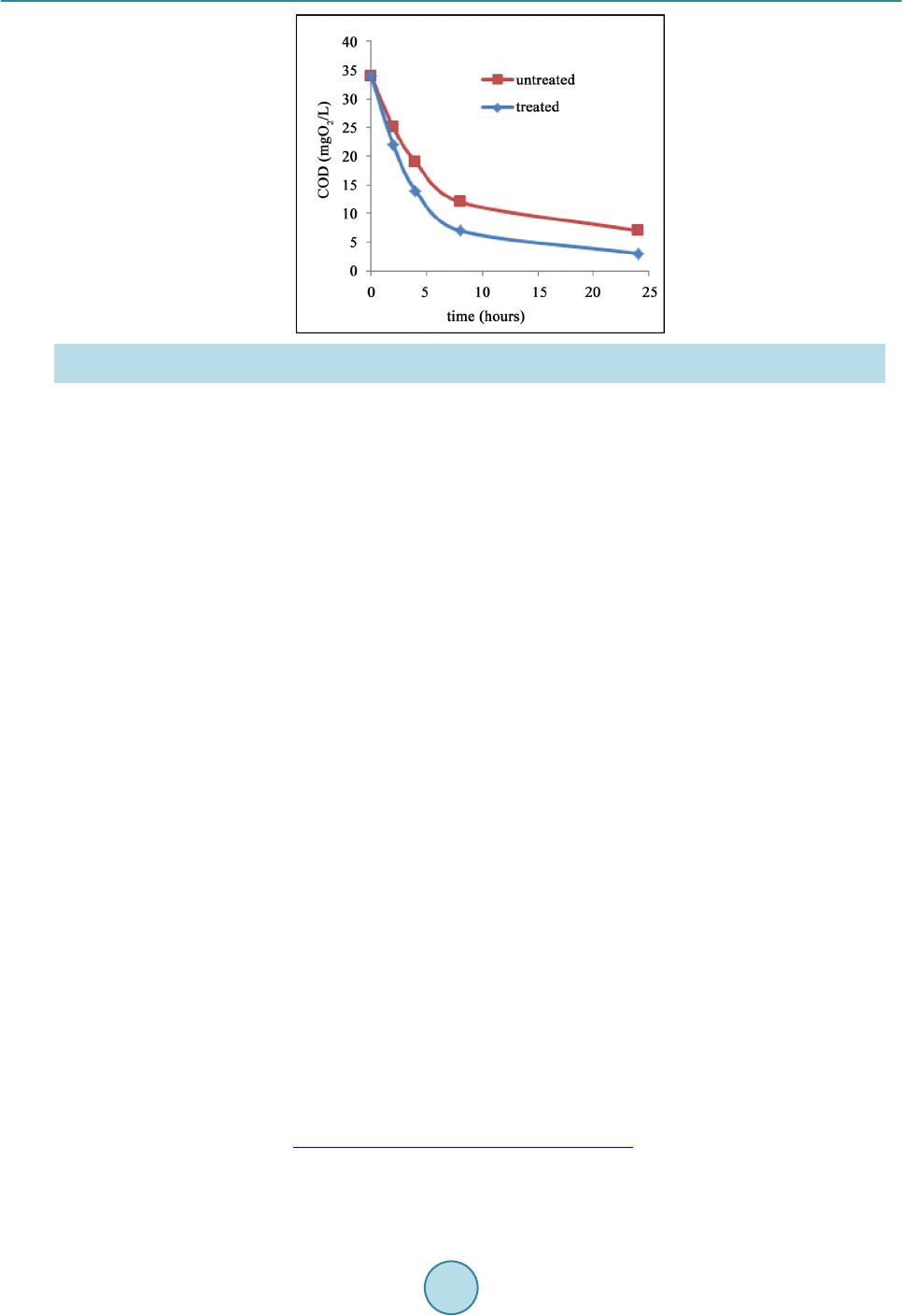 W. Chouchene, N. Bellakhal Figure 7 . Evolution of Chemical oxygen demand (COD) of cochi neal red in interaction with a thin layer of birnessite un- treated and treated by humid air pl asma (C = 8.25 × 10−6 mol∙ L−1, V = 200 ml, pH = 3, Q = 650 L∙h−1; d = 5 cm). of CR. Results ob tained sho wed that, when the initial CR conce ntratio n is 8.25 × 10−6 mol∙L−1 t he treatment is effec- tive at about 95% for CR solution in interaction with thin layer of birnessite treated by humid air plasma and about 80% for thin layer of birnessite untreated. However at a concentration of 3.3 × 10−5 mol∙L−1, only 60% of the color disapp ear ed even after 24 h for CR solution in inter actio n with thin layer of birnessite tre ated b y humid air plasma and about 50% for thin layer of birnessite untre ated. The results show that the effectivene ss of treat- ment increased with CR solution in interactio n with thin layer of bir nessite treated by humid air p lasma and CR discoloration increased with increase the initial CR concentration. 3.6. Mineralization Effici en cy Mineralization of treated Cochineal Red solutions are followed by measuring the chemical oxygen demand (COD) values to evaluate the organic carbon content of the solution. Electrolysis of initial Cochineal Red solu- tion leads to a gradual decrease in chemical oxygen demand ( COD) with electrical charge Q consumed, indicat- ing the mineral izatio n. The Figure 7 shows that the mineralization process reached a ratio 80% at 24 h for t hin layer of birnessite untreated compared to 95% for thin layer of birnessite treated by humid air plasma. The de- gradation of Cochineal Red is not only a breaking of the azo band, but also degradation of the aromatic rings [10]. It is also to be mentioned that final degradation products were mineral ions such , and accord ing to the substituent groups included in the initial mole cule, a s well as CO2 a nd H2O [10] [38] [39]. 4. Conclusion This paper is devoted to the surface treatment of thin layer of birnessite by an humid air plasma, in fact, it has been well established that oxidative degradation of organic matter by thin layers of birnessite is via a surface mechanism [10] [40] [41]. Furthermore, this surface treatment has been incre a si ng s i g ni fic a ntl y t he e ff ic ie nc y o f the treatment of the Coc hineal Red solution fro m 80% to 95%. T hese exp erimental res ults ar e important because Cochineal Red is e xtensivel y employed in man y industries. So, bir nessite thin layer tre ated b y humid air plas ma appears as a very interesting material for the development of a simple and ecological method applied to the re- mediation o f wastewater co ntaining Coc hinea l Red. References [1] Guyer, G.T. and Ince, N.H. (2003) Degradation and Toxicity Reduction of Textile Dyestuff by Ultrasound. Ultrasonics Sonochemistry, 10, 235-240. http://dx.doi.org/10.1016/S1350-4177(03)00089-0 [2] Sau er , T., Nero, G.C., Jose, H. J. and Moreira, R.F.P.M. (2002) Kinetics of Photocatalytic Degradation of Reactive Dyes in a TiO2 Slurry Reactor. Journal of Photochemistry and Photobiology A: Chemistry, 149, 147-154.  W. Chouchene, N. Bellakhal http://dx.doi.org/10.1016/S1010-6030(02)00015-1 [3] Cisneros, R. L., Espinoza, A.G. and Litter, M.I. (2002) Photodegradation of an Azo Dye of the Textile Industry. Che- mosphere, 48, 393. [4] Karkmaz, M., Puzenat, E., Guillard, C. and Herrmann, J.M . (2004) Photocatalyticdegradation of the Alimentary Azo Dye Amaranth: Mineralization of the Azo Group to Nitrogen. Applied Catalysis, 51, 183. [5] Brillas, E., Boye, B., Sirés, I., Garrido, J.A., Rodríguez, R.M. , Arias, C., Cabot, P.L. and Comninellis, C. (2004) Elec- trochemical Destruction of Chlorophenoxy Herbicides by Anodic Oxidation and Electro-Fenton Using a Boron-Doped Diamond Electrode. Electrochi m ica Acta, 49, 4487-4496. http://dx.doi.org/10.1016/j.electacta.2004.05.006 [6] Kesrao ui, A. , Oturan, N., Bel lakhal, N., Dachraoui, M. and Oturan, M.A. (2008) Experimental Design Methodology Applied to Electro-Fenton Treatment for Degradation of Herbicide Chlortoluron. Applied Catalysis B: Environmental, 78, 334-341. http://dx.doi.org/10.1016/j.apcatb.2007.09.032 [7] Arslan, I., Balciogul, I.A. and Bahnemann, D.W. (2000) Advanced Chemicaloxidatio n of Reactive Dyes in Simulated Dyehouse Effluent by Ferrioxalate-Fenton/UV-A and TiO2/UV—A Processes. D yes Pigmen ts, 47, 207. [8] Nam, S., Renganathan, V. and Tratnyek, P.G. (2001) Substituant Effects on Azo Dye Oxidation by the FeIII-EDTA- H2O2 System. Chemosphere, 45, 59. [9] Ashraf, S., Rau f, A. and Alhadrami, S. (2006) Degradati on of Meth yl RedFen ton’s Reagent and the Effect o f Vario us Salts. Dyes Pigments, 69, 80. [10] Zaied, M., Peulon, S., Bellakhal, N., Desmazieres, B. and Chausse, A. (2011) Studies of N-Demethylation Oxidative and Degradation of Methylene Blue by Thin Layers of Birnessite Electrodeposited onto SnO2. Applied Catalysis B: Environmental, 101, 441-450. http://dx.doi.org/10.1016/j.apcatb.2010.10.014 [11] Post, J. E. (1999) Manganese Oxide Minerals: Crystal Structures and Economic and Environmental Significance. Pro- ceedings of the National Academy of Sciences of the United S ta tes of America, 96, 3447-3454. http://dx.doi.org/10.1073/pnas.96.7.3447 [12] Mao, L., Arihara, K., Sotomura, T. and Ohsaka, T. (2004) A Novel Alkaline Air Electrode Based on a Combined Use of Cobalt Hexadecafluoro-Phthalocyanine and Manganese Oxide. Electrochimica Acta, 49, 2515-2521. http://dx.doi.org/10.1016/j.electacta.2004.02.007 [13] Machefaux, E., Verb aer , A. an d Guyomard, D. (2006) Electrochemical Synthesis of New Substituted Manganese Oxides for Lithium Battery Applications. Journal of Power Sources, 157, 443-447. http://dx.doi.org/10.1016/j.jpowsour.2005.07.035 [14] Feng, X.H., Zhai, L.M. , Tan, W.F., Liu, F. and He, J.Z. (2007) Adsorption and Redox Reactions of Heavy Metals on Synthesized Mn Oxide Minerals. Envir onm e ntal Polluti on , 147, 366-373. http://dx.doi.org/10.1016/j.envpol.2006.05.028 [15] Chowdhury, A.N., Azam, M.S., Aktaruzza man, M. and Rahim, A. (2009) Oxidative and Antibacterial Activity of Mn3O4. Journal of Hazardous Materials, 172, 1229-1235. http://dx.doi.org/10.1016/j.jhazmat.2009.07.129 [16] Lesueur, H., C zer nichowski, A. and Chapelle, J. (1988) Dispositif de Génération de Plasmas Basse Température par Formation de Décharges Electriques Glissant es . French Patent No. 2639172. [17] Fridman, A., P etrousov, R., Chapelle, J., Carnier, L.M ., Czernichowski, A. , Lesueur, H. and Stevefelt, J. (1994) Modèle physique de l’arc glissant. Journal de Physique III, 4, 1449-1465. http://dx.doi.org/10.1051/jp3:1994213 [18] Benstaali , B., Moussa, D., Addou, A. and Brisset, J.L. (1998) Plasma Tre atment of Aqueous Solutes: Some Chemical Properties of a Gliding Arc in Humid Air. The European Physical Journal Applied Physics, 4, 171-179. http://dx.doi.org/10.1051/epjap:1998258 [19] Marouf-Khelifa, K., Abdelmalek, F., Khelifa, A., Belhadj, M. and Addou, A. (2006) Reduction of Nitrite by Sulfamic Acid and Sodium Azide from Aqueous Solutions Treated by Gliding Arc Discharge. Separation and Purification Technology, 50, 373-379. http://dx.doi.org/10.1016/j.seppur.2005.12.012 [20] Benstaali , B., Chéron, B.G., Addou, A. and Brisset, J.L. (1998) Plasma Treat ment o f Aqueou s Solutes: S o me Chemical Properties of a Gliding Arc in Humid Air. The European Physical Journal Applied Physics, 4, 939-944. [21] Benstaali , B., Boubert, P., Chéro n, B.G., Addou, A. and Brisset , J .L. (2002) Density and Rotational Temperatures Measurements of the NO and OH Radicals Produced by a Gliding Arc in Humid Air and Their Interaction with Aqueous Solutions. Plasma Chemistry and Pla sma Processing, 22, 553-571. http://dx.doi.org/10.1023/A:1021371529955 [22] Imamura, A. and H irao, K. (1979) A Molecular Orbital Approach to the Electrophilicity of H and OH Radicals. Bulle- tin of the Chem i c al Soc i e t y of Ja pan, 52, 287-292. http://dx.doi.org/10.1246/bcsj.52.287 [23] Marouf-Khelifa, K., Abd elmalek, F., Khelifa, A., Belhadj, M., Addou, A. and Br i sset, J.L. (2006) Reduction of Nitrite by Sulfamic Acid and Sodium Azide from Aqueous Solutions Treated by Gliding Arc Discharge. Separation and Puri-  W. Chouchene, N. Bellakhal fication Technology, 50, 373-379. http://dx.doi.org/10.1016/j.seppur.2005.12.012 [24] Larabi-Gruet, N., Peulon, S. and Lacroix, A. (2008) Studies of Electrodeposition from Mn(II) Species of Thin Layers of Birnessite onto Transparent Semiconductor. Electrochimica Acta, 53, 7281-7287. http://dx.doi.org/10.1016/j.electacta.2008.03.080 [25] Shin, J.Y., Buzgo, C.M. an d Cheney, M. A. (2000) Mechanochemical Degradation of Atrazine Adsorbed on Four Syn- theti c Manganese Oxides. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 172, 113-123. http://dx.doi.org/10.1016/S0927-7757(00)00574-4 [26] Barrett, K.A. and McBr ide, M. (2005) Oxidative Degradation of Glyphosate and Aminomethylphosphonate by Manganes e Oxide. Environmental Science & Technology, 39, 9223-9228. http://dx.doi.org/10.1021/es051342d [27] Zaied, M., Chutet, E., Peulon, S., Bellakhal, N., Desmazieres, B., Dachrao ui, M. and Chauss ee, A. (2011) Spontaneous Oxidative Degradation of Indigo Carmine by Thin Films of Birnessite Electrodeposited onto SnO2. Applied Catalysis B: Environmental, 107, 42-51. http://dx.doi.org/10.1016/j.apcatb.2011.06.035 [28] Li, H., Lee, L.S., Schul ze, D .G. an d Guest, C.A. (2003) Role of Soil Manganese i n the O xidat ion of Aromatic A mines. Envir onm e n t al Sc i e nc e & Tec hnology, 37, 2686-2693. http://dx.doi.org/10.1021/es0209518 [29] Zhang, H. and Huang, C.H. (2003) Oxidative Transformation of Triclosan and Chlorophene by Manganese Oxides. Envir onm e n t al Sc i e nc e & Tec hnology, 37, 2421-2430. http://dx.doi.org/10.1021/es026190q [30] Nowack, B. and Stone , A.T. (2003) Manganese-Catalyzed Degradation of Phosphonic Acids. Environmental Chemi- stry Lett er s, 1, 24-31. http://dx.doi.org/10.1007/s10311-002-0014-3 [31] Gaillot, A.C. (2002) Caractérisatio n structurale de la birnessite: Influence du protocole de synthèse. Universit é Joseph Fourier, Grenoble, 17-78. [32] Peulon, S., Barai ze, Q. and Chausse, A. (2007) Iron Compounds Electrodeposited onto a Transparent Semiconductor: Synthesis and Characterisation by UV-Vis Spectroscopy. Electrochimica Acta, 52, 7681-7688. http://dx.doi.org/10.1016/j.electacta.2006.12.084 [33] Peulon, S. and Lincot, D. (1998) Mechanistic Study of Cathodic Electrodeposition of Zinc Oxide and Zinc Hydro- xychloride Films from Oxygenated Aqueous Zinc Chloride Solutions. Journal of The Electrochemical Society, 145, 864-874. http://dx.doi.org/10.1149/1.1838359 [34] Ndjeri, M., Peulon, S., Schlegel, M.L. and Chausse, A. (2011) In Situ Grazing-Incidence X-Ray Diffraction during Electrodeposition of Birnessite Thin Films: Identification of Solid Precursors. Electrochemistry Communications, 13, 491-494. http://dx.doi.org/10.1016/j.elecom.2011.02.029 [35] Shin, J.Y. and Cheney, M.A. (2004) Abiotic Transformation of Atrazine in Aqueous Suspension of Four Synthetic Manganes e Oxides. Colloids and Surfaces A: Physic oc he m ic al and Engi ne e r i ng As pe c t s , 242, 85-92. http://dx.doi.org/10.1016/j.colsurfa.2004.04.061 [36] Kang, K.H., Lim, D.M. and Shin, H.S. (2008) A Novel Solution for Hydroxylated PAHs Removal by Oxidative Coupling Reaction Using Mn Oxide. Water Science and T echnology, 58, 171-178. http://dx.doi.org/10.2166/wst.2008.637 [37] Subramanian, V., Zhu, H. and Wei, B. (2006) Nanostructured MnO2: Hydrothermal Synthesis and Electrochemical Properties as a Supercapacitor Electrod e Material. Journal of Power Sources, 159, 361-364. http://dx.doi.org/10.1016/j.jpowsour.2006.04.012 [38] Joseph, J., Destaillats, H., Hung, H. and Hoffman, M. (2000) The Sonochemical Degradation of Azobenzene and Related Azo Dyes: Rates Enhancements via F enton ’s Reactions. The Journal of Physical Chemistry A, 104, 301-307. http://dx.doi.org/10.1021/jp992354m [39] Tanak, K., Padermole, K. and Hisanaga, T. (2000) Photocatalytic Degradation of Commercial Azo Dyes. Water Re- search, 34, 327-333. http://dx.doi.org/10.1016/S0043-1354(99)00093-7 [40] Chowdhury, A.N., Azam, M.S., Aktaruzza man, M. and Rahim, A. (2009) Oxidative and Antibacterial Activity of Mn3O4. Journal of Hazardous Materials, 172, 1229-1235. http://dx.doi.org/10.1016/j.jhazmat.2009.07.129 [41] Stone, A.T. and Morgan, J.J. (1984) Reduction and Dissolution of Manganese(III) and Manganese(IV) Oxides by Or- ganics. 1. Reaction with Hydroquinone. Environmental Science & Technology, 18, 450-456. http://dx.doi.org/10.1021/es00124a011
|