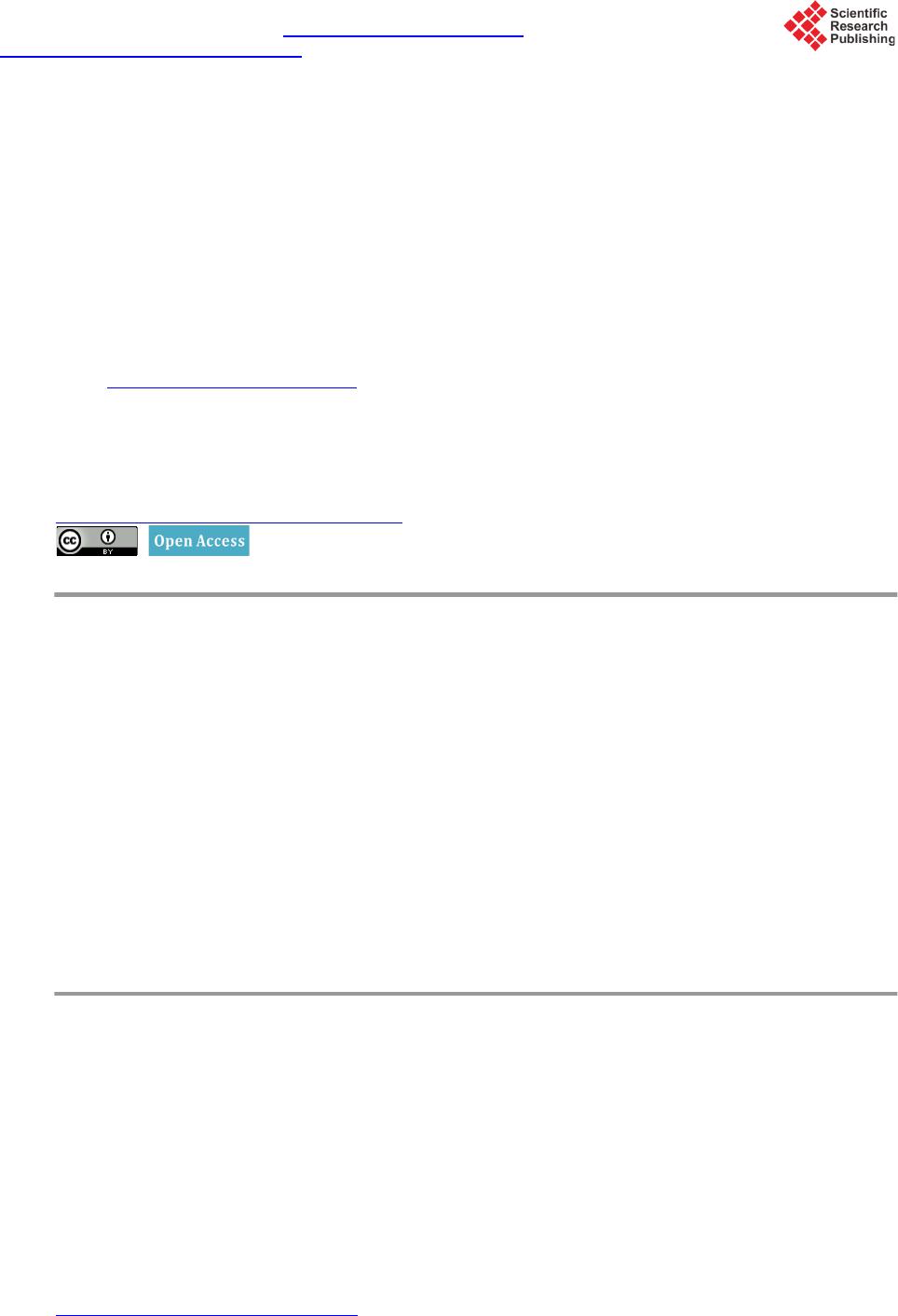
 Open Journal of Composite Materials, 2015, 5, 101-109 Published Online October 2015 in SciRes. http://www.scirp.org/journal/ojcm http://dx.doi.org/10.4236/ojcm.2015.54013 How to cite this paper: Todoroki, A., Sawada, T., Mizutani, Y. and Suzuki, Y. (2015) Supercapacitor Consisting of a Form Core Sandwich with Woven Carbon Fiber Skin. Open Journal of Composite Materials, 5, 101-109. http://dx.doi.org/10.4236/ojcm.2015.54013 Supercapacitor Consisting of a Form Core Sandwich with Woven Carbon Fiber Skin Akira Todoroki1, Tomohiro Sawada2, Yoshihiro Mizutani1, Yoshiro Suzuki1 1Department of Mechanical Sciences of Engineering, Tokyo Institute of Technology, Tokyo, Japan 2Tokyo Institute of Technology, Tokyo, Japan Email: atodorok@ginza.mes.titech.ac.jp Received 31 August 2015; accepted 4 October 2015; published 8 October 2015 Copyright © 2015 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Structural capacitors are composite structures that function as energy storage capacitors. Parallel plate-type capacitors have the advantage of high voltage resistance, but are limited by low capa- citance. An electric double-layer capacitor with a composite structure using a solid polymer elec- trolyte matrix with a glass fiber fabric separator has recently been developed. However, the solid polymer electrolyte caused the capacitor to possess high internal resistance. In the present study, a new design of supercapacitor using a form core sandwich with high water retention is proposed and experimentally investigated. Activated carbon sheets are used as electrodes on the form core sandwich to make a supercapacitor. Woven carbon fabric is used as lead wires of the supercapa- citor. The resulting supercapacitor displays a low surface resistance of 810 Ωcm2 and high areal capacitance of 520 mF/cm2. Keywords Composi tes, Woven Carbon Fabric, Capacitor, Supercapacitor, Sandwich, Form Core 1. Introduction Multifunctional composites have been developed to integrate the function of one structure with that of another for applications such as energy storage, antennas and sensors. Multifunctional composites make it possible to reduce the number of parts and required space of a system [1]. Structural capacitors are composite structures that behave as energ y storage capacitors. Luo and Chung [2] fabricated structural capacitors using a paper interlayer that exhibited an areal capacitance of 12 nF/cm2. Meanwhile, Lin and Sodano [3] made a cylindrical capacitor composed of piezoelectric material BaTiO3, which can be used to reinforce composites. Carlson et al. [4] [5] used a paper and polymer sheet as a dielectric separator in a capacitor, and obtained an areal capacitance of 25 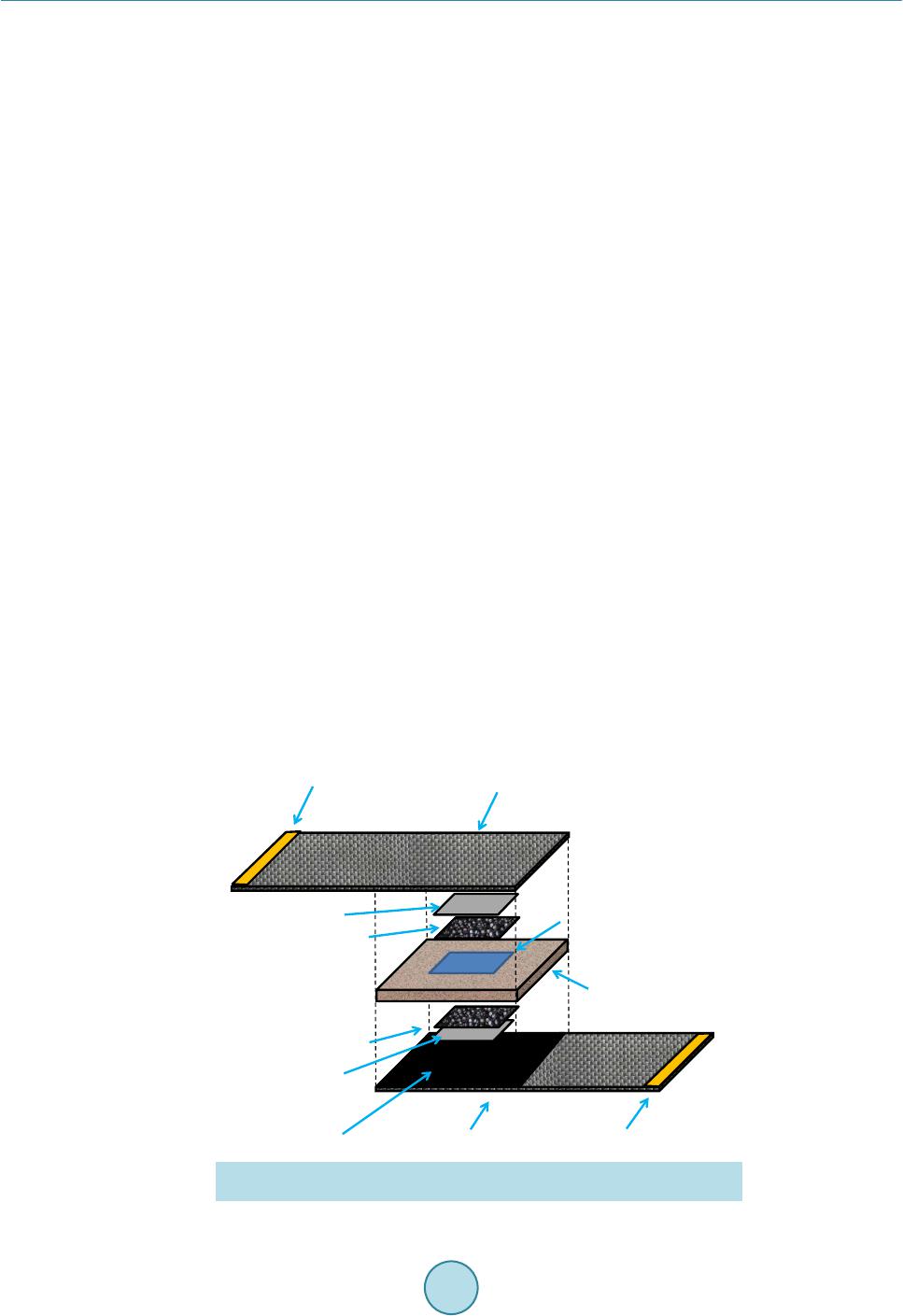 A. Todoroki et al. nF/cm2. O’Brien and colleagues also developed a parallel plate-type structural capacitor [6], which exhibited high voltage resistance but low capacitance. Shirshova et al. [7] [8] developed an electric double-layer capacitor (EDLC) or supercapacitor with a compo- site structure consisting of a solid polymer electrolyte matrix and glass fiber fabric separator. The areal capacit- ance of this supercapacitor was approximately 8.9 mF/cm2. Qian et al. [9] improved the supercapacitor using a carbon aerogel, obtaining an areal capacitance of 640 mF/cm2. Although the modified supercapacitor exhibited a higher areal capacitance by around the order of 1 F/cm2 than that without the carbon aerogel, its voltage resis- tance was smaller than that of a film capacitor. The voltage resistance of a supercapacitor is approximately 1 V, while that of a film capacitor is approximately 10 kV. The extremely high voltage charged to a capacitor is not safe for regeneration capacitors in automobiles during service because it may easily cause an electrical short if damaged. Therefore, a supercapacitor is preferable over a film capacitor for regenerated energy storage in auto- mobiles. In the structural supercapacitor developed by Shirshova et al. [7] [8], the solid polymer electrolyte caused the capacitor to possess high internal resistance and thus low power density. A new method to produce a supercapa- citor with lower internal resist ance is desired . One method to achieve this is to use liq uid electrol yte solution in- stead of a solid polymer electrolyte. However, using a liquid electrolyte solution is a considerable drawback from the structural viewpoint because it decreases the stiffness and strength of the structure. Recent develop- ment of supercapacitors is reviewed in the reference [10]. In the p r ese nt st udy, a ne w structur e c onsi sti ng o f a fo r m c o re sandwic h wit h hi gh wate r rete nti on i s p ro p o sed and the possibility of a supercapacitor with such a structure is experimentally investigated. Because activated carbon sheets are used as electrodes in the supercapacitor, the present approach is not strictly speaking a struc- tural capacitor. Woven carbon fabric is used simply as lead wires of the supercapacitor with a form core sand- wich structure. This new structure is expected to have lo w internal resistance and high capacitance. We examine the electric properties of the supercapacitor composed of a form core sandwich co mposite; its mechanical prop- erties will be evaluated elsewhere. 2. Concept of a Capacitor Composed of a Form Core Sandwich Composite The concept of the form core sandwich capacitor is depicted schematically in Figure 1. The skin of the sand- wich structure was a woven fabric of carbon fiber-reinforced polymer (CFRP). The woven CFRP sheets act as struc tural support and lead wires, as well as protecting the supercapacitor. The woven CFRP fabric was infused with epoxy resin. After curing, part of the CFRP surface was polished with sand paper to make an electric con- tact with the electrode. Figure 1. Schematic representation of a form-core capacitor for woven car- bon skin composites. Woven carbon cloth Polished area Copper tape electr ode Copper tape electr ode Woven carbon cloth Carbon tape Activated ca rbon film Carbon tape Activated ca rbon film Form core with r esin Form core with e lectr oly te  A. Todoroki et al. The electrodes of the supercapacitor must have a large surface area to realize large capacitance. Usually, elec- trodes are composed of materials like activated carbon or carbon nanotubes to realize large surface area. In the present research, activated carbon sheets were used as electrodes to minimize cost. To form an electrical contact with the woven CFRP fabric, double-sided carbon adhesive tape was used, as shown in Figure 1. The central square area o f the thin form core was immersed in liquid electrolyte solutio n. This immersed region was used as the supercapacitor. Because the liquid electrolyte solution and activated carbon electrodes are separated from the structural wo- ven CFRP fabric, this new supercapacitor system does not have problems from a structural point of vie w. I n ad- dition, because this system uses liquid electrolyte solution, the internal resistance of the capacitor is lower than that of a system using an electrolyte polymer matrix. This supercapacitor system should also have high capacit- ance without the drawbac k of structural degradation that affects film capacitors. The main design challenges facing this new supercapacitor system are to find an appropriate form core to re- tain the liquid electrolyte solution, deter mine how to seal up the capacitor in the form core sandwich structure, and establish ho w to include the liquid e lectrol yte solution in the for m core sand wich after it is made. T he cap a- citance of the new supercapacitor system is experi mentally measured in the present study. 3. Fabrication of the Form Core Sandwich Composite 3.1. Selection of a Form Core The new syste m need s to i ncl ude an op ti mum for m core t ha t has the ab ilit y to ho ld elec trol yte solutio n while re- taini ng io n cond uctivi ty in t he thr ough -thic kness d irecti on o f the fo rm. T hree t ypes o f form core materials were expe rime nt ally i nvestigat ed, a s s hown in Figure 2. The first form core was composed of polyvinyl chloride (Divinycell H80, BASF Japan Ltd, Tokyo, Japan) with a thickness of 4 mm (Figure 2(a)). The second form core was made of polymethacrylimide (Rohacell, IG51, Dicel-Evo nik Ltd, Tokyo, Ja pan) wit h a thickness o f 1 mm (F igure 2(b)). T he third form core was com- posed of polystyrene (moisture absorption type, Sekisui Plastic Co. Ltd, Osaka, Japan) with a thickness of 0.4 mm (Figure 2(c)). Both Divinycell and Rohacell are typically used in lightweight sandwich structures for air- craft. Both have high compressive strength and are commercially available in many countries as typical form core sandwich materials. The water-absorbent polystyrene form core is specially designed to keep water inside the form, and is typically used in trays to keep food warm in Japan. The commercially available polystyrene form had open cells on the top surface, while the botto m surface was closed to prevent leakage of water. In the present study, the solid polystyrene bottom surface was mechanically removed to obtain a water-permeable form core. To investigate the ability of the form cores to keep electrolyte solution inside them while retaining ion con- ductivity in the through-thickness direction, water colored with red ink was added dropwise on the surface of each form core. A uniform surface area with dimensions of 20 × 20 mm was first prepared using masking tape, (a) (b) (c) Figure 2. Three types of form used in the present study. (a) Polyvinyl chloride (Divinicell) form (4 mm thickness); (b) Polymethacrylimide ( Rohacell ) form (1 mm thickness); (c) Polystyrene (moisture absorp- tion type) form (0.4 mm thickness). 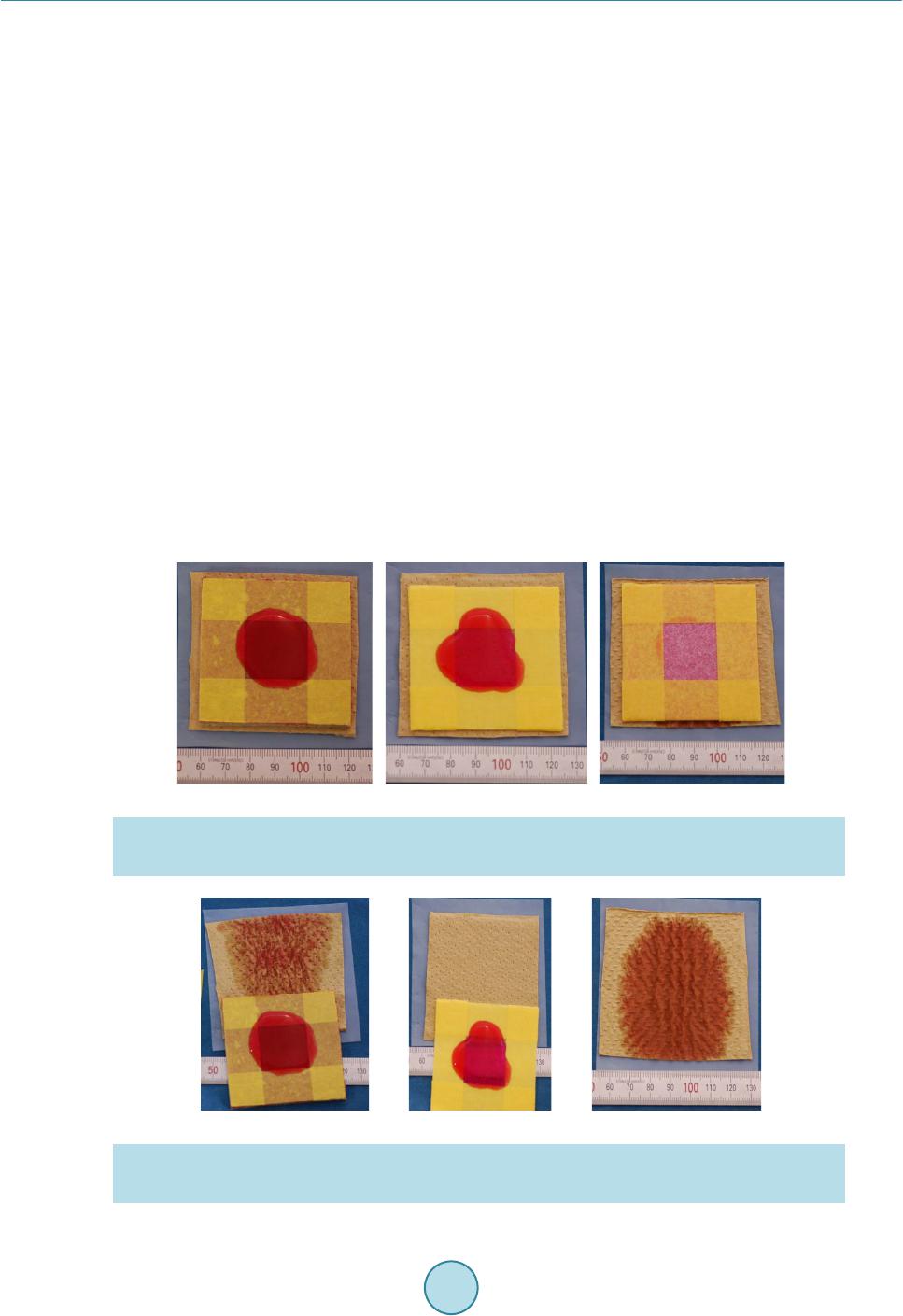 A. Todoroki et al. as illustrated in F igure 3. The surface of each form core after the addition of red ink is also displayed in Figure 3. The Divinycell had residual red ink on its top surface (Figure 3(a)), as did the Rohacell (Figure 3 (b)). In contrast, the po lyst yrene for m absorbed all of the red i nk, as shown i n F igure 3( c) . Figure 4 shows the ink leaked from the back surface of each form core onto a sheet of paper placed there. The Divinyc ell form core had some water permeability (Figure 4(a)), while the Rohacell was not water perme- able because the paper was completely dry (Figure 4 (b)). These indicate that these form cores are not approp ri- ate because the paths for ions are small number or there is no path. Meanwhile, Figure 4( c) reveals that the po- lystyrene form core was completely water permeable. These results show that the polystyrene form core is ap- propriate for keeping liquid electrolyte solution inside it when both surfaces are completely sealed. Moreover, the polystyrene form is very thin (0.4 mm thick), so we can make a thin sand wich panel using this polyst yrene form core. Therefore, we used the polystyrene form core to construct the s andwich st ruct ure in the pr esent s tudy. 3.2. Woven CFRP Cloth with an Electrically Conductive Surface In the supercapacitor developed in the present paper, a single ply woven CFRP cloth is u sed as lead wires. Be- cause the electrodes in the supercapacitor are activated carbon sheets, the woven CFRP cloth needs to exhibit electrical conductivity. To realize electrical contact to the carbon fibers, the surface resin of the woven CFRP composite must be removed. We polished the CFRP cloth with sandpaper to make electrodes as reported pre- viously [11]. The woven CFRP composite cloth plate with an area of 250 mm2 was made by resin infusion molding. The woven CFRP cloth (SA-3102, 13 μm thickness, Sakai Ovex Co. Ltd., Fukui, Japan) had a thickness of 0.013 mm. The epoxy resin (Z2/H02, GH Craft Ltd., Gotenba, Japan) was cured at room temperature. After curin g, sa mples of the woven CFRP plate that were 100 mm long and 50 mm wide were made. Half of each sample (50 × 50 mm) was polished using sandpaper to remove the surface resin. The removal of resin in (a) (b) (c) Figure 3. Water-holding capability test results (a drop of red ink is placed on the surface of three types of fo r m) . (a) Polyvinyl chloride (Divinicell) form (4 mm thickness); (b) Polymethacrylimide (Rohacell) form (1 mm thickness); (c) Polystyrene (moisture absorption type) form (0.4 mm thickness). (a) (b) (c) Figure 4 . Permeated water result s of the thr ee types of form core. (a) Polyvinyl chloride (Divinicell) form (4 mm thickness); (b) Polymethacrylimide (Rohacell) form (1 mm thickness); (c) Polystyrene (moisture ab- sorption type) form (0.4 mm thickness).  A. Todoroki et al. the polished area of the plate was confirmed using a digital microscope (KH-1300, HiroxInc, Tokyo Japan) as shown in Figure 5. In Figure 5, the right side is the unpolished area and the left side has been polished. The conductivity of the polished ar ea was confirmed using a LCR meter (3522, Hioki Co. Ltd., Japan). The other end of the woven CFRP plate was also polished and used as an electrode. Using copper tape, an electrode was made at the end of the woven CFRP plate . As shown i n Figure 1, double-sided carbo n adhesive tape with a thickness of 0.16 mm was attached onto the surface of the polished woven CFRP plate. T he other side of the carbon adhe- sive tape was attached to an activated carbon sheet (40 × 40 × 0.15 mm, Nippon Valqua Industries Ltd., Tokyo Japan), as illustrated in Fi gure 1. 3.3. Fabrication of the Supercapacitor Containing a Form Core Sandwich Composite A square polystyrene form core with sides of 60 mm was prepared. The polystyrene form core was 10 mm wider than the widt h of the woven CFRP plate. This was to prevent unexpected electrical shorting between the top and bottom woven CFRP plates. This extra width can be removed after fabrication of the supercapacitor. The center area of the polystyrene form core (40 × 4 0 mm) was le ft vacant to fill wit h liquid electr olyte sol u- tion after making the sandwich structure. To prevent leakage of the liquid electrolyte solution from the four sides of the form core, the surrounding polystyrene form core was infused with epoxy resin (Z2/HO2). The cen- tral vacant square of the polystyrene form core was aligned with the activated carbon sheet attached to the wo- ven CFRP plate, and the woven CFRP plate was bonded with epoxy resin, as shown in Figure 6. As the woven 0/90 fabric cloth has isotropic electric conductance, fiber direction is not important for the electrical point of view. In the present stud y, the fiber direction is aligned to the specimen longitudina l directio n and transverse d i- rection as shown in Figure 6. The dimension of the vacant area of the foam core should be opti mized with con- sidering mechanical properties. That is our future work. Another woven CFRP plate was similarly bonded to the other side of the polystyrene form core except for an opening left to fill the central vacant area of the polystyrene form core with liquid electrolyte solution. A sheet of polytetrafluoroethylene was used to make this opening, as depicted in Figure 7. The sample was sandwiched between aluminum plates and held using vices during curing. Figure 5. Surface observation of the polished area made to realize electric contact. Figure 6. Configuration of form core pre-preprocess to make a form- core capacitor. 0.5 mm Polished area Normal area Vacant area 40×40 mm 2 Normal CFRP area Copper tape Epoxy impregnated form core  A. Todoroki et al. Figure 7. Process to make insertion opening for electrolyte. There are several kinds of liquid electrolyte solution available to use in supercapacitors. Liquid organic elec- trolyte solutions possess higher voltage strength than liquid inorganic electrolyte solutions. This means that the total stored energy of the supercapacitor using an organic electrolyte solution is hi gher than that using an inor- ganic electro l yte solution. However, organic electrolyte solutions are easily degraded by mo isture. In the present study, a 10 wt% solution of co pper sulfate was used as the electr olyte to allo w easy handling d uring the fabric a- tion process. The solution of copper sulfate (approximately 640 mm3) was injected into the opening of the sam- ple, and t hen the opening was clos ed using epo xy resin (Z2 /HO2). 4. Measurement of the Capacitance of the Supercapacitor with a Form Core Sandwich Structure 4.1. Measurement Methods Two methods were used to measure the capacitance of the supercapacitor with a form core sandwich structure in the present study. The first was cyclic voltammetry, and the second was chronoamperometry. Cyclic voltamme- try uses constant rate voltage increases and decreases to determine capacitance by measuring the current during the voltage sweeps. The capacitance C(t) at time (t) can be calculated as follows, (1) where V(t) is the voltage at t, and I(t) is the electric current at t. Chronoamperometry uses a constant voltage and measures changes in electric current. This method requires a circuit model to identify parameters such as capacitance and internal resistance. In the present study, a circuit model including a capacitor and two resistors was used, as shown in Figure 8. Non-linea r parameter fitting was performed and the two resistors and capacitor were identified. The electric current of the circuit in Figure 8 can be calculated as follo ws [8], ( ) e 1e t t aa s sp VV It R RR ττ − − =+− + (2) (3) where Rs is the resistor connected in series and Rp is the resistor connected in parallel in Figure 8, and C is the capacitor. Cyclic voltammetry gives reliable capacitance measurements, while chronoamperometry allows in- ternal resistance to be determined. A potentiostat/galvanostat (Versastat 4, Princeton Applied Research, Oak Ridge USA) was used to measure the capacitance of the sample. In the present study, because the electrolyte was a solution of copper sulfate, the maximum voltage was s et to 0.5 V to pre vent insulation breakdown of the E DLC. 4.2. Results and Discussion Figure 9 shows the results obtained by cyclic voltammetry. The abscissa is the charged voltage and the ordinate Polytetrafluoro ethylene s heet (insertion opening for electrolyte) 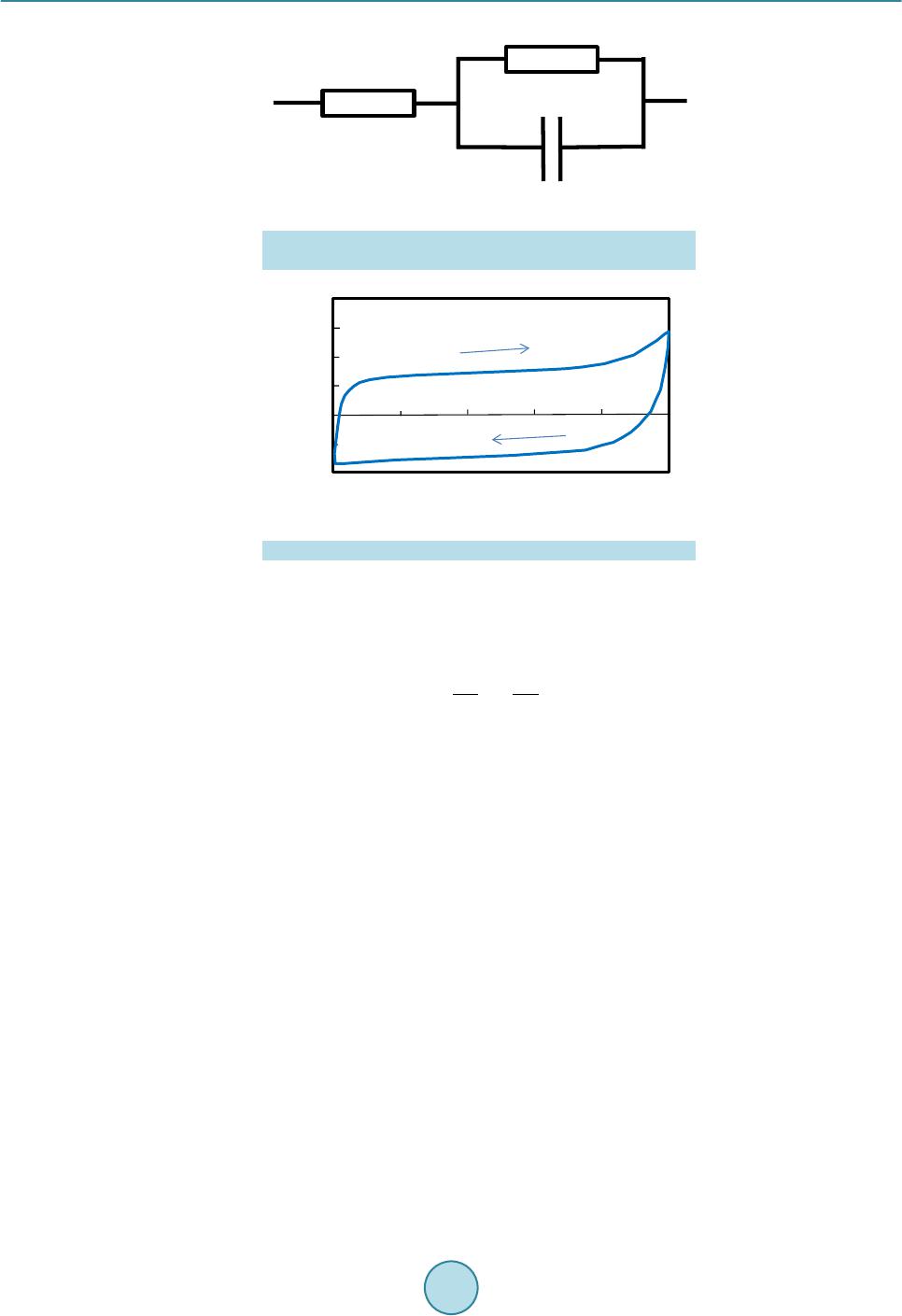 A. Todoroki et al. Figure 8. Circuit model to meas ure capacitan ce and resist ances for the chrono amperometry. Figure 9. M eas ured results using the cyclic volta mmetry. is the measured current. The voltage change rate was 0.1 mV/s, and three cycles were performed. The electric current-voltage curve is almost rectangular in shape. Let us consider the capacitance: (4) (5) where Q is electrical charge, V is voltage, C is capacitance, and I is electric current. Equation (5) sho ws that ca- pacitance can be easily calculated when the voltage change ratio is constant. In the present study, the capacit- ance was calculated using an increasing ti me period of voltage. T he measured capacitance was 3.1 mF. Because the area of the sample was 40 × 40 mm = 16 c m2, the areal capacitance is 520 mF/cm2, which is approximately 106 times higher tha n that of a film capacitor. This indicates that an EDLC has successfully been fabricated, al- though the capacitance is a little b it smaller than that achieved by Qian and co -wo rkers [9]. The maximum capa- citance of this structure can be improved using electrodes that have a larger surface area such as carbon nano- tubes [12] [13]. The adva nta ges of thi s ne w syst em ar e eas y ha ndli ng dur in g the fabr ica tion p ro cess a nd it s lig ht weight because of the form core. Figure 10 displays the results of chronoamperometry measurements. The abscissa shows time and the ordi- nate is measured electric current. In chronoampero metr y measurements, a step voltage of 0.5 V was applied for 3600 s. The blue curve is the measur ed r esults a nd the red curve shows the re sults o f cur ve fitt ing using M icro- soft Exce l to Equation (2). Rs is 50.7 Ω, and Rp is 490 Ω. The measured capacitance is 3.6 mF, which is consis- tent with the value of 3.1 mF measured by cyclic voltammetry. Because the curve fitting is poor at short t, the capacitance determined by chronoamperometry is not accurate. However, chronoamperometry gives relatively accurate resistances whe n the curve fitting is performed using a long t. Table 1 presents the energy density and power density of the supercapacitor with a form core sandwich structure. Because copper sulfate solution undergoes voltage strength around 1 V, the voltage limit was set to 0.8 V. The energy and power densities in Ta ble 1 are the converted values to 0.8 V. The electrode area is only 16 cm2, so the extremely high capacitance obtained indicates that the developed form core sandwich structure behaves as an EDLC. We intend to report the mechanical properties of this new system soon. 0.8 0.6 0.4 0.2 0 -0.2 -0.400.1 0.2 0.30.4 0.5 Voltage , V Current, mA  A. Todoroki et al. Figure 10. Measured resu lts using the ch ronoamperometry to measure capacitance. Table 1. Measured results of form-core su percapacit or composi tes (converted to 0.8 V). Are al capaci ta nce [mF/cm2] 520 Specific capacitance [mF/g] 845 Surface resistance [Ω/cm2] 810 Energy density [mWh/kg] 57.1 Peak power density [mW/kg] 563 5. Conclusions In the present study, a supercapacitor with a new form core sandwich composite structure is proposed. Because this method uses liquid electrolyte solution to make the EDLC, the internal resistance is small, and extremely high capacitance is obtained. T he results are summarized as follows. 1) A wa te r-absorbent polystyrene form core with a thickness of 0.4 mm is appropriate for keeping liquid elec- tro lyte solution in the sandwich struc ture. 2) The developed supercapacitor exhibits high capacitance compared with that of film-type structural capaci- tors, and realizes EDLC with small internal resistances. High areal capacitance of 520 mF/cm2 and low surface resistance of 810 Ωcm2 were obtained. References [1] Gibson, R.F. (2010) A Review of Recent Research on Mechanics of Multifunctional Composite Materials and Struc- tures. Composit e Str uc t ur e s , 92, 2793-2810. http://dx.doi.org/10.1016/j.compstruct.2010.05.003 [2] Luo, X.C. and Chung, D.D.L. (2001) Carbon-Fiber/Polymer-Matrix Composites as Capacitors. Composites Science and Technology, 61, 885-888. http://dx.doi.org/10.1016/S0266-3538(00)00166-4 [3] Lin, Y.R. and Sodano, H.A. (2009) Characterization of M ultifunctional Structural Capacitors for Embedded Energy Storage. Journal of Applied Physics, 106, Article ID: 114108. http://dx.doi.org/10.1063/1.3267482 [4] Carlson, T., Ordéus, D., Wysocki, M. and Asp, L.E. (2010) Structural Capacitor Materials Made from Carbon Fibre Epoxy Composites. Composites Science and Technology , 70, 1135-1140. http://dx.doi.org/10.1016/j.compscitech.2010.02.028 [5] Carlson, T., Ordéus, D., Wysocki, M. and Asp, L.E. (2011) CFRP S tructural Capacito r Materials f or Automotive Ap- plication. Plastics, Rubber and Composites, 40, 311 -316. http://dx.doi.org/10.1179/174328911X12948334590286 [6] O’Brien , D.J., Baechie, D.M. and Wetzel, E.D. (2011) Design and Performance of Mu ltifunctional Structural Compo- site Capacitors. Journal of Composite Materials, 45, 27 97-2809. http://dx.doi.org/10.1177/0021998311412207 [7] Sh irshova, N., Qian, H ., Shaffer, M.S.P., Steinke, J.H.G., Greenhalgh, E.S., Curtis, P.T., Kucernak, A. and Bismarck, A. (2013) Structural Composite Supercapacitor. Composites Part A: Applied Science and Manufacturing, 46, 96-107. http://dx.doi.org/10.1016/j.compositesa.2012.10.007 [8] Shirshova, N., Bismarck, A., Carreyette, S., Fontana, Q.P.V., Greenhalgh, E.S., Jacobsson, P., Johansson, P., Marc- zewski, M.J., Kalinka, G., Kucernak, A.R.J., Sheers, J., Shaffer M.S.P., Steinke E.S. and Wienrich, M. (2013) Struc- tural Supercapacitor Electrolytes Based on Bicontinuous Ionic Liquid-Epoxy Resin System. Journal of Materials Chemistry A, 1, 15300-15309. http://dx.doi.org/10.1039/c3ta13163g 14 12 10 8 6 4 2 001000 2000 3000 Time, s ec Electric cu rrent, m A Measured Curve-fit model  A. Todoroki et al. [9] Qian, H., Kucern ak, A.R.J., Greenh algh, E.S., Bismarck, A. and Shaffer, M.S.P. (2013) Multifunctional St ructural Su- percapacitor Composites Based on Carbon Aerogel Modified High Performance Carbon Fiber Fabric. Applied Mate- rials and Interfaces, 5, 6113-6122. [10] Asp, L.E. and Greenhalgh, E.S. (2014) Structural Power Composites. Composites Science and Technology, 101, 41-61. http://dx.doi.org/10.1016/j.compscitech.2014.06.020 [11] Todoroki, A., Suzuki, K., Mizutani, Y. and Matsuzaki, R. (2010) Durability Estimates of Copper Plated Electrodes for Self-sensing CFRP Composites. Journal of Solid Mechanics and Materials Engineering, 4, 610-620. http://dx.doi.org/10.1299/jmmp.4.610 [12] Futaba, D.N., Hata, K., Yamada, T., Hiraoka, T., Hayamizu, Y., Kakudate, Y., Tanaike, O., Hatori, H., Yumura, M. and Iijima, S. (2006) Shape-Engineerable and Highly Densely Pac ked Sin gle-Walled Carbon Nanotubes and Th ei r Ap- plication as Super-Capacitor Electrodes. Nature Materials, 5, 98 7-994. http://dx.doi.org/10.1038/nmat1782 [13] Yan, J., Wei, T., Shao, B., Ma, F., Fan, Z., Zhang, M., Zheng, C., Shang, Y., Qian, W. and Wei, F. (2010) Electro- chemical Properties of Graph ene Nanosheet/ Carbon Black Composit es as Electrodes for Supercapacitors. Carbon, 48, 1731-1737. http://dx.doi.org/10.1016/j.carbon.2010.01.014
|