Journal of Water Resource and Protection
Vol.6 No.4(2014), Article ID:44135,7 pages DOI:10.4236/jwarp.2014.64030
Preparation of Iron Ferrocyanide-Supported Nanofiber Membrane for Purification of Cesium-Contaminated Water
Yasuhito Mukai, Ayana Mizuno
Department of Chemical Engineering, Nagoya University, Nagoya, Japan
Email: mukai@nuce.nagoya-u.ac.jp
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 7 January 2014; revised 9 February 2014; accepted 4 March 2014
ABSTRACT
The earthquake in northeastern Japan that occurred on March 11, 2011 brought about the nuclear accident, resulting in the detection of radioactive cesium in soil and water over a wide region around Fukusihma. In this study, with the aim of the establishment of an effective method for removing cesium from water contaminated with cesium, the functionalized membrane with large cesium adsorption capacity per unit mass was prepared by combining nanofibers having a large specific surface area with iron ferrocyanide having a high selectivity for cesium adsorption. The nanofiber membrane made of polyacrylonitrile (PAN) was used as a base material of the functionalized membrane. Nanofiber membranes were immersed in the dispersions of iron ferrocyanide with various concentrations and pH values. After taking it out, it was dried at various temperatures and then non-immobilized iron ferrocyanide was completely removed through cleaning. As a result of the evaluation of completed affinity membranes, the amount of iron ferrocyanide immobilized by the nanofiber membrane increased significantly with the increase in the iron ferrocyanide concentration but subsequently showed a tendency to decrease rapidly, resulting in a distinct maximum at the iron ferrocyanide concentration of 3 wt%. And, the supported amount of iron ferrocyanide to the nanofiber membrane increased as pH became lower. Moreover, it was found that as high temperature as possible without exceeding the glass transition temperature of PAN was optimal as a drying temperature of prepared affinity membrane.
Keywords:Nanofiber Membrane; Iron Ferrocyanide; Cesium; Adsorption

1. Introduction
On March 11, 2011, a severe earthquake struck northeastern Japan. It caused the nuclear reactor accident, and consequently radioactive materials were released into the surrounding environment with the explosion of the nuclear reactor, and the seawater used for cooling of the nuclear reactor flew out into the sea with radioactive materials. As a result, a large amount of radioactive materials, mainly cesium 134 and cesium 137, dispersed over a large region around Fukushima and polluted the atmosphere, the forest, the ocean, the soil, and so on. Therefore, the introduction of technology to purify polluted environmental water and wastewater discharged after washing polluted soil is urgently needed. Currently various approaches including a zeolite adsorption method are being tried, but the definitive technology has not been established yet.
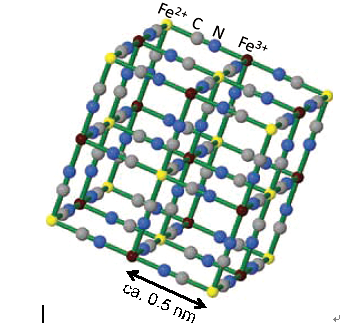
Meanwhile, iron ferrocyanide applicable to the removal of radioactive cesium from the contaminated water is now attracting attention as an alternative to conventional zeolite adsorption method [1] . Iron ferrocyanide has a unique 3D framework structure as illustrated in Figure 1, which can capture radioactive cesium ion selectively [2] . The most typical handling method of iron ferrocyanide is the direct addition to the contaminated water and subsequent coagulation-sedimentation. However, because this method is followed by solid-liquid separation, the handling becomes quite complex. Therefore, it is desirable from the standpoint of ease in handling to immobilize iron ferrocyanide.
For immobilization of iron ferrocyanide, the base material with larger specific surface area is required to minimize the amount of secondary waste containing radioactive materials. From this point of view, membrane-like nanofiber nonwoven cloth with extremely large specific surface area is one of the most promising base materials [3] . In recent years, the nanofiber membrane is being applied as a base material of the functionalized membrane [4] -[6] , and is being developed as a high-performance filter medium for water treatment [7] -[9] . Therefore, iron ferrocyanide-supported nanofiber membrane can be applied to not only adsorption of cesium but also filtration of colloidal or suspended particles onto which cesium is adsorbed.
In this study, with the aim of establishing an effective method for removing cesium from the contaminated water, the functionalized membrane having both large adsorption capacity and high selectivity for cesium ion was prepared by immobilizing iron ferrocyanide onto the surface of nanofibers. In order to provide a fundamental understanding, the preparation conditions for supporting as much iron ferrocyanide as possible on the surface of nanofibers were explored.
2. Materials and Methods
2.1. Materials for Adsorption
The adsorbent used was highly-dispersed iron ferrocyanide suspension supplied by Kanto Chemical Co. It is viscous and blue aqueous suspension containing 10% iron ferrocyanide particle with a diameter of 10 to 20 nm. Since it is a nanoparticle, it is appropriate for immobilization on the surface of nanofibers.
On the other hand, the adsorbate used for evaluation of the adsorption performance of iron ferrocyanide or prepared membrane was cesium chloride supplied by Kanto Chemical Co. Non-radioactive cesium chloride was used for experiment because the use of radioactive cesium was extremely hazardous. The cesium ion solution was prepared by adding cesium chloride to ultrapure deionized water with agitation.
Polyaluminum chloride (PAC) supplied by Taki Chemical Co. was used as a flocculant for iron ferrocyanide in the adsorption test. This is one of the most popular flocculants to be applied to such general water treatment as surface water treatment, effluent treatment and sludge dewatering because it has very high flocculation performance for various sorts of particles.
2.2. Nanofiber Membrane
The nanofiber membrane supplied by Japan Vilene Co. was used as a base material for immobilizing iron ferrocyanide. This membrane was made of polyacrylonitrile (PAN) and produced by an electrospinning method. Two kinds of nanofiber membranes with different fiber diameters of 220 and 411 nm and the same deposition weight per unit base area of 17 g/m2 were used. The specific surface area calculated from the fiber diameter and the density of PAN (1.17 g/cm3) was 15.5 m2/g for 220 nm fiber, and 8.32 m2/g for 411 nm fiber, respectively. An example of their SEM photographs is shown in Figure 2.
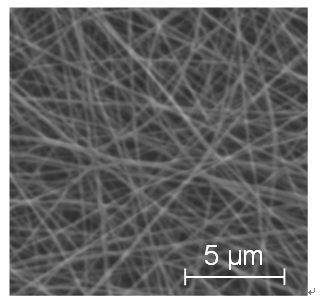
Figure 2. SEM photograph of nanofiber membrane used.
2.3. Cesium Adsorption Test of Iron Ferrocyanide
In order to evaluate the cesium adsorption performance of iron ferrocyanide, the static adsorption test was conducted by mixing various concentrations of cesium chloride and iron ferrocyanide. The iron ferrocyanide dispersion and the cesium chloride solution with twice the concentration of desired one were prepared by diluting them with ultrapure deionized water, respectively. By mixing the same amount of these two fluids with agitation of 300 rpm by a magnetic stirrer, the cesium adsorption test of iron ferrocyanide was started. After 30 minutes when the adsorption amount of cesium absolutely reached an equilibrium, dispersed iron ferrocyanide was flocculated by the addition of PAC to separate iron ferrocyanide from unadsorbed cesium solution. This mixture was centrifuged under 3300 rpm for 5 minutes and then the supernatant was sampled immediately. The concentration CCs* of cesium ion in the supernatant was measured by an atomic absorption spectrophotometer. The adsorption amount qCs of cesium to iron ferrocyanide was calculated from the difference between the cesium concentrations of solutions before and after the adsorption test.
2.4. Preparation of Iron Ferrocyanide-Supported Membrane
The nanofiber membrane was cut into 3 cm square and preweighed. Nanofiber membranes were immersed in the dispersions of iron ferrocyanide with various concentrations CIF and pH values in the petri dishes with the lid and shaked under 80 rpm for 2 hours. After taking them out, they were dried for 2 hours at various temperatures T and then non-immobilized iron ferrocyanide was completely removed through water washing and subsequent ultrasonic cleaning for 2 hours. Finally, the functionalized nanofiber membrane was completed after drying for 2 hours at 50˚C, and then its weight was measured to estimate the amount qIF of iron ferrocyanide immobilized by the nanofiber membrane from the difference from the previous weight. In this series of operations, the conditions that can immobilize more iron ferrocyanide on the surface of nanofibers were searched.
2.5. Cesium Adsorption Test of Prepared Membrane
Prepared iron ferrocyanide-supported nanofiber membranes were soaked in the cesium chloride solution and shaked under 80 rpm for 2 hours. The adsorption amount qCs of cesium onto the functionalized nanofiber membrane was obtained by measuring the cesium ion concentrations before and after the adsorption test, which also led to the evaluation of the amount of iron ferrocyanide immobilized by the nanofiber membrane.
3. Results and Discussion
3.1. Adsorption Isotherm of Iron Ferrocyanide
The adsorption amount qCs of cesium per unit mass of iron ferrocyanide measured by the static adsorption test is plotted against the equilibrium cesium concentration CCs* in Figure 3. This figure clearly demonstrates that the adsorption behavior can be described by the following Langmuir adsorption isotherm equation:
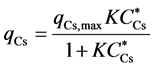 , (1)
, (1)
which indicates the saturated adsorption amount qCs,max of cesium to iron ferrocyanide is estimated to be 84 mg-Cs/g-IF.
The saturated cesium adsorption amount of generally-used zeolite has been reported to be approximately 200 mg-Cs/g-ZL, which is larger than that of iron ferrocyanide [1] . It is known, however, that the cesium selectivity of zeolaite decreases in such saline environment as seawater. Meanwhile, iron ferrocyanide keeps a stable cesium selectivity even in a solution containing a variety of ionic species [10] .
3.2. Effect of Dispersion Concentration and Fiber Diameter
For investigation of the effect of the concentration of iron ferrocyanide dispersion on the immobilization of iron ferrocyanide, the functionalized nanofiber membranes were prepared under various conditions of iron ferrocyanide concentration. In Figure 4, the photographs of prepared affinity membranes are shown. The membrane prepared at lower or higher iron ferrocyanide concentration turns a comparably pale blue color, while that at intermediate concentration is stained bright blue. This indicates the existence of the optimal iron ferrocyanide concentration.
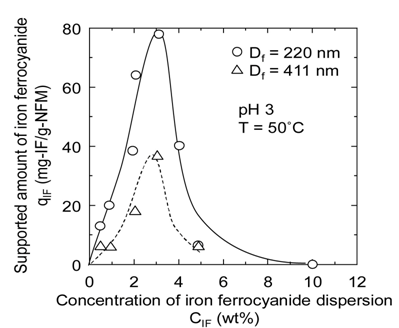
In Figure 5(a), the supported amount qIF of iron ferrocyanide per unit mass of the nanofiber membrane is shown against the initial concentration CIF of iron ferrocyanide dispersion used for preparation of the affinity membrane. In Figure 5(b), the adsorption amount qCs of cesium onto the affinity membrane is also shown against CIF. The initial concentration CCs,0 of cesium ion in the adsorption test was adjusted to 24 ppm. This concentration was determined in consideration of the measurement accuracy of the atomic absorption spectrophotometer used in this study even though it is much higher than the cesium concentration of real contaminated water. Both qIF and qCs increase significantly with the increase in CIF and subsequently show a tendency to decrease rapidly for any fiber diameter, resulting in both maximums of qIF and qCs at CIF of 3 wt%. This is because the increase in the viscosity of iron ferrocyanide dispersion and the aggregation of iron ferrocyanide occur under conditions of high concentration, and consequently iron ferrocyanide becomes impenetrable to the inside of the nanofiber membrane.
The nanofiber membrane with fiber diameter of 220 nm has about twice specific surface area of the nanofiber membrane with fiber diameter of 411 nm, and therefore the values of qIF of the former membrane were also about twice those of the latter membrane. However, the increase of qCs with a twofold increase in specific surface area was considerably less than twice. This may be attributed to the deactivation of iron ferrocyanide accompanying close contact between iron ferrocyanide particles. Thus, using thinner fibers is effective for attaining a higher adsorption performance of cesium, but further improvement of the supporting method of iron ferrocyanide onto nanofibers is required to make the most of their performance.
3.3. Effect of Dispersion pH
The effect of pH of the iron ferrocyanide dispersion on the preparation of the affinity membrane was investi-
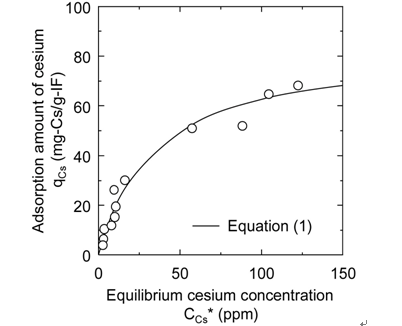
Figure 3. Adsorption isotherm of iron ferrocyanide.
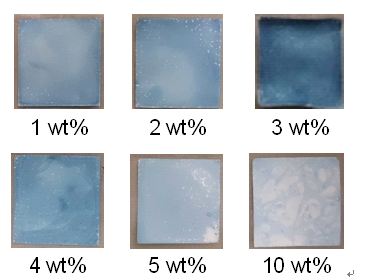
Figure 4. Photographs of prepared membranes.
 (a)
(a) 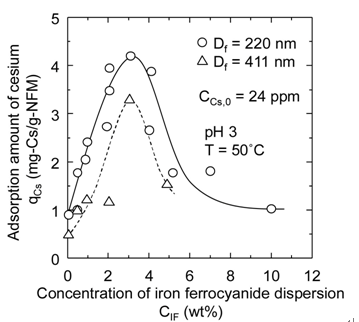 (b)
(b)
Figure 5. Effect of dispersion concentration on (a) qIF and (b) qCs.
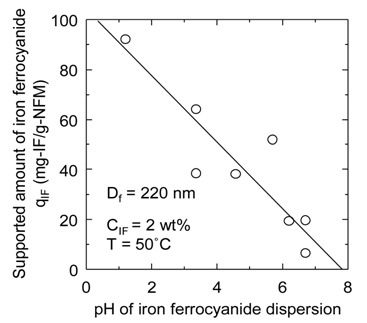
gated. In Figure 6(a), the supported amount qIF of iron ferrocyanide onto the nanofiber membrane is plotted against pH of the iron ferrocyanide dispersion used for preparation of the affinity membrane. In Figure 6(b), the adsorption amount qCs of cesium onto the affinity membrane is also plotted against pH. As indicated in these figures, both qIF and qCs increase as pH becomes lower. This is because the negative charge on the surface of PAN nanofibers, which interferes with the approach of iron ferrocyanide, is neutralized by hydrogen ions in an acidic atmosphere. On the other hand, in an alkaline atmosphere iron ferrocyanide is at risk of releasing toxic
 (a)
(a)  (b)
(b)
Figure 6. Effect of dispersion pH on (a) qIF and (b) qCs.
 (a)
(a) 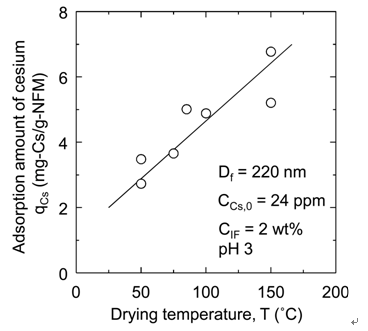 (b)
(b)
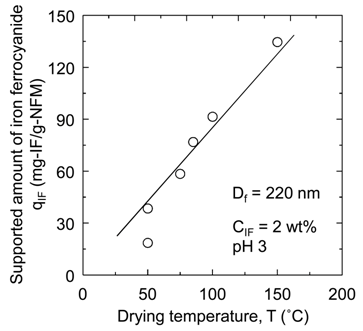
Figure 7. Effect of drying temperature on (a) qIF and (b) qCs.
and hazardous cyanide due to decomposition. Thus, it is more effective to lower the dispersion pH.
3.4. Effect of Drying Temperature
The affinity membranes were prepared at various drying temperatures (T = 50˚C to 150˚C) in the drying process after immersing a nanofiber membrane in the iron ferrocyanide dispersion. In Figure 7(a), the supported amount qIF of iron ferrocyanide onto the nanofiber membrane is shown against the drying temperature T. In Figure 7(b), the adsorption amount qCs of cesium onto the affinity membrane is also shown against the drying temperature T. It is clearly demonstrated that both qIF and qCs increase as the temperature rises. However, when the nanofiber membrane after soaking was dried at a temperature above the glass transition temperature 104˚C of PAN, the breakage of the nanofiber membrane occurred in subsequent washing process. Therefore, it is optimal to dry it at as high temperature as possible without exceeding this limit temperature.
4. Conclusions
In order to evaluate the cesium adsorption performance of iron ferrocyanide, the static adsorption tests were conducted by mixing various concentrations of non-radioactive cesium chloride and iron ferrocyanide. The adsorption behavior could be described by the Langmuir adsorption isotherm equation, which indicated the saturated adsorption amount of cesium to the iron ferrocyanide is 84 mg-Cs/g-IF.
The optimal conditions for immobilizing as much iron ferrocyanide as possible on the surface of nanofibers were explored to prepare the functionalized nanofiber membrane with higher cesium adsorption performance. As a result, it was found that the functionalized membrane containing more iron ferrocyanide could be prepared when the concentration of iron ferrocyanide dispersion was adjusted to 3 wt%, the nanofiber membrane composed of thinner fibers was employed, the pH environment of iron ferrocyanide dispersion was kept acidic, and the drying temperature was set to near 104˚C which is the glass transition temperature of PAN.
Acknowledgements
This work has been financially supported in part by the Kurita Water and Environment Foundation, and Tatematsu Foundation. In addition, the authors have received the valuable suggestions and guidance from Dr. Yoshihiro Yamashita at the University of Shiga Prefecture. The authors would like to acknowledge with sincere gratitude their supports leading to the publication of this article.
References
- Saito, K. (2012) Countermeasures against Radioactive Substances Following the Great East Japan Earthquake. NTS Inc., Tokyo.
- Yoshino, K. (2013) Prussian Blue: New Applications and Its Nanoparticles. The Chemical Times, 229, 23-27.
- Hongu, T. (2006) Nanofiber Technology. Nikkan Kogyo Shinbun Ltd., Tokyo.
- Ma, Z.W., Kotaki, M. and Ramakrishna, S. (2005) Electrospun Cellulose Nanofiber as Affinity Membrane. Journal of Membrane Science, 265, 115-123. http://dx.doi.org/10.1016/j.memsci.2005.04.044
- Teng, M., Li, F., Zhang, B. and Taha, A.A. (2011) Electrospun Cyclodextrin-Functionalized Mesoporous Polyvinyl Alcohol/SiO2 Nanofiber Membranes as a Highly Efficient Adsorbent for Indigo Carmine Dye. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 385, 229-234. http://dx.doi.org/10.1016/j.colsurfa.2011.06.020
- Li, X., Zhang, C., Zhao, R., Lu, X., Xu, X., Jia, X., Wang, C. and Li, L. (2013) Efficient Adsorption of Gold Ions from Aqueous Systems with Thioamide-Group Chelating Nanofiber Membranes. Chemical Engineering Journal, 229, 420-428. http://dx.doi.org/10.1016/j.cej.2013.06.022
- Mukai, Y. (2011) Preliminary Study to Apply Nanofiber Nonwoven Cloth as Filter Media for Water Treatment. Nanofiber, 2, 20-25.
- Yoon, K., Hsiao, B.S. and Chu, B. (2008) Functional Nanofibers for Environmental Applications. Journal of Materials Chemistry, 18, 5326-5334. http://dx.doi.org/10.1039/b804128h
- Feng, C., Khulbe, K.C., Matsuura, T., Tabe, S. and Ismail, A.F. (2013) Preparation and Characterization of Electrospun Nanofiber Membranes and Their Possible Applications in Water Treatment. Separation and Purification Technology, 102, 118-135. http://dx.doi.org/10.1016/j.seppur.2012.09.037
- Hiraoka, H. and Kamiya, D. (2013) Cesium Adsorbent Using Iron Ferrocyanide. Annual Research Report of Toagosei “TREND”, 16, 31-36.