Journal of Biomedical Science and Engineering
Vol.7 No.6(2014), Article ID:45894,7 pages DOI:10.4236/jbise.2014.76038
Regenerative Surgery for the Rehabilitation of a Patient after Surgery and Radiation Therapy for Head and Neck Cancer: A Case Report
M. Scala, A. Orsi, A. Rattaro, M. Trapasso, Susanna Polotto*, F. Spagnolo, P. Mereu, P. L. Santi
Department of Plastic Surgery, I.R.C.C.S. Azienda Ospedaliera Universitaria San Martino-IST, Genova, Italy
Email: *polotto.susanna@gmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 3 April 2014; revised 6 May 2014; accepted 14 May 2014
ABSTRACT
Oral cancer is usually treated combining surgery, radiation therapy and chemotherapy, following effective therapeutic protocols. Severe maxillary and mandibular bone atrophy resulting after therapies are usually treated with autologous bone grafting procedures even though these techniques often present a significant risk of postoperative complications and disadvantages. We describe the case of a 59-year-old woman presenting severe bone defect after being treated with surgery and radiotherapy for recurrent oral verrucous squamous cell carcinoma. We performed bone regeneration using Platelet-Derived Growth Factors (PDGF) in combination with autologous bone chips. Our procedure of bone regeneration allowed the placement of dental implants and the achievement of a good aesthetic and functional result. Regenerative surgery may enable the regeneration of substantial bone defects. Moreover, PDGF application decreases the risk of implant failure in irradiated patients.
Keywords:Regenerative Surgery, Radiation Therapy, Head and Neck, Squamous Cell Carcinoma, Platelet-Derived Growth Factors, Bone Chips

1. Introduction
Post-surgical bone defects are usually treated with autologous bone grafting procedures that may be considered the gold standard approach. However the need for a donor site, the surgical and immune-rejection risks and the unpredictable reabsorption rate represent some limitations to its use [1] -[3] .
Moreover, radiotherapy side effects may cause considerable complications in oral rehabilitation and dental occlusion restoration. In fact, while many patients are not able to bear the use of traditional removable prostheses, dental implants placement leads to a consistent patient’ quality of life improvement. Head and neck surgery and radiation therapy are not considered an absolute contraindication for dental implant positioning anymore, even though there is a significant difference in success rate between irradiated and non-irradiated patients [4] . In fact, successful osseointegration is significantly affected by total radiation dose: although there is no consensus in guidelines about dental implants positioning in irradiated patients, in literature it is stated that usually doses higher than 60 - 66 Gy are related to a higher implant failure rate, doses in the 50 - 60 Gy range do not contraindicate implantology and doses lower than 45 Gy are not associated with implant failure [4] -[6] . Our patient underwent 24 radiotherapy sessions with a 50 Gy range, thus comporting a symptomatic second degree mucositis (G2) pharmacologically treated, and severe maxillary and mandibular bone atrophy and maxillary sinus floor thinning. Bone radiation therapy leads to alteration of the function of osteoblasts and osteoclasts during bone repair and remodeling process and to the formation of hypovascularised and fibrotic tissue [7] . Major complications for dental implant placement in irradiated patients are delayed healing and osteoradionecrosis; moreover, in literature the relative risk of dental implant failure in irradiated bone is valued to be 2 or 3 times greater than that of non-irradiated ones [6] .
For this reason we tried a new surgical approach, in order to enable the regeneration of bone defects. In fact, three factors are needed to allow bone regeneration: a supporting structure (carrier), cells stimulated by growth factors (GF) and extracellular matrix material (ECM), i.e. signal molecules mediating the healing process [8] .
We performed bone reconstruction using PDGF, namely the Platelet Rich Plasma (PRP) or its gel form, the Platelet Gel (PG), in combination with autologous bone chips, as bone chips may be mixed with PDGF to become osteoinductive [9] .
We obtain PG from a single unit (450 cc) of patient’s whole blood with an easy and safe procedure [10] . The addition of cryoprecipitate, thrombin and excess of Ca++ to PRP triggers the coagulation with the PDGF released by the α-granules, through the promotion of mesenchymal stem cell recruitment and the initiation of cell proliferation, chemotaxis, and differentiation [11] [12] .
2. Case Report
We describe the case of a 59-year-old woman who underwent radical surgical excision of recurrent verrucous squamous cell carcinoma of the left cheek, which comported also the surgical removal of a block of alveolar process of left hemimandible starting from the second premolar to the second molar and a conservative neck dissection. The surgical treatment was completed by adjuvant radiation therapy.
Two years after surgery the patient showed severe maxillar bone atrophy with extreme bilateral thinning of the maxillary sinus floor associated with mandibular bone defect; as evidenced by orthopantomography (Figure 1) there was also a retraction of the left cheek that made the positioning of the dental implants impossible due to lack of space.
The patient had head and neck CT-scan and PET negative for malignancy so we decided to use regenerative surgery techniques to fill post-surgical and post-radiotherapy defects, with the ambitious goal to place a number of dental implants sufficient to allow a satisfactory functional and aesthetic rehabilitation.
The patient underwent left mandibular bone reconstruction using PG, thus allowing the positioning of three dental implants. Six months later we performed left maxillary sinus elevation with PG in combination with autologous bone chips; twelve months later again, we performed the right maxillary sinus elevation using the same surgical technique (Figure 2).
Finally, four dental implants were placed in the right hemimandible. One year later we performed a second left maxillary sinus elevation using PG: in fact the sinus was divided in two parts by a bone septum and required two surgical procedures to obtain an acceptable thickness. The biological sinus lift has allowed us to regenerate a block of bone measuring 10 mm by 10 mm within both maxillary sinuses. Three months later two dental implants were positioned in the left hemimaxilla and two in the right one (Figure 3).
Then, asubmucosal expander was positioned for three months in order to obtain the necessary space to settle both teeth’s crowns, given the scar retraction and fibrosis caused by tumor removal on the left side.
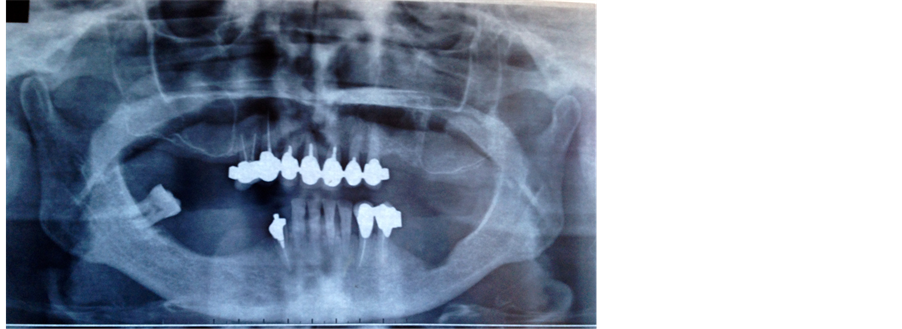
Figure 1. Orthopantomography shows severe maxillary atrophy with extreme bilateral thinning of the maxillary sinus floor associated with mandibular atrophy.
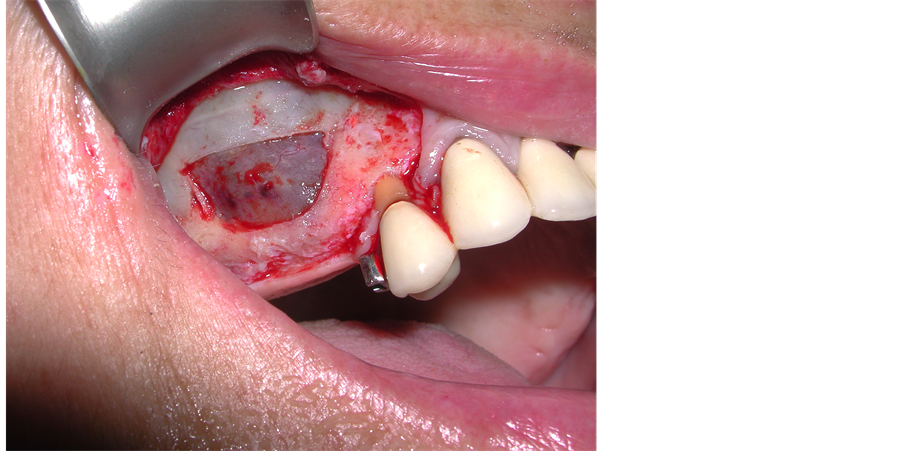
Figure 2. Bilateral maxillary sinus elevation with Platelet Gel in combination with bone autologous grafting.
At our follow up visit, three years later dental implant surgery, the patient is recurrence-free and all implants are correctly osseointe grated and functioning, with an optimal improving of patient quality of life (Figure 4).
3. Discussion
Head and neck cancer (HNC) is the 6th most common cancer in the world (6% of all cancer cases) and 8th most common cause of cancer’ death worldwide, including tumour localized in the oral cavity (40%), larynx (25%) and pharynx (15%). In 90% of the cases the histological type is squamous cell carcinoma of head and neck (HNSCC), that is tumour growing from epithelial cells of mouth, pharynx, larynx and nose [13] . Most common risk factors are smoking, alcohol abuse, oral tobacco, betel nut, pan or gutkha chewing, wrong alimentation, Ebstein-Barr Virus (EBV) or Human Papilloma Virus (HPV) 16 and 18 infection. Mostly men and patients >55 years are more subjected to HNC [14] [15] . A multi-modality treatment is frequently required in HNSCC, consisting of surgery, radiation therapy (RT), chemotherapy and photodynamic therapy, depending on tumour stage [16] . A fundamental thing to consider in the beginning is HPV presence in tumour cells as it is one of the most important independent prognostic factor in HNSCC; it is present in 26% of all HNSCC. In fact, HPV infection is
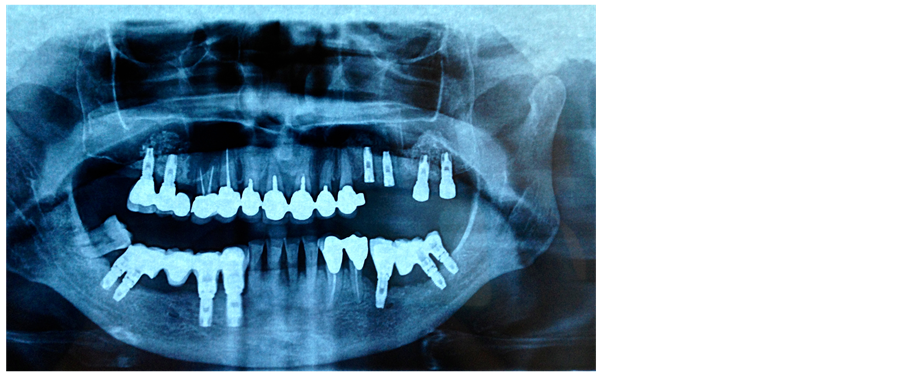
Figure 3. Orthopantomography shows the placement of dental implants and the achievement of a good functional result.
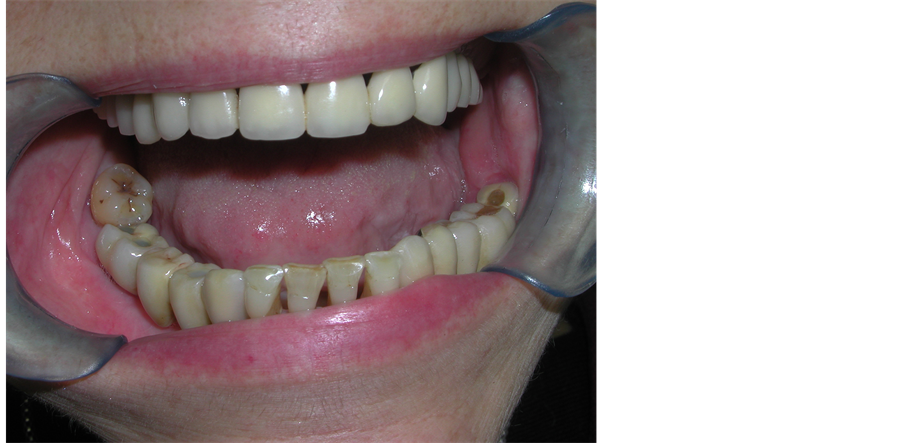
Figure 4. Three-year post-operative follow up result after implant positioning.
associated with less aggressive tumour, higher survival rate, better loco-regional control and lower second primary cancer rates or recurrence. It basically means that it should be necessary to intensify the treatment and introduce new chemotherapic agents for induction therapy for HPV negative patients [14] . At an early stage, HNSCC are mostly treated with surgery associated to postoperative radiotherapy (standard treatment consists of 3 - 7 weeks, 5 days a week), with a high percentage of survival and low recurrence rate, depending on its location [17] . Improvement in non-surgical treatment has led to less invasive and organ-conserving surgery, with a consisting enhancement in speech, swallowing and preservation of other functions proper of the region involved in tumour growth. Unfortunately, about 60% of patients with HNSCC are diagnosed with stages III and IV (locally advanced) [18] ; without any treatment the median survival is <4 months, for this reason combined treatment with surgery, radiotherapy and chemotherapy is required. Chemotherapy may be applied as neo-adjuvant therapy, in association with radiotherapy, as adjuvant therapy after surgery or palliative therapy in inoperable tumours. For inoperable local-regional advanced HNSCC chemo-radiotherapy is the standard of care which allows an organ conserving management [19] ; an alternative is a chemotherapy alone, mostly with platinum-based agent or with fluorouracil (5-FU). In particular chemo-radiotherapy withcis-platinum leads to higher complete remission rates (65% - 70%) than other protocols and platinum-based concurrent chemoradiotherapy improve patient survival of 8% - 11% compared to radiotherapy alone, even if it comports higher toxicity [18] . Some recent clinical studies comparing organ-sparing radiation therapy associated or not to chemotherapy underline that patients’ quality of life is worst in the group treated with chemo-radiotherapy, especially for some long-term life-altering side effects of radiotherapy as prostration, nausea, cough, dysphagia, cutaneous irritation, alopecia, pharyngitis, aphonia, decrease in hunger, taste and smell, disphagia, osteoradionecrosis, soft tissue fibrosis, xerostomia and speech problems [19] . Chemotherapy side effects are mostly nausea and vomiting, diarrea, dermatitis, mucositis, anemia, hypoalbuminemia, neutropenia, neurotoxicity, nephrotoxicity and myelosupression [18] [19] . Because of these important side effects of radiotherapy and chemotherapy, these treatments cannot be applied as standard treatment for many patients with comorbidity, as they comport delays in irradiation protocols, reduction in radiation doses or discontinuation in chemotherapy. When the Gross Tumour Volume (GTV) is irradiated to a radical dose which is typically 70 Gray (Gy), the Planning Target Volume (PTV) which includes the free margin beyond the gross disease to irradiate, ranges from 0 to 3 - 5 mm, to a maximum of 1.5 - 2 cm, according to several clinical studies, even if the long term morbidity related to the irradiation of large volume of non-involved tissue beyond GTV, especially in HPV-positive patients, leads to a shrinkage of PTV and to intensify the radiation dose completely within the GTV, thanks to brachytherapy or stereotactic body radiotherapy techniques. Moreover, when possible, given the benefit in preserving as much salivary tissue as possible, radiation therapy with contralateral parotid sparing is routine in lymph-node negative neck and it is important to consider the risk-benefit ratio of the omolateral parotid preservation. As micro-metastases require relatively low doses of irradiation for complete sterilization, concurrent chemotherapy has a radiosensiting effect and there is a relatively low risk of metastases in contralateral neck, it is possible to apply conservative dosing strategy (≤56 GY) in uninvolved nodal regions, sparing salivary glands and swallowing structures. Moreover, Intensity Modulated Radiation Therapy (IMRT) is a notable improvement towards standard radiotherapy treatments, as it is an highly conformal technique which allows organ sparing, ensuring at the same time an adequate radiation dose to target structures. Whole neck IMRT has become more commonly used than split-field IMRT, as it preserves low anterior neck (LAN) field, leading to lower swallowing and other side effects due to larynx and pharyngeal constrictors irradiation. Also dose-painting and Simultaneous Integrated Boost (SIB) has improved patients’ quality of life [20] . The development of target therapies led to the combination of radiotherapy and cetuximab, an IgG1 chimeric monoclonal antibody which binds specifically to EGFR (Epithelial Growth Factor Receptor), a target molecule which is over expressed in 80% - 90% of HNSCC. Its expression increases as precancerous lesions progress to invasive cancer, with tumour size, stage, progression, risk recurrence increase, RT low sensivity and poor prognosis [21] [22] . It is the only molecular-targeting agent approved to be used in combination with chemotherapy in recurrent or metastatic HNSCC, with radiotherapy in loco-regionally advanced onesor as monotherapy for recurrent/metastatic HNSCC that has become resistant to chemotherapy [22] [23] . It significantly improves survival rates and loco-regional control when compared with radiotherapy alone, but there is no clinical study proving his efficacy in comparison with standard chemotherapy. Also photodynamic therapy (PDT) can be applied to early staged tumours or as palliative therapy; its main side effects are photosensibilization, pain, difficult in swallowing and bleeding. For the treatment of metastatic/recurrent HNSCC, standard therapy is multidisciplinary approach: mainly chemotherapy associated or not with cetuximab. Therapeutic options and prognosis greatly depends on site, extension and previous treatment. Surgery represent the first option when possible (local recurrence) as it is generally the only curative option for patients with recurrent upper aerodigestive tract HNSCC. Factors associated with poor outcomes are positive surgical margins, advanced initial and recurrent tumour stage, lack of disease-free interval following previous treatment, presence of concomitant recurrent neck disease. This treatment is mostly associated with significant patient morbidity and poor long-term survival; for these reasons an accurate patients’ selection is fundamental. Patients with metastatic disease are not usually offered salvage surgery, unless they have isolated metastasis: in this case they can undergo metastasectomy. If patients have not been previously radiotreated, radiotherapy after surgery, often applied to patients with positive surgical margins and regional lymph node extracapsular disease extension, can be applied. On the contrary, re-irradiation involves a high percentage of morbidity and mortality (33% and 10%) withoutany demonstrable effect on overall survival. In alternative, successful savage can be obtained with chemoembolization, metastasectomy or palliative chemotherapy; for example in patients with isolated metastases in the lung or liver stereotactic irradiationor laparoscopic surgical resection is possible, for isolated liver metastases chemoembolization or radiofrequency ablation is also possible [15] [23] .
4. Conclusion
Regenerative surgery may represent a valid alternative to enable the regeneration of bone defects and manage particularly challenging clinical issues, especially in head and neck cancer surgery. As these techniques are minimally invasive, guaranteeing a good quality of life is particularly useful when there is a shortage of donor tissue.
Disclosure
M. Scala, A. Orsi, A. Rattaro, M. Trapasso, S. Polotto, F. Spagnolo, P. Mereu, P. L. Santi do not have any financial interest in any of the products, devices, or drugs mentioned in this article.
References
- Farré-Guasch, E., Prins, H.J., Overman, J.R., Ten Bruggenkate, C.M., Schulten, E.A., Helder, M.N. and Klein-Nulend, J. (2012) Human Maxillary Sinus Floor Elevation as a Model for Bone Regeneration. Enabling the Application of OneStep Surgical Procedures. Tissue Engineering Part B Review.
- Schmitt, C., Karasholi, T., Lutz, R., Wiltfang, J., Neukam, F.W. and Schlegel, K.A. (2012) Long-Term Changes in Graft Height after Maxillary Sinus Augmentation, Onlay Bone Grafting, and Combination of Both Techniques: A Long-Term Retrospective Cohort Study. Clinical Oral Implants Research.
- De Oliveira, E., Silva, M., Pelegrine, A.A., Alves Pinheiro da Silva, A., Manhães Júnior, L.R., de Mello, E., Oliveira, R., Gaiba França, S., Aloise, A.C. and Ferreira, L.M. (2012) Xenograft Enriched with Autologous Bone Marrow in Inlay Reconstructions: A Tomographic and Histomorphometric Study in Rabbit Calvaria. International Journal of Biomaterials. http://dx.doi.org/10.1155/2012/170520
- Carini, F., Pisapia, V., Monai, D., Barbano, L. and Porcaro, G. (2012) Implant Rehabilitation in Patients Irradiated for Head and Neck Cancer: Role of Intensity-Moduled Radiotherapy (IMRT) in Planning the Insertion Site. Annali di Stomatologia (Roma), 3, 8-20.
- Alsaadi, G., Quirynen, M., Komarek, A. and van Steenberghe, D. (2008) Impact of Local and Systemic Factors on the Incidence of Late Oral Implant Loss. Clinical Oral Implants Research, 19, 670-676.
- Tanaka, T.I., Chan, H.L., Tindle, D.I., MacEachern, M. and Oh, T.J. (2012) Updated Clinical Considerations for Dental Implant Therapy in Irradiated Head and Neck Cancer Patients. Journal of Prosthodontics, 22, 432-438. http://dx.doi.org/10.1111/jopr.12028
- Chen, H., Liu, N., Xu, X., Qu, X. and Lu, E. (2013) Smoking, Radiotherapy, Diabetes and Osteoporosis as Risk Factors for Dental Implant Failure: A Meta-Analysis. PloS One, 8, 8.
- De Girolamo, L., Sartori, M.F., Arrigoni, E., Rimondini, L., Albisetti, W., Weinstein, R.L. and Brini, A.T. (2008) Human Adipose-Derived Stem Cells as Future Tools in Tissue Regeneration: Osteogenic Differentiation and Cell-Scaffold Interaction. International Journal of Artif Organs, 31, 467-479.
- Scala, M., Gipponi, M.,Pasetti, S., Dellachà, E., Ligorio, M., Villa, G., Margarino, G., Giannini, G. and Strada, P. (2007) Clinical Applications of Autologous Cryoplatelet Gel for the Reconstruction of the Maxillary Sinus. A New Approach for the Treatment of Chronic Oro-sinusal Fistula. In Vivo, 21, 541-547.
- Dominiak, M., Lysiak-Drwal, K., Solski, L., Zywicka, B., Rybak, Z. and Gedrange, T. (2012) Evaluation of Healing Processes of Intraosseous Defects with and without Guided Bone Regeneration and Platelet Rich Plasma. An Animal Study. Annals of Anatomy, 194, 549-555.
- Scala, M., Spagnolo, F., Trapasso, M., Strada, P., Moresco, L. and Santi, P.L. (2012) Association of Vacuum-Assisted Closure and Platelet Gel for the Definitive Surgical Repair of an Enterocutaneous Fistula: A Case Report. In Vivo, 26, 147-150.
- Müller, A.M., Mehrkens, A., Schäfer, D.J., Jaquiery, C., Güven, S., Lehmicke, M., Martinetti, R., Farhadi, I., Jakob, M., Scherberich, A. and Martin, I. (2010) Towards an Intraoperative Engineering of Osteogenic and Vasculogenic Grafts from the Stromal Vascular Fraction of Human Adipose Tissue. European Cells and Materials, 19, 127-135.
- Oliveira, K.G., Von Zeidler, S.V., Podestà, J.R.V., Sena, A., Souza, E.D., Lenzi, J., Bissoli, N.S. and Gouvea, S.A. (2014) Influence of Pain Severity on the Quality of Life in Patients with Head and Neck Cancer before Antineoplastic Therapy. BMC Cancer, 14, 39. http://dx.doi.org/10.1186/1471-2407-14-39
- Ramshankar, V. and Krishnamurthy, A. (2012) Human Papilloma Virus in Head and Neck Cancers-Role and Relevance in Clinical Management. Indian Journal of Surgical Oncology, 4, 59-66. http://dx.doi.org/10.1007/s13193-012-0196-5
- Deeken, J.F., Newkirk, K., Harter, K.W., Marshall, M.B., Banovac, F., Johnson, L., Wang, H., Wang, Y., Zhuang, T., Jay, A.K., Berkowitz, F., Esposito, G., Kallakury, B. and Davidson, B. (2014) Multimodality Treatment for Patients with Metastatic/Recurrent HPV-Positive Head and Neck Squamous Cell Carcinoma May Lead to Significant Improvement in Overall Survival.
- Balermpas, P., Bauer, C., Fraunholz, I., Ottinger, A., Wagenblast, J., Stöver, T., Seitz, O., Fokas, E., Rödel, C. and Weiss, C. (2014) Concomitant Chemoradiotherapy versus Induction Chemotherapy Followed by Radiotherapy as Definitive, First Line Treatment of Squamous Cell Carcinoma of the Head and Neck. Strahlentherapie und Onkologie.
- Mak, M.P. and Glisson, B.S. (2014) Is There Still a Role for Induction Chemotherapy in Locally Advanced Head and Neck Cancer? Current Opinion in Oncology, 26.
- Schlumpf, M., Fischer, C., Naehrig, D., Rochlitz, C. and Buess, M. (2013) Results of Concurrent Radio-Chemotherapy for the Treatment of Head and Neck Squamous Cell Carcinoma in Everyday Clinical Practice with Special Reference to Early Mortality. BMC Cancer, 13, 610. http://dx.doi.org/10.1186/1471-2407-13-610
- Klein, J., Livergant, J. and Ringash, J. (2014) Health Related Quality of Life in Head and Neck Cancer Treated with Radiation Therapy with or without Chemotherapy. A Systematic Review. Oral Oncology, 50, 254-262. http://dx.doi.org/10.1016/j.oraloncology.2014.01.015
- Dandekar, V., Morgan, T., Turian, J., Fidler, M.J., Showel, J., Nielsen, T., Coleman, J., Diaz, A. and Sher, D.J. (2014) Patterns-of-Failure after Helical Tomotherapy-Based Chemoradiotherapy for Head and Neck Cancer: Implications for CTV Margin, Elective Nodal Dose and Bilateral Parotid Sparing. Oral Oncology, in Press. http://dx.doi.org/10.1016/j.oraloncology.2014.02.009
- Yokota, T. (2013) Is Biomarker Research Advancing in the Era of Personalized Medicine for Head and Neck Cancer? International Journal of Oncology, 19, 211-219.
- Fujii, M. (2014) Recent Multidisciplinary Approach with Molecular Targeted Drugs for Advanced Head and Neck Cancer. International Journal of Oncology, 19, 220-229.
NOTES

*Corresponding author.

