Journal of Cancer Therapy
Vol.5 No.3(2014), Article ID:44114,17 pages DOI:10.4236/jct.2014.53034
Skin-Sparing Mastectomy and Breast Reconstruction: An Update for Clinical Practice
Abdul Kasem, Christina Choy, Kefah Mokbel*
The London Breast Institute, The Princess Grace Hospital, London, UK
Email: *kefahmokbel@hotmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 20 January 2014; revised 19 February 2014; accepted 27 February 2014
ABSTRACT
Aim: To provide an up-to-date review of the literature on skin-sparing mastectomy (SSM) for breast cancer (BC). The article also reviews the oncological safety, effects of radiotherapy (RT) on immediate breast reconstruction (IBR), the indications for preserving the nipple-areola complex (NAC) and the emerging role of allogenic grafts as adjuncts to implant in IBR. Methods: Review of the English literature from 1965 to 2013 was carried out using Medline and PubMed research engines. Results: SSM is oncologically safe in appropriately selected cases of invasive breast cancer (IBC) and ductal carcinoma in-situ (DCIS) including IBC < 5 cm, multi-centric tumours, DCIS and for risk-reduction surgery. Inflammatory breast cancer and tumours with extensive skin involvement represent contra-indications to SSM due to an unacceptable risk of local recurrence. Prior breast irradiation or the need for post-mastectomy radiotherapy (PMRT) do not preclude SSM with IBR, however the aesthetic outcome may be compromised by radiation. Preservation of the nippleareola complex (NAC) has aesthetic and psychological benefits and is safe for peripherally located node negative unifocal tumours. An intraoperative frozen section protocol for the retro-areolar tissue should be performed when NAC preservation is considered. The advent of acellular dermal matrix has enhanced the scope of implant-based immediate reconstruction following SSM. Cell-assisted fat transfer is emerging as a promising technique to optimise the aesthetics outcome. There is no sufficient evidence to support the role of endoscopic mastectomy in clinical practice. Conclusion: Numerous retrospective and prospective studies show that SSM is oncolgically safe in appropriately selected cases and is aesthetically superior to non-SSM mastectomy. New techniques such as the use of acellular dermal matrix (ADM) and cell-assisted fat transfer have increased the use of implants for volume replacement following SSM. In the absence of randomized clinical trials, an updated systematic meta-analysis of published studies is required in order to consolidate the evidence.
Keywords:Skin-Sparing Mastectomy; Breast Reconstruction

1. Introduction
Breast-conserving surgery with adjuvant radiotherapy is a safe alternative to mastectomy for the majority of women with early breast cancer (BC) [1] . However, up to one third of patients require a mastectomy for large or multi-focal tumours (particularly where breast conservation would lead to a poor cosmetic outcome), local recurrence after previous radiotherapy and patient preference [2] .
The primary aim of surgical treatment of breast cancer is to achieve local control of disease with a good aesthetic result and to provide prognostic information in order to guide adjuvant treatment recommendations. For patients who require or opt to have mastectomy for breast cancer, breast reconstruction improves cosmesis and minimizes the psychological impact of mastectomy. However the optimal timing weather is immediate or delay breast reconstruction remains controversial with guidance from level-1 evidence lacking. Delay breast reconstruction may result in a poorly contoured breast, with prominent scars and a paddle of skin that is of a different colour and texture [3] . Delayed reconstruction mandates at least a second surgical procedure.
Skin-sparing mastectomy (SSM), a type of mastectomy where incisions are planned to maximise skin preservation and facilitate breast reconstruction, was described by Toth and Lappert in 1991 [4] . SSM involving enbloc removal of the breast gland, nipple-areola complex (NAC), may also include previous biopsy sites and the skin overlying superficial tumours. The native breast skin envelope and infra-mammary fold are preserved [5] . Using the native skin envelope optimises the final contour of the reconstructed breast reducing the need for contralateral breast adjustment in order to achieve symmetry. Scarring and donor skin requirements (e.g. in flap based reconstructions) are minimised [6] . A combined oncological and reconstructive approach is acceptable to patients, cost-effective and reduces the number of hospital admissions and time away from home or work [7] . This approach has been advocated as an effective treatment option for patients with early-stage BC and DCIS which is not amenable to breast conserving therapy. SSM and IBR have also been shown to be particularly valuable in patients who develop breast cancer following augmentation mammoplasty who might otherwise have a poor cosmetic result with breast conserving surgery and adjuvant radiotherapy. SSM and IBR can allow the omission of adjuvant RT in most cases and are associated with an excellent cosmetic outcome [8] .
Acceptance and popularity of SSM and IBR are increasing amongst patients and many surgeons. A 2008 survey of Californian surgeons performing surgery for breast cancer has demonstrated a change in attitudes towards SSM. Ninety per cent of those surveys were satisfied with the oncological adequacy of the technique in early breast-cancer and 70% in agreement that the cosmetic results of SSM with IBR are superior to standard mastectomy [9] .
However the flaps of the skin envelop of breast may contain glandular breast tissue and may harbour residual disease thus raising questions regarding the oncological safety of SSM. There has been no single randomised controlled trial comparing oncological outcome of SSM versus non-SSM and such trials are not currently feasible due to ethical and methodological considerations.
The evidence on oncological adequacy of SSM will be presented in this article. In addition to survival and the risk of local recurrence (LR), post-operative morbidity, local control, cosmesis, patient satisfaction and an assessment of functional disturbance and psychological morbidity are important outcome measures [10] .
Controversies including the impact of radiotherapy (RT) on IBR, preservation of the nipple-areola complex (NAC) and the role of endoscopic mastectomy are reviewed. The use of allogenic biological grafts (such as Alloderm®, DermaMatrix®,Permacol®, SurgiMend®, Allomax®, FlexHD®, DermaCell®, Veritas®, and Strattice®) in immediate reconstruction using sub-pectoral implants is also discussed.
2. Search Methodology
Articles were identified by searches of MEDLINE and PubMed up to July 2013 using the terms: “Skin Sparing Mastectomy” or “Subcutaneous Mastectomy” or “Nipple Sparing Mastectomy” or “Areola Sparing mastectomy” or “Endoscopic Mastectomy” and “Breast Cancer” or “DCIS” and “Oncological Safety” or “Aesthetic” or “Cosmesis” and “Morbidity” or “Mortality” and “Sentinel Lymph Node Biopsy” or “Radiotherapy” and “Evidence” and “Recurrence”. Studies identified were screened for those that focused on Skin Sparing Mastectomy. All randomized controlled trials and large retrospective series were included. The reference articles in this review were selected to provide a balanced and representative overview of a complex subject with an extensive base of published work.
3. Surgical Considerations
The management of women with IBC after matectomy should take place within the context of a multidisciplinary team. Techniques such as SSM and IBR are demanding and should be undertaken by breast surgeons with oncoplastic training or as a joint procedure between a breast and a plastic surgeon. Oncological principles should not be compromised. The incisions used to perform SSM will be influenced by various factors including breast size and the degree of ptosis, NAC size, tumour location, position of biopsy sites, need for axillary intervention, reconstructive technique and the preference of the patient and the surgeon. Many patients are suitable for a peri-areolar approach where the only breast skin excised is the NAC [5] [11] (Figure 1). If the NAC is small and the breast is large, a peri-areolar incision with lateral and medial extensions or a larger ellipse including the NAC can be used. Wise-pattern incisions, akin to those used for reduction mammoplasty, can be required for large breasts with moderate/severe ptosis [12] . If necessary, symmetry may be achieved by performing a simultaneous, or delayed, contralateral reduction mammoplasty or mastopexy. NAC-preserving SSM can be performed through an inframammary, infero-lateral or periareolar (with or without medial and lateral extensions) incisions.
Some surgeons advocate subcutaneous injection of saline-adrenaline solution (1:25,000) when performing SSM. This is haemostatic and results in some hydro-dissection of the plane between the subcutaneous tissue and the mammary gland. Skin flaps are carefully elevated at the plane separating the subcutaneous fat from the underlying breast tissue and the dissection is extended to the edges of the breast tissue circumferentially, preserving the infra-mammary fold [13] . High intensity fibre-optic light sources and longer instruments can be useful during this stage as access could be limited. The parenchyma can then be mobilised by dissecting the pectoral fascia from the underlying musculature. In order to protect the viability of the preserved envelope, skin flaps must be sufficiently thick to maintain the sub-dermal vascular plexus and yet without leaving breast tissue behind. Care is required to limit excessive use of electro-cautery and flap margins should be assessed for bleeding. Native skin flap necrosis (partial or complete) has been estimated to occur in 11% of cases, which is similar to conventional mastectomy [14] .
Sentinel lymph node biopsy (SLNB) and/or axillary node clearance (ANC) can be performed safely through the same incision [6] [11] [15] . In larger breasts an additional axillary incision will sometimes be required [3] . SLNB should be performed in clinically node negative patients with IBC and high-risk DCIS. Many surgeons perform intra-operative assessment of the sentinel node (frozen section, imprint cytology or one step nucleic acid amplification—OSNA) and perform simultaneous ANC if the SNLB is positive. However, intra-operative analysis can be associated with false negatives and ANC is more difficult to perform as a second operation following IBR, with potential harm to the vascular pedicle and viability of a flap-based reconstructed breast [16] [17] .
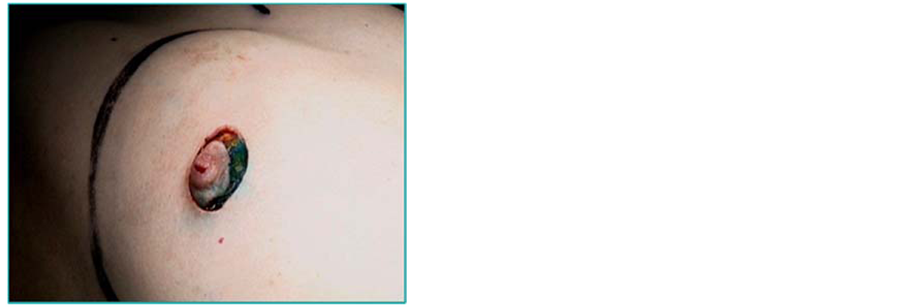
Figure 1. The incision of SSM.
It should be noted however that the false negatives associated with the SLNB are usually due to the presence of micrometastases or isolated tumour cells. This minimal nodal disease does not usually require ANC. An alternative approach is to perform a day-case sentinel node biopsy one or two weeks beforehand so that nodal status is accurately established prior to SSM and IBR [18] .
Endoscopic video-assisted surgery has been successfully employed for a range of aesthetic and plastic surgery procedures including those relating to the breast. The technique has been extended to the treatment of both benign and malignant breast conditions [19] . Yamashita et al. have described the use of a 2.5 cm axillary incision and skin traction to create a working space, allowing partial or total glandular resection and SLNB+/−ANC to be successfully performed. Operating times and blood loss were comparable to open surgery and all surgical margins were clear. The conversion rate to open surgery was low. Wounds healed with minimal scarring and the approach was associated with high levels of patient satisfaction [20] . Similarly, small peri-areolar incisions can be made to facilitate endoscopic breast conserving surgery for early-stage IBC [21] [22] . Some studies have also reported immediate reconstruction with endoscopic harvest of the latissimusdorsi (LD) flap [23] or endoscopic creating pocket for prosthetic implant [24] . Kitamura et al. compared patients undergoing SSM and IBR, using an implant, with or without endoscopic assistance. The addition of endoscopy was associated with a longer operating time but significantly smaller scars and greater patient satisfaction [25] . It is noteworthy that these reports stem from relatively small studies from single institution and superiority over conventional techniques has not been demonstrated. Furthermore, it is critical that the surgical margins and oncological outcomes need to be examined carefully in the context of endoscopic mastectomy.
4. Reconstructive Options Following SSM
The options of various reconstructive techniques following SSM require careful consideration of several patient related factors and tumour related factors. Patient factors include breast size, ptosis, areola size, patient preference and expectation, general health and smoking status. Tumour related factors include size, location and proximity to the NAC. Preference and experience of the operating surgeon is also taken into account. Occasionally patients may decline any form of reconstruction after mastectomy. Sometimes the prognosis may be so poor that any breast reconstruction is unwarranted [26] . For those who are suitable and choose to have breast reconstruction, prosthetic implants and/or autologous tissue or fat could be used. First is to reconstruction the breast mound and then concerns about the nipple and areola complex could be addressed [27] .
4.1. Prosthetic Implants
Conventional mastectomy is associated with significant loss of the native skin envelope, complicating implant only reconstruction. It is therefore necessary to use an expandable implant or a tissue expander and to exchange this at a second operation once the remaining skin has stretched to accommodate the desired fixed volume implant. Although expandable implants are still commonly used, the advent of SSM has meant that implant-based reconstruction can now often be performed in a single stage procedure, or if a two stage procedure is needed, the number of inflations can be minimised. The implant is positioned in a sub-muscular pocket superiorly and in a sub-fascial pocket inferiorly. The results of this approach in terms of breast volume, shape and symmetry are favourable with complication rates as low as 8.3% [28] . A prospective study by Woerdeman et al. reported 400 implant only reconstructions following SSM, identifying patient related risk factors associated with complications. The study recommended particular caution in obese patients who smoke (32% loss of implant) and in large breasted women (27% loss of implant) [29] . In a separate study, the same authors found patient age and experience of the operating surgeon to be associated with a greater risk of post-operative complications [30] .
4.2. The Role of Acellular Dermal Matrix
Acellular dermal matrix (ADM) products such as Alloderm®, DermaMatrix®, Permacol®, SurgiMend®, Allomax®, FlexHD®, DermaCell®, Veritas®, and Strattice® have recently emerged as useful adjuncts in implantbased reconstruction following SSM [31] . These products are derived from the human dermis (Alloderm®, FlexHD®, Allomax®, DermaCell®), porcine dermis (Strattice®), bovine dermis (SurgiMend®) or bovine pericardium (Veritas®) through special processing procedures which remove cellular contents and leave a sterile acellular matrix of natural biological components that promotes rapid revascularization and cell repopulation. ADMs have been shown to incorporate well into tissues with no rejection and their use seems to be associated with a lower risk of capsule formation but a higher incidence of seromas and infection [32] . The various ADM products vary in their physical properties (such as tensile strength, thickness, and elasticity), incorporation rate, associated inflammatory reactions, the need for orientation and antibiotics impregnation. The ideal ADM does not exist. Comparisons between the various types of ADM on clinical efficacy, complications and costs are required to determine the best available ADM to use in clinical practice [31] . In the USA, Alloderm is currently the most widely used in breast surgery with a very low incidence of inflammatory reactions. Based on their experience, the authors of this review article prefer to use SurgiMend due to its favourable physical properties and good handling, high incorporation rate and extremely low incidence of inflammatory reactions (Figure 2).
ADMs are sutured to the lower edge of the pectoralis major muscle superiorly and to the infra-mammary fold inferiorly thus providing cover and support to the lower pole of the implant. This approach allows the use of a larger implant volume, improves lower pole projection and facilitates faster tissue expansion. Although prior or post-mastectomy radiation decreases the extent of incorporation into the host tissues due to reduced neovascularisation, however RT does not preclude the use of ADM in breast reconstruction and the authors of this article prefer using the ADM prior to RT. Often this group of patients also required adjuvant chemotherapy. Hence there is time during the chemotherapy to allow adequate incorporation of ADM before the blood supply is compromised by RT (Figure 3).
A recent study which compared use of ADM versus conventional sub-pectoral implant, revealed significantly larger initial fill volumes and fewer sessions required for inflation with no differences in complication rates between the two groups [33] . A non-randomised large series of 361 women undergoing implant with or without ADM did not reveal any differences in infection rates between two groups [34] . However, ADM was associated with adherence to skin in all cases, particularly after RT.
More recently a titanium-coated polypropylene mesh (TCPM) has been introduced as a biocompatible synthetic alternative to biological ADMs [35] . This relatively cheaper type of matrix (TiLoop® Bra) is unsuitable for cases with thin skin and subcutaneous tissue.
In women with large breasts undergoing reduction pattern SSM, a de-epithelialised dermal flap (Autoderm) can be used to cover the lower pole of the implant thus providing a valid alternative to allogenic grafts with an acceptable complications rate [36] .
4.3. Autologous Flaps
The LD flap, introduced by Schneider in 1977, was once the standard for autologous breast reconstruction [37] . A variety of other flaps and prosthetic materials are now available but the LD myocutaneous flap is still in common usage. The LD is often combined with an implant for use in a single-stage procedure, where it serves to improve coverage of the prosthesis and improve the final breast contour [38] (Figure 4).
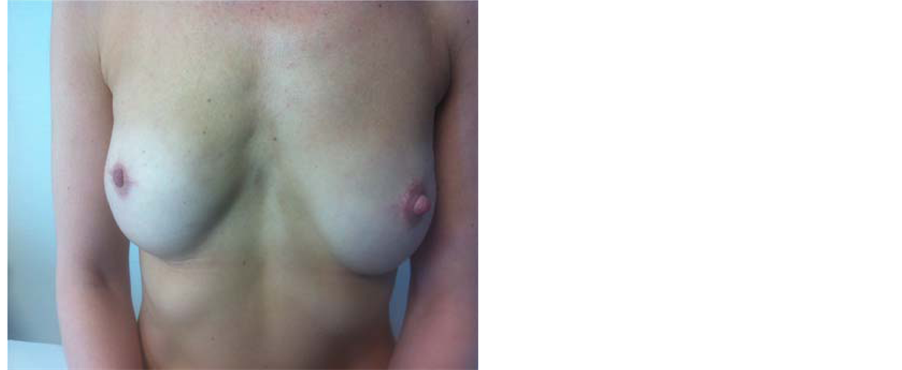
Figure 2. This 45 years old woman underwent bilateral SSM and immediate breast reconstruction using an implant and acellular dermal matrix.
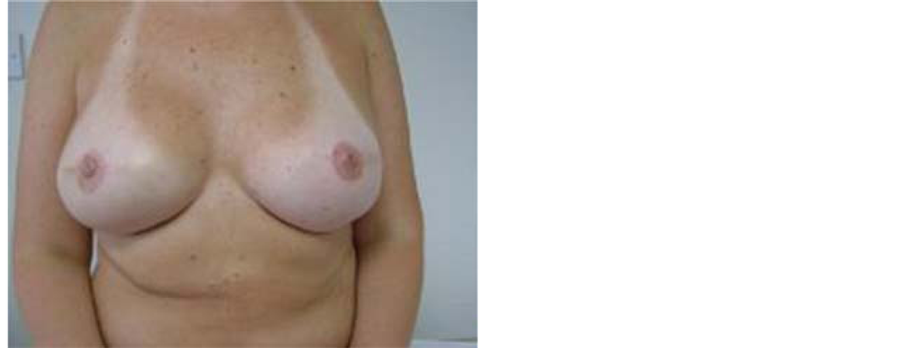
Figure 3. Bilateral SSM and immediate implant based reconstruction with subsequent nipple reconstruction (using local flaps) in a 47 years old woman (a BRCA2 gene carrier with bilateral breast cancers). The patient also had adjuvant chemotherapy and radiotherapy to the right breast.
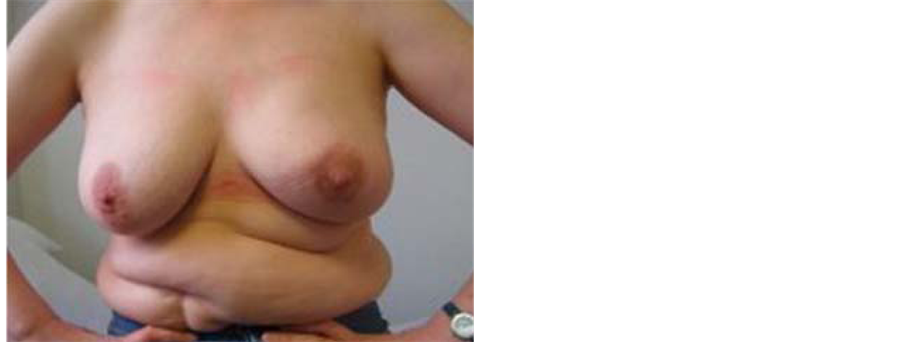
Figure 4. This 50 years old lady underwent left SSM and immediate breast reconstruction using the LD flap and an implant. She subsequently had nipple reconstruction using a local flap and micropigmentation.
The TRAM (Transverse Rectus Abdominus Myocutaneous flap) [39] can be used either as a local pedicle or a free tissue transfer flap [40] . In one study, comparing 85 patients undergoing TRAMs, implant-based or LD reconstructions, the mobility and consistency of the reconstructed breast are more closely resembled the natural breast in patients with TRAM flaps [41] . Patients with TRAM flap reconstruction also report high levels of satisfaction with good aesthetic result [42] . However, TRAM flap reconstruction causes bulging at the lower inner part of the breast and can be associated with donor site complications such as abdominal wall weakness and has been largely replaced by muscle-sparing free flaps such as the deep inferior epigastric perforator (DIEP) flap derived from the abdominal wall.
The DIEP flap, described by Holmstrom [43] and popularised by Blondeel [44] , allows large volume tissue transfer with donor site advantages over the TRAM flap. Preservation of total abdominal musculature and aponeurotic layers reduces complications such as muscular weakness, asymmetry, bulging and hernias [45] . The DIEP and superficial inferior epigastric artery (SIEA) flaps are able to transfer the same tissue from the abdomen as the TRAM flap without sacrificing the rectus muscle or fascia [27] . The neo-breast consists primarily of fat and skin like the native organ and furthermore patients benefit from an improved abdominal contour. However, the procedure can be technically demanding and the limited surgical access associated with SSM can cause difficulties in the identification and dissection of recipient vessels.
In a review of a series of 30 breast reconstructions with DIEP flap, with an average follow-up of 29 months, breast skin complications were noted in 2 patients manifesting as small areas of necrosis. Partial flap losses were observed in 2 patients representing less than 15% of the total area. There was only one case of total flap loss and one case of local recurrence during the follow-up period. Patients reported high levels of satisfaction with the outcome [46] . In a study of 42 patients, SSM and IBR using the DIEP flap was compared to breast conservation and found to be associated with comparable quality of life and significantly better cosmetic outcome [47] .
The use of soft tissue from the gluteal region as a free flap was introduced by Fujino et al. in 1975 [48] . In 1995, the free superior gluteal artery perforator (S-GAP) flap was used for autologous breast reconstruction [49] . This allowed free transfer of skin and fat without sacrifice of the gluteus maximus, thereby minimising donor site complications. In addition it is sometimes possible to anastomose nerves, harvested with the flap, to local branches at the recipient site and provide limited sensation to the flap [44] . This approach is particularly useful when the abdomen is not suitable nor preferable as a donor site. In a study of 142 GAP flaps, the superior-GAP flap was found to have significant advantages over the inferior-GAP flap. Guerra et al reported low morbidity, 98% flap survival, good cosmetic results and high levels of patient satisfaction [50] .
Several other free flaps have been described for autologous breast reconstruction including the myocutaneous transverse upper gracilis free flap (TUG) [51] [52] . There are also reports in the literature of free omental flaps, harvested laparoscopically, being used for IBR following SSM [53] .
Myocutaneous flaps could allow replacement of the small area of excised skin if required during SSM and aim to replace the approximate the total volume of breast tissue removed with or without the assistance of a prosthetic implant.
In a survey of 453 female plastic surgeons from USA and Canada questioned on which method of reconstruction they would choose if they had breast cancer, 66% preferred implant based over autologous breast reconstruction [54] .
4.4. The Role of Fat Transfer
Autologous fat transfer has recently gained increasing popularity as an adjunct technique to reconstructive breast surgery [55] . Autologous fat can be used as whole fat or processed, in order to enhance the numbers of regenerative adipose cells in the harvested fat prior to injecting it into the breast. The processing techniques include, saline washing, centrifugation and/or enzymatic degradation and aims to reduce the incidence of fat necrosis and reabsorption. Serial lipofilling seems to be a safe and effective technique that can improve the contours of the reconstructed breast and create a subcutaneous plane in the irradiated breast, thus reducing implant related complications, such as capsule formation [56] . Asymmetric defects can be also corrected using lipomodelling. However, the published studies are relatively small and have a short clinical follow-up duration. Therefore, larger studies, with longer clinical follow-up, are required in order to determine the long-term efficacy and safety of this technique.
5. Oncological Considerations
The main oncological concern in SSM is that residual breast tissue within the skin envelope may manifest later as LR. Histological studies following conventional mastectomy have confirmed the presence of residual glandular tissue in 5% of all biopsies taken from the operating field [57] . Torresan et al. performed histological analysis of skin sparing mastectomy flaps from patients with invasive breast cancer who were marked up for SSM and then had conventional mastectomy performed and found the prevalence of residual breast tissue to be 59.5% and residual disease to be 9.5% in the portion of the specimen that would have been left in-situ had the patient undergone SSM. The presence of breast tissue and residual disease was significantly associated with skin flaps thicker than 5 mm [58] [59] .
Ho et al. performed histological examinations of the skin and subcutaneous tissue of 30 conventional mastectomy specimens and found that the skin flaps (excluding the NAC) were involved in 23% (7 of 30) of cases. In 5 cases, the skin involved was situated directly over the tumour. Significant risk factors for this were skin tethering, large tumour size and peri-neural infiltration [60] . Despite this, the incidence of LR following SSM for IBC has been investigated by Lanitis et al and found to comparable to conventional mastectomy [61] .
Although there are no large randomised trials comparing conventional mastectomy to SSM, a meta-analysis of 9 observational studies was published in 2010. In this study, 3739 patients were included, 30% of women underwent SSM. The median follow-up in the included studies was between 15 and 101 months [62] . All the included studies were retrospective and non-randomised, but there was no significant difference in stage or grade between the 2 groups. This meta-analysis found no significant difference in local recurrence rate between SSM and standard mastectomy (SSM 3.8% - 10.4% vs conventional mastectomy 1.7% - 11.5%). The meta-analysis showed that the incidence of distant relapse was lower in the SSM group however this finding should be interpreted with caution since the tumour grade was not adequately assessed in the studies analysed [62] . The B06 trial (a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer) found a roughly equivalent 10% local recurrence rate at 20 years in the mastectomy treated group [1] .
A large retrospective series from a single centre reported outcomes in 1810 patients undergoing either SSM (799 patients) or standard mastectomy (1011 patients). After adjusting for disease stage and age there was no significant difference in disease-free survival or local recurrence rates [63] .
Many patients requiring mastectomy have T3 tumours. Though evidence for the safety of SSM for these tumours is less clear, the results of early studies are encouraging. In a study of 38 patients with tumours considered to be at high risk of LR, only one case (2.6%) developed a LR after SSM and IBR after a median 53 months of follow-up, despite 10 (26%) patients had systemic recurrences [64] . Foster et al performed SSM and IBR on a prospective cohort of 25 patients with stage 2b or 3 disease and reported an overall post-operative complication rate of 13% and only one LR after 4 years of follow-up [65] .
Neo-adjuvant chemotherapy may be administered to patients with T3 tumours in an attempt to down-stage disease. This may facilitate SSM in a breast that may otherwise have required conventional mastectomy. Alternatively, it may even facilitate breast conservation surgery, avoiding the need for a mastectomy altogether. Radiotherapy should be given to all patients with locally advanced or high risk breast cancer, however its timing in relation to treatments such as SSM and IBR has yet to be established.
It is unlikely that large multi-centre randomised studies comparing conventional mastectomy and SSM will take place. Although the above studies are retrospective and usually with relatively small number of patients, there is reasonable evidence that SSM is a safe oncological operation for T1, T2 and multi-centric tumours. Moreover, there is evidence that SSM combined with IBR does not significantly delay adjuvant therapy, as some clinicians had feared [66] .
The predicting and risk factors for LR after SSM are related to both tumour and patient characteristics. LR may be a reflection of the underlying tumour biology rather than the amount of skin preserved during SSM or the choice of reconstructive technique. Many of these studies found that tumour size, stage, lymph node positivity and poor differentiation were all risk factors for LR [7] [67] . The management of LR after SSM and IBR can be problematic and LR is associated with poor prognosis [63] . LR can be treated locally with surgical excision and RT and systemically with chemotherapy or endocrine therapy [61] . Removal of the reconstructed breast/ implant may be required. The anatomical location of recurrence has been shown to have prognostic implications. A study by Langstein et al. showed that cutaneous or subcutaneous LR was associated with a better overall survival and lower risk of distant metastases and better response to treatment than LR within the chest wall [68] .
6. SSM for DCIS
A mastectomy may be necessary for extensive, multi-focal or recurrent DCIS.
The incidence of LR following conventional mastectomy for DCIS ranges from 1% - 3% [69] , with cure rates of approximately 98% (breast cancer-specific mortality of 0.59%) [70] . SSM and IBR is a good option for women undergoing mastectomy for DCIS. Radiotherapy may adversely affect the appearance of a reconstructed breast but is not usually required in DCIS [71] .
In one series of 95 patients undergoing SSM and IBR for DCIS, 93 (98%) were alive and disease free after a median follow-up of 3.7 years. The overall local recurrence rate was 3 of 93 patients (3%) [72] . A retrospective review of 223 patients with DCIS treated by SSM and IBR, the same author reported an LR rate of 3.3%, with high tumour grade and surgical margins <1 mm as risk factors with a mean follow-up of 82 months [69] . Finally, in a retrospective long-term follow-up study of 44 patients who underwent SSM and IBR for DCIS, there were no local or distant recurrences after 9.8 years [73] . These retrospective studies have all demonstrated that SSM and IBR for DCIS is oncologically safe with low local recurrence rates. However, prospective randomised data to confirm these findings are lacking. There is no evidence that RT after mastectomy is indicated for patients undergoing SSM for DCIS with margins greater than 5 mm, however its potential role in women with close of positive margins for high grade DCIS is not known [74] .
7. Preservation of the Nipple-Areola Complex
It has traditionally been held that the NAC should be removed as the NAC and its adjacent ducts may contain tumour cells that have spread along the ducts from the primary tumour. This concept was based on older studies that demonstrated occult tumour in the region of the NAC [13] . Recent evidence has shown that the risk of tumour involvement in the NAC has been overestimated [75] -[77] . In many patients removal of the NAC may be an over-treatment and hence some surgeons have attempted preservation in view of the cosmetic and psychological benefits (Figures 5).
In a retrospective series of 286 SSM specimens, 16 (5.6%) were found to contain tumour in the NAC [75] . Nodal positivity, sub-areolar tumour location and multi-centricity were significant risk factors. If multi-centric and sub-areolar tumours were excluded, the NAC was involved in 3% of cases. Another series of 140 mastectomies also found tumour size and nodal positivity to be risk factors for NAC involvement. The primary tumour was situated within 2.5 cm of the areola in all 22 cases in which the NAC was positive [76] . A retrospective study of 217 mastectomy specimens reported NAC tumour involvement in 23 cases (10.6%) [77] .
Frozen sections of retro-areolar tissue can be used to attempt to preserve the NAC. In one study of 112 women who underwent SSM with breast cancer more than 2 cm from the NAC, frozen sections of the subareolar tissue were negative for tumour in 61 cases (54.5%) and hence enabling NAC preservation. The NAC was excised in the other 51 cases [78] . The cosmetic results after SSM and IBR (using LD or TRAM flaps) were independently evaluated as excellent or good in 102 out of these 112 (91%) patients. However the cosmetic satisfaction was significantly better after preservation of the NAC (P = 0.001). Six (5.4%) recurrences occurred in these 112 patients who underwent SSM compared with 11 (8.2%) of 134 patients who had undergone conventional mastectomy during the same 6-year period, although this difference is likely to reflect differences in the cases chosen for conventional mastectomy. Only one LR occurred in the NAC preservation group [78] .
In another retrospective study of 219 mastectomies, 20% of NACs were found to be involved, consisting of 9.4% of stage 1 - 2 tumours and 30% of stage 3 tumours. The NAC was involved in only 2.5% of peripheral tumours vs. 68% of central lesions [79] . Tumour size and distance to the nipple on mammography have been identified as independent predictors of NAC involvement that may aid pre-operative planning [80] . Axillary lymph node involvement and lymphovascular invasion have also been shown to be significant risk factors [81] . An-
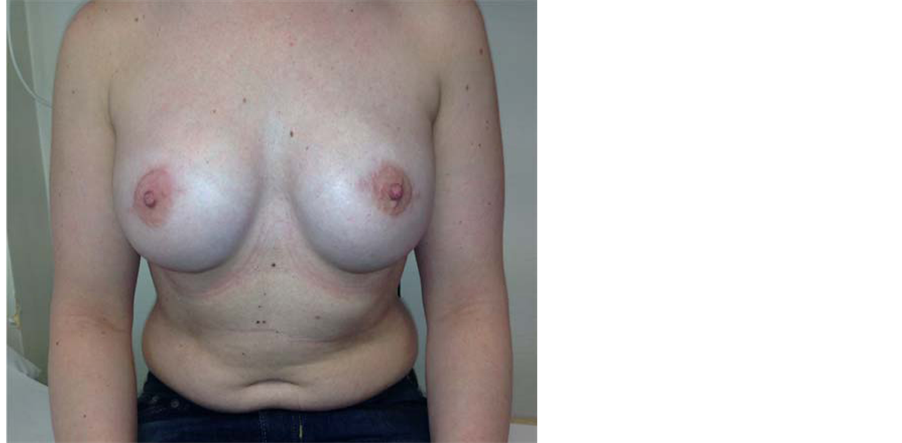
Figure 5. Bilateral skin and nipple areolar sparing mastectomy with IBR with Becker expandable implants and aellular dermal matrix (surgimend).
other study of 115 cases undergoing NAC sparing mastectomy (NSM) for prophylaxis (75 women) or for breast cancer (40 women), the occult nipple involvement rate was only 5.2% [82] .
Some patients will lose part or all of the NAC after NSM due to impaired NAC vascular supply. This has been reported to occur in between 6 and 33% of cases [83] -[85] . Crowe et al reported 54 SSM in 44 patients with preservation of NAC that was clinically thought to be disease-free. Nipple core biopsy frozen sections were performed. 6 out of these 54 biopsies were positive for tumour, necessitating removal of NAC. Of the remaining 48 women with NSM, 3 cases had partial NAC loss [85] . Nipple sparing mastectomy (NSM) performed through a skin incision on the lateral aspect of the breast, rather than a peri-areolar skin incision, has been shown to reduce the risk of ischaemic complications for the nipple (2.8% vs. 59.7%). In addition to ischaemic complications, impaired sensitivity of the NAC seems to affect most patients to some extent, though some resolution with time can occur [86] .
In a recent comprehensive review of the literature on NSM, Mallon et al. concluded that NSM is appropriate in carefully selected patients and all patients should have retroareolar tissue sampling. The review demonstrates strong evidence that NSM is appropriate in cases of well circumscribed single or multifocal lesions (grade 1 to 2) that have a tumor-to-nipple distance greater than 2 cm. Tumors should not have lymphovascular invasion, axillary node metastasis, or human epidermal growth factor receptor-2 positivity [87] .
Therefore, it would appear oncologically safe to perform SSM with NAC preservation for prophylactic cases, for DCIS and for smaller tumours of low stage provided the tumour is not close to the NAC and a frozen section of the subareolar tissue protocol is followed. Patients with clinically apparent involvement of the NAC or adjacent skin, bloody nipple discharge, inflammatory or retro-areolar cancers should not be offered NAC preservation.
Patients must be informed that NAC excision may still be required if residual carcinoma is identified on definitive histology of the retro-areolar tissue or if significant necrosis of nipple occurs.
Some groups have reported the use of radiotherapy to the NAC in addition to employing a frozen-section protocol [88] [89] . One study of 106 NSM with intra-operative RT to the NAC in which two thirds were done for invasive disease, reported one LR at a mean follow-up of 13 months. Around 10% of patients experienced partial NAC necrosis and 5% total NAC necrosis [88] . In another study of NAC preservation, 10 patients were administered RT to NAC post-operatively. Around half of these experienced some loss of nipple sensation or non significant necrosis [89] . More randomised studies with long term follow-up would be required to determine if these approaches improve local control and see if ischaemic complications could be minimised.
An alternative to NAC-sparing SSM is to remove the nipple but preserve the areola—areola-sparing mastectomy (ASM). In an analysis of 217 mastectomy specimens the areola was involved in 2 of 23 cases that NAC was involved with tumour. This represented 0.9% of all the mastectomy specimens [77] . Access for ASM is facilitated by medial and lateral extensions to the skin incision encircling the nipple. This may achieve a superior aesthetic outcome compared to conventional SSM, only requiring a subsequent nipple reconstruction, if requested by the patient. The patient could save the need of nipple areolar tattooing after the further nipple reconstruction. Unfortunately, nipple reconstruction using one of the conventional local flap techniques is problematic in this situation.
In a series of 17 ASM with IBR, the only reported complication was a wound infection in one patient. Although 10 of the cases were done for prophylaxis, there was no local or distant recurrence over median follow-up of 24 months [90] .
If the NAC need removal for oncological reason, various options of nipple reconstruction are available such as using local tissue flap (Figure 6) or nipple sharing technique (removing a wedged graft of nipple from contralateral breast and be transferred and grated onto a deepithelized new nipple position in the reconstructed breast) (Figure 7). Reconstruction of NAC using labial skin is also reported. Subsequent tattooing of nipple areolar area could then follow.
8. Radiotherapy and SSM
Most women undergoing mastectomy for breast cancer do not require post-mastectomy radiotherapy (PMRT). However, patients with four or more positive regional lymph nodes and large tumours (>5 cm) will be offered RT in view of the proven reduction in LR and improved survival [91] . There is limited data from a sub-group analysis of Danish trials [92] suggesting there is a survival benefit in patients with 1 - 3 positive nodes. The
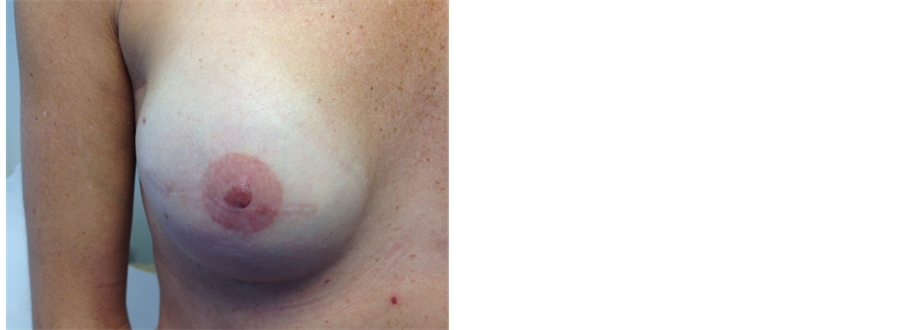
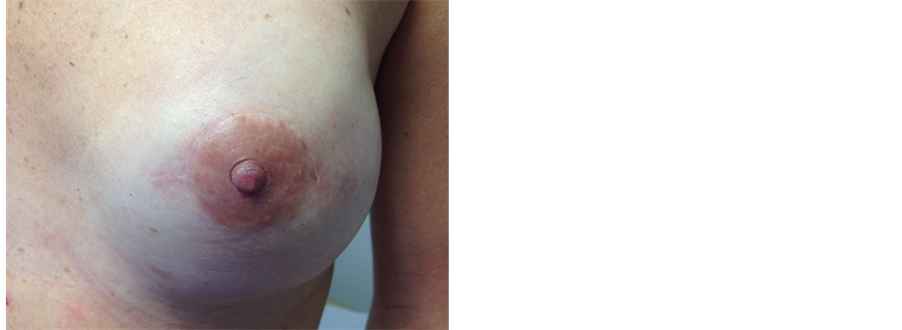
Figure 6. This 38 years old health care professional had right skin-sparing mastectomy and immediate reconstruction using an implant and ADM (SurgiMend) and subsequent reconstruction and micropigmentation of nipple (local flap and tattooing). On the left she underwent left nipple-sparing mastectomy and immediate reconstruction using implant and ADM as a single procedure (prophylactic).
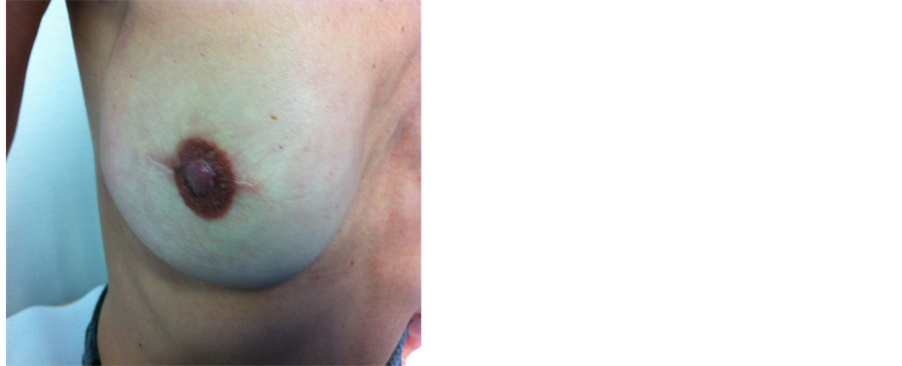
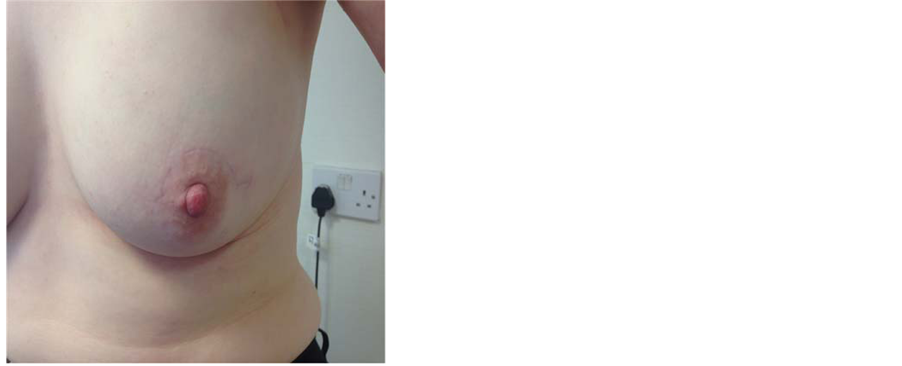
Figure 7. A patient with bilateral SSM and implant reconstruction with acellular dermal matrix—Surgimend. The nipple-areola complex was preserved on the left and reconstructed of the right nipple using the nipple-sharing technique. A wedge of left nipple was removed inferiorly and grafted onto the right side. The right areolar had subsequent tattooing.
“SUPREMO” trial has been designed to address the benefit in this patient group and in other patients thought to be at intermediate risk of local recurrence [93] . The frequency of PMRT is therefore increasing, which has complicated the planning of IBR as post-reconstruction radiotherapy is associated with local complications [94] . In addition to the potential detrimental effect on cosmesis, the planning and delivery of PMRT can be complicated by the presence of the reconstructed breast itself [95] .
The overall complication rate of PMRT following autologous breast reconstruction ranges from 5% to 16% [13] . Although results from individual series vary, complications following IBR and PMRT occur in a high proportion of patients [96] . A study of immediate TRAM reconstructions showed the commonest complications were fat necrosis (16%) and radiation fibrosis (11%) [97] . Tran et al. have recommended that patients who require PMRT should undergo delayed free TRAM flap reconstruction in order to avoid significant late complications such as fat necrosis, volume loss and flap contracture [98] . Fat necrosis leads to volume loss and hardening of the reconstructed breast and particularly occurs when PMRT is given after IBR using free tissue transfer of skin and fat only (e.g. DIEP flap).
For implant or implant-assisted IBR, PMRT can lead to higher rates of significant capsular-contracture resulting in a poor aesthetic outcome. One study compared 39 irradiated implant reconstructed breasts with 338 non-irradiated reconstructions and found a significant negative effect on the reconstructive outcome with PMRT. The main complications were capsular contracture and post-operative pain, 43% of patients required a capsulotomy [99] . In another retrospective study on IBR with expander or implant, 68 receiving RT were compared with 75 non-irradiated IBRs. Capsular contracture rates were 68% vs. 40% respectively, patient satisfaction was 67% vs 88%. However, 72% of those irradiated said that they would choose the same form of reconstruction again [100] .
A systematic review of capsular contracture revealed that various factors are involved in the development of clinically significant capsule formation. Although capsulotomy or capsulectomy is possible, the recurrence rate is high [101] . This has led some surgeons to recommend that implants-based IBR should be avoided if PMRT is likely [96] . Alternative approaches are an autologous IBR or a delayed breast reconstruction. More recently, an “immediate-delayed” reconstructive technique has been suggested. A temporary tissue expander can be used deep to the pectoralis major muscle at the time of SSM. Following PMRT, delayed reconstruction can then be done, removing the tissue expander and using a myocutaneous flap and/or implant [13] [96] . This approach could avoid the potential radiotherapy delivery problems and cosmetic disadvantages associated with IBR followed by PMRT [96] . Prior to mastectomy, the radiological assessment of tumour size combined with analysis of core-biopsies from the primary tumour and techniques such as SNLB could be used to assess the likelihood of PMRT, thereby facilitating the selection of patients for immediate-delayed reconstruction. However, randomised controlled trials are also required to compare immediate-delayed reconstruction to conventional mastectomy and delayed reconstruction for those women who require PMRT.
Pre-operative radiotherapy can also affect the outcome of SSM and IBR. Hultman and Daiza reported the effects of previous radiotherapy on subsequent SSM and IBR in 37 breasts [102] . TRAMs, LDs and implants were included. 9 patients (24%) had a SSM flap complication of which 5 required re-operations. Adjuvant treatment with chemotherapy or radiotherapy was not delayed in these patients. Previous irradiation and diabetes were found to be significant risk factors for complications [102] . Chang et al. found a significantly higher frequency of native flap complication and capsular contracture in women who had received pre-operative radiotherapy prior to SSM. TRAM flap reconstruction was also found to be superior to LD + implant after SSM in this group of patients [103] . These studies are small and larger studies with longer follow-up are required to verify their findings. However, it would appear that women with previously irradiated breasts can still benefit from the advantages of SSM and IBR, as long as the risk of various complications is explained [71] .
9. Conclusions
Despite their shortcomings, numerous retrospective and prospective studies have supported the growing evidence that SSM is oncologically safe for early-stage IBC and DCIS with aesthetic and psychological benefits. In the absence of randomised control trials, there is a need for an updated systematic meta-analysis of published studies in order to confirm the oncological safety with overall and disease-free survivals as primary endpoints.
In carefully selected cases, the NAC can be preserved provided that the tumour is node negative, and located more than 2 cm away from the nipple and an intraoperative frozen-section of retroareolar tissue is examined. The optimal cosmetic outcome is found in women who have not had prior RT and those who do not need PMRT. Though neither representing a contraindication to SSM and IBR, the optimal integration of IBR and RT has yet to be determined. Good outcomes require appropriate patient selection, a coordinated oncoplastic and multidisciplinary approach. Patients should be appropriately counselled. Photographs and audit data can be used to illustrate the likely outcome and potential complications.
References
- Fisher, B., Anderson, S., Bryant, J., et al. (2002) Twenty-Year Follow-Up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy Plus Irradiation for the Treatment of Invasive Breast Cancer. The New England Journal of Medicine, 347, 1233-1241. http://dx.doi.org/10.1056/NEJMoa022152
- (2009) NICE Guideline CG80. Early and Locally Advanced Breast Cancer: Diagnosis and Treatment. http://guidance.nice.org.uk/CG80/Guidance/pdf/English
- Hidalgo, D.A. (1998) Aesthetic Refinement in Breast Reconstruction: Complete Skin-Sparing Mastectomy with Autogenous Tissue Transfer. Plastic and Reconstructive Surgery, 102, 63. http://dx.doi.org/10.1097/00006534-199807000-00009
- Toth, B.A. and Lappert, P. (1991) Modified Skin Incisions for Mastectomy: The Need for Plastic Surgical Input in PreOperative Planning. Plastic and Reconstructive Surgery, 87, 1048-1053. http://dx.doi.org/10.1097/00006534-199106000-00006
- Carlson, G.W. (1996) Skin Sparing Mastectomy: Anatomic and Technical Considerations. The American Surgeon, 62, 151-155.
- Simmons, R.M. and Adamovich, T.L. (2003) Skin-Sparing Mastectomy. Surgical Clinics of North America, 83, 885- 899. http://dx.doi.org/10.1016/S0039-6109(03)00035-5
- Singletary, S.E. (1996) Skin-Sparing Mastectomy with Immediate Breast Reconstruction: The M. D. Anderson Cancer Center Experience. Annals of Surgical Oncology, 3, 411-416. http://dx.doi.org/10.1007/BF02305673
- Carlson, G.W., Losken, A., Moore, B., Thornton, J., Elliott, M., Bolitho, G. and Denson, D.D. (2001) Results of Immediate Breast Reconstruction after Skin-Sparing Mastectomy. Annals of Plastic Surgery, 46, 222-228. http://dx.doi.org/10.1097/00000637-200103000-00003
- Shen, J., Ellenhorn, J., Qian, D., Kulber, D. and Aronowitz, J. (2008) Skin-Sparing Mastectomy: A Survey Based Approach to Defining Standard of Care. The American Surgeon, 74, 902-905.
- Gendy, R.K., Able, J.A. and Rainsbury, R.M. (2003) Impact of Skin-Sparing Mastectomy with Immediate Reconstruction and Breast-Sparing Reconstruction with Miniflaps on the Outcomes of Oncoplastic Breast Surgery. British Journal of Surgery, 90, 433-439. http://dx.doi.org/10.1002/bjs.4060
- Shrotria, S. (2001) The Peri-Areolar Incision—Gateway to the Breast! European Journal of Surgical Oncology, 27, 601-603. http://dx.doi.org/10.1053/ejso.2001.1051
- Hammond, D.C., Capraro, P.A., Ozolins, E.B. and Arnold, J.F. (2002) Use of a Skin-Sparing Reduction Pattern to Create a Combination Skin-Muscle Flap Pocket in Immediate Breast Reconstruction. Plastic and Reconstructive Surgery, 110, 206-211. http://dx.doi.org/10.1097/00006534-200207000-00035
- Singletary, S.E. and Robb, G.L. (2003) Oncologic Safety of Skin-Sparing Mastectomy. Annals of Surgical Oncology, 10, 95-97. http://dx.doi.org/10.1245/ASO.2003.01.910
- Carlson, G.W., Bostwick 3rd, J., Styblo, T.N., et al. (1997) Skin Sparing Mastectomy, Oncologic and Reconstructive Considerations. Annals of Surgery, 225, 570-575. http://dx.doi.org/10.1097/00000658-199705000-00013
- Stradling, B.L., Ahn, M., Angelats, J. and Gabram, S.G. (2001) Skin-Sparing Mastectomy with Sentinel Lymph Node Dissection: Less Is More. Archives of Surgery, 136, 1069-1075. http://dx.doi.org/10.1001/archsurg.136.9.1069
- Meretoja, T.J., Jahkola, T.A., Toivonen, T.S., Krogerus, L.A., Heikkila, P.S., von Smitten, K.A. and Leidenius, M.H. (2007) Sentinel Node Biopsy with Intraoperative Diagnosis in Patients Undergoing Skin-Sparing Mastectomy and Immediate Breast Reconstruction. European Journal of Surgical Oncology, 33, 1146-1149. http://dx.doi.org/10.1016/j.ejso.2007.03.009
- Kuerer, H.M., Krishnamurthy, S. and Kronowitz, S.J. (2002) Important Technical Considerations for Skin-Sparing Mastectomy with Sentinel Lymph Node Dissection. Archives of Surgery, 137, 747. http://dx.doi.org/10.1001/archsurg.137.6.747-a
- Schrenk, P., Woelfl, S., Bogner, S., Moser, F. and Wayand, W. (2005) The Use of Sentinel Node Biopsy in Breast Cancer Patients Undergoing Skin Sparing Mastectomy and Immediate Autologous Reconstruction. Plastic and Reconstructive Surgery, 116, 1278-1286. http://dx.doi.org/10.1097/01.prs.0000181515.11529.9a
- Friedlander, L.D., Sundin, J. and Bakshandeh, N. (1995) Endoscopy Mastectomy and Breast Reconstruction: Endoscopic Breast Surgery. Aesthetic Plastic Surgery, 19, 27-29. http://dx.doi.org/10.1007/BF00209307
- Yamashita, K. and Shimizu, K. (2006) Endoscopic Video-Assisted Breast Surgery: Procedures and Short-Term Results. Journal of Nippon Medical School, 73, 193-202. http://dx.doi.org/10.1272/jnms.73.193
- Lee, E.K., Kook, S.H., Park, Y.L. and Bae, W.G. (2006) Endoscopy-Assisted Breast-Conserving Surgery for Early Breast Cancer. World Journal of Surgery, 30, 957-964. http://dx.doi.org/10.1007/s00268-005-0202-y
- Tamaki, Y., Tsukamoto, Y., Miyoushi, Y., Taguchi, T. and Noguchi, S. (2002) Overview: Video Assisted Breast Surgery. Biomedicine & Pharmacotherapy, 56, 187s-191s. http://dx.doi.org/10.1016/S0753-3322(02)00206-8
- Nakajima, H., Sakaguchi, K., Mizuta, N., Hachimine, T., Ohe, S. and Sawai, K. (2002) Video-Assisted Total Glandectomy and Immediate Reconstruction for Breast Cancer. Biomedicine & Pharmacotherapy, 56, 205s-208s. http://dx.doi.org/10.1016/S0753-3322(02)00281-0
- Ho, W.S., Ying, S.Y. and Chan, A.C. (2002) Endoscopic-Assisted Subcutaneous Mastectomy and Axillary Dissection with Immediate Mammary Prosthesis Reconstruction for Early Breast Cancer. Surgical Endoscopy, 16, 302-306. http://dx.doi.org/10.1007/s004640000203
- Kitamura, K., Ishida, M., Inoue, H., Kinoshita, J., Hashizume, M. and Sugimachi, K. (2002) Early Results of an Endoscope-Assisted Subcutaneous Mastectomy and Reconstruction for Breast Cancer. Surgery, 131, S324-329. http://dx.doi.org/10.1067/msy.2002.120120
- Kroll, S.S. (1997) Immediate Breast Reconstruction. A Review. Annales Chirurgiae et Gynaecologiae, 86, 5-12.
- Granzow, J.W., Levine, J.L., Chiu, E.S. and Allen, R.J. (2006) Breast Reconstruction Using Perforator Flaps. Journal of Surgical Oncology, 94, 441-454. http://dx.doi.org/10.1002/jso.20481
- Salgarello, M., Seccia, A. and Eugenio, F. (2004) Immediate Breast Reconstruction with Anatomical Permanent Expandable Implants after Skin-Sparing Mastectomy: Aesthetic and Technical Refinements. Annals of Plastic Surgery, 52, 358-366. http://dx.doi.org/10.1097/01.sap.0000105914.69611.c7
- Woerdeman, L.A., Hage, J.J., Hofland, M.M. and Rutgers, E.J. (2007) A Prospective Assessment of Surgical Risk Factors in 400 Cases of Skin-Sparing Mastectomy and Immediate Breast Reconstruction with Implants to Establish Selection Criteria. Plastic and Reconstructive Surgery, 119, 455-463. http://dx.doi.org/10.1097/01.prs.0000246379.99318.74
- Woerdeman, L.A., Hage, J.J., Smeulders, M.J., Rutgers, E.J. and van der Horst, C.M. (2006) Skin-Sparing Mastectomy and Immediate Breast Reconstruction by Use of Implants: An Assessment of Risk Factors for Complications and Cancer Control in 120 Patients. Plastic and Reconstructive Surgery, 118, 321-332. http://dx.doi.org/10.1097/01.prs.0000234049.91710.ba
- Ibrahim, A.M., Ayeni, O.A., Hughes, K.B., Lee, B.T., Slavin, S.A. and Lin, S.J. (2013) Acellular Dermal Matrices in Breast Surgery: A Comprehensive Review. Annals of Plastic Surgery, 70, 732-738. http://dx.doi.org/10.1097/SAP.0b013e31824b3d30
- Ho, G., Nguyen, T.J., Shahabi, A., Hwang, B.H., Chan, L.S. and Wong, A.K. (2012) A Systematic Review and Meta-Analysis of Complications Associated with Acellular Dermal Matrix-Assisted Breast Reconstruction. Annals of Plastic Surgery, 68, 346-356. http://dx.doi.org/10.1097/SAP.0b013e31823f3cd9
- Sbitany, H., Sandeen, S.N., Amalfi, A.N., et al. (2009) Acellular Dermis-Assisted Prosthetic Breast Reconstruction versus Complete Submuscular Coverage: A Head-To-Head Comparison of Outcomes. Plastic and Reconstructive Surgery, 124, 1735-1740. http://dx.doi.org/10.1097/PRS.0b013e3181bf803d
- Nahabedian, M.Y. (2009) Postmastectomy Reconstruction: An Approach to Patient Selection. Plastic and Reconstructive Surgery, 124, 43-52. http://dx.doi.org/10.1097/PRS.0b013e31818b8c23
- Dieterich, M., Reimer, T., Dieterich, H., Stubert, J. and Gerber, B. (2012) A Short-Term Follow-Up of Implant Based Breast Reconstruction Using a Titanium-Coated Polypropylene Mesh (TiLoop(®) Bra). European Journal of Surgical Oncology, 38, 1225-1230. http://dx.doi.org/10.1016/j.ejso.2012.08.026
- Selber, J.C., Clemens, M.W., Oates, S. and Baumann, D.P. (2013) Autoderm: An Alternative Bioprosthetic for Breast Reconstruction. Plastic and Reconstructive Surgery, 131, 985-987.
- Schneider, W.J., Hill, H.L. and Brown, R.G. (1977) Latissimus Dorsi Myocutaneous Flap for Breast Reconstruction. British Journal of Plastic Surgery, 30, 277-281. http://dx.doi.org/10.1016/0007-1226(77)90117-5
- de la Torre, J.I., Fix, R.J., Gardner, P.M. and Vasconez, L.O. (2001) Reconstruction with the Latissimus Dorsi Flap after Skin-Sparing Mastectomy. Annals of Plastic Surgery, 46, 229-233. http://dx.doi.org/10.1097/00000637-200103000-00004
- Hartrampf, C.R., Scheflan, M. and Black, P.W. (1982) Breast Reconstruction with a Transverse Abdominal Island Flap. Plastic & Reconstructive Surgery, 69, 216-224. http://dx.doi.org/10.1097/00006534-198202000-00006
- Arnez, Z.M., Bajec, J., Bardsley, A.F., Scamp, T. and Webster, M.H. (1991) Experience with 50 Free TRAM Flap Breast Reconstructions. Plastic & Reconstructive Surgery, 87, 470-482. http://dx.doi.org/10.1097/00006534-199103000-00012
- Drucker-Zertuche, M. and Robles-Vidal, C. (2007) A 7 Year Experience with Immediate Breast Reconstruction after Skin Sparing Mastectomy for Cancer. European Journal of Surgical Oncology, 33, 140-146. http://dx.doi.org/10.1016/j.ejso.2006.10.010
- Gherardini, G., Thomas, R., Basoccu, G., Zaccheddu, R., Fortunato, L., Cortino, P., Evans, G.R., Matarasso, A., D’Aiuto, M. and D’Aiuto, G. (2001) Immediate Breast Reconstruction with the Transverse Rectus Abdominis Musculocutaneous Flap after Skin-Sparing Mastectomy. International Surgery, 86, 246-251.
- Holmstrom, H. (1979) The Free Abdominoplasty Flap and Its Use in Breast Reconstruction. Journal of Plastic Surgery and Hand Surgery, 13, 423-427. http://dx.doi.org/10.3109/02844317909013092
- Blondeel, P.N. (1999) The Sensate Free Superior Gluteal Artery Perforator (S-GAP) Flap: A Valuable Alternative in Autologous Breast Reconstruction. British Journal of Plastic Surgery, 52, 185-193. http://dx.doi.org/10.1054/bjps.1998.3032
- Yano, K., Hosokawa, K., Nakai, K., Kubo, T., Hattori, R., Taguchi, T., Tamaki, Y. and Noguchi, S. (2003) SkinSparing Mastectomy and Immediate Reconstruction with a Deep Inferior Epigastric Perforator Flap. Breast Cancer, 10, 275-280. http://dx.doi.org/10.1007/BF02966729
- Munhoz, A.M., Arruda, E., Montag, E., Aldrighi, C., Aldrighi, J.M., Gemperli, R. and Ferreira, M.C. (2007) Immediate Skin-Sparing Mastectomy Reconstruction with Deep Inferior Epigastric Perforator (DIEP) Flap. Technical Aspects and Outcome. Breast Journal, 13, 470-478. http://dx.doi.org/10.1111/j.1524-4741.2007.00467.x
- Cocquyt, V.F., Blondeel, P.N., Depypere, H.T., Van De Sijpe, K.A., Daems, K.K., Monstrey, S.J. and Van Belle, S.J. (2003) Better Cosmetic Results and Comparable Quality of Life after Skin-Sparing Mastectomy and Immediate Autologous Breast Reconstruction Compared to Breast Conservative Treatment. British Journal of Plastic Surgery, 56, 462- 470. http://dx.doi.org/10.1016/S0007-1226(03)00198-X
- Fujino, T., Harasina, T. and Aoyagi, F. (1975) Reconstruction for Aplasia of the Breast and Pectoral Region by Microvascular Transfer of a Free Flap from the Buttock. Plastic & Reconstructive Surgery, 56, 178-181. http://dx.doi.org/10.1097/00006534-197508000-00010
- Allen, R.J. and Tucker Jr., C. (1995) Superior Gluteal Artery Perforator Free Flap for Breast Reconstruction. Plastic & Reconstructive Surgery, 95, 1207-1212. http://dx.doi.org/10.1097/00006534-199506000-00010
- Guerra, A.B., Metzinger, S.E., Bidros, R.S., Gill, P.S., Dupin, C.L. and Allen, R.J. (2004) Breast Reconstruction with Gluteal Artery Perforator (GAP) Flaps: A Critical Analysis of 142 Cases. Annals of Plastic Surgery, 52, 118-125. http://dx.doi.org/10.1097/01.sap.0000095437.43805.d1
- Arnez, Z.M., Pogorelec, D., Planinsek, F. and Ahcan, U. (2004) Breast Reconstruction by the Free Transverse Gracilis (TUG) Flap. British Journal of Plastic Surgery, 57, 20-26. http://dx.doi.org/10.1016/j.bjps.2003.10.007
- Wechselberger, G. and Schoeller, T. (2004) The Transverse Myocutaneous Gracilis Free Flap: A Valuable Tissue Source in Autologous Breast Reconstruction. Plastic & Reconstructive Surgery, 114, 69-73. http://dx.doi.org/10.1097/01.PRS.0000127797.62020.D4
- Jimenez, A.G., St Germain, P., Sirois, M., Hatheway, M. and Lethbridge, R. (2002) Free Omental Flap for Skin-Sparing Breast Reconstruction Harvested Laparoscopically. Plastic & Reconstructive Surgery, 110, 545-551. http://dx.doi.org/10.1097/00006534-200208000-00028
- Sbitany, H., Amalfi, A.N. and Langstein, H.N. (2009) Preferences in Choosing between Breast Reconstruction Options: A Survey of Female Plastic Surgeons. Plastic & Reconstructive Surgery, 124, 1781-1789.
- De Blacam, C., Monoh, A.O., Colakoglu, S., Tobias, A.M. and Lee, B.T. (2011) Evaluation of Clinical Outcomes and Aesthetic Results after Autologous Fat Grafting for Countour Deformities of the Reconstructed Breast. Plastic & Reconstructive Surgery, 128, 411e-418e.
- Panettiere, P., Marchetti, L. and Accorsi, D. (2009) The Serial Free Fat Transfer in Irradiated Prosthetic Breast Reconstructions. Aesthetic Plastic Surgery, 33, 695-700.
- Barton Jr., F.E., English, J.M., Kingsley, W.B. and Fietz, M. (1991) Glandular Excision in Total Glandular Mastectomy and Modified Radical Mastectomy: A Comparison. Plastic & Reconstructive Surgery, 88, 389-394. http://dx.doi.org/10.1097/00006534-199109000-00001
- Torresan, R.Z., dos Santos, C.C., Okamura, H. and Alvarenga, M. (2005) Evaluation of Residual Glandular Tissue after Skin-Sparing Mastectomies. Annals of Surgical Oncology, 12, 1037-1044. http://dx.doi.org/10.1245/ASO.2005.11.027
- Torresan, R.Z., Cabello dos Santos, C., Brenelli, H., Okamura, H. and Alvarenga, M. (2005) Residual Glandular Tissue after Skin-Sparing Mastectomies. Breast Journal, 11, 374-375. http://dx.doi.org/10.1111/j.1075-122X.2005.00029.x
- Ho, C.M., Mak, C.K., Lau, Y., Cheung, W.Y., Chan, M.C. and Hung, W.K. (2003) Skin Involvement in Invasive Breast Carcinoma: Safety of Skin-Sparing Mastectomy. Annals of Surgical Oncology, 10, 102-107. http://dx.doi.org/10.1245/ASO.2003.05.001
- Newman, L.A., Kuerer, H.M., Hunt, K.K., Kroll, S.S., Ames, F.C., Ross, M.I., Feig, B.W. and Singletary, S.E. (1998) Presentation, Treatment, and Outcome of Local Recurrence after Skin-Sparing Mastectomy and Immediate Breast Reconstruction. Annals of Surgical Oncology, 5, 620-626. http://dx.doi.org/10.1007/BF02303832
- Lanitis, S., Tekkis, P.P., Sgourakis, G., Dimopoulos, N., Al Mufti, R. and Hadjiminas, D.J. (2010) Comparison of Skin-Sparing Mastectomy versus Non-Skin-Sparing Mastectomy for Breast Cancer: A Meta-Analysis of Observational Studies. Annals of Surgery, 251, 632-639. http://dx.doi.org/10.1097/SLA.0b013e3181d35bf8
- Yi, M., Kronowitz, S.J., Meric-Bernstam, F., Feig, B.W., Symmans, W.F., Lucci, A., Ross, M.I., Babiera, G.V., Kuerer, H.M. and Hunt, K.K. (2011) Local, Regional, and Systemic Recurrence Rates in Patients Undergoing Skin-Sparing Mastectomy Compared with Conventional Mastectomy. Cancer, 117, 916-924. http://dx.doi.org/10.1002/cncr.25505
- Downes, K.J., Glatt, B.S., Kanchwala, S.K., Mick, R., Fraker, D.L., Fox, K.R., Solin, L.J., Bucky, L.P. and Czerniecki, B.J. (2005) Skin-Sparing Mastectomy and Immediate Reconstruction Is an Acceptable Treatment Option for Patients with High-Risk Breast Carcinoma. Cancer, 103, 906-913. http://dx.doi.org/10.1002/cncr.20851
- Foster, R.D., Esserman, L.J., Anthony, J.P., Hwang, E.S. and Do, H. (2002) Skin-Sparing Mastectomy and Immediate Breast Reconstruction: A Prospective Cohort Study for the Treatment of Advanced Stages of Breast Carcinoma. Annals of Surgical Oncology, 9, 462-466. http://dx.doi.org/10.1007/BF02557269
- Allweis, T.M., Boisvert, M.E., Otero, S.E., Perry, D.J., Dubin, N.H. and Priebat, D.A. (2002) Immediate Reconstruction after Mastectomy for Breast Cancer Does not Prolong the Time to Starting Adjuvant Chemotherapy. American Journal of Surgery, 183, 218-221. http://dx.doi.org/10.1016/S0002-9610(02)00793-6
- Rainsbury, R.M. (2006) Skin-Sparing Mastectomy. British Journal of Surgery, 93, 276-281. http://dx.doi.org/10.1002/bjs.5257
- Langstein, H.N., Cheng, M.H., Singletary, S.E., Robb, G.L., Hoy, E., Smith, T.L. and Kroll, S.S. (2003) Breast Cancer Recurrence after Immediate Reconstruction: Patterns and Significances. Plastic & Reconstructive Surgery, 111, 712-720. http://dx.doi.org/10.1097/01.PRS.0000041441.42563.95
- Carlson, G.W., Page, A., Johnson, E., Nicholson, K., Styblo, T.M. and Wood, W.C. (2007) Local Recurrence of Ductal Carcinoma in Situ after Skin-Sparing Mastectomy. Journal of the American College of Surgeons, 204, 1074-1078. http://dx.doi.org/10.1016/j.jamcollsurg.2007.01.063
- Mokbel, K. (2003) Towards Optimal Management of Ductal Carcinoma in Situ of the Breast. European Journal of Surgical Oncology, 29, 191-197. http://dx.doi.org/10.1053/ejso.2002.1425
- Mokbel, R. and Mokbel, K. (2006) Skin-Sparing Mastectomy and Radiotherapy: An Update. International Seminars in Surgical Oncology, 3, 35. http://dx.doi.org/10.1186/1477-7800-3-35
- Rubio, I.T., Mirza, N., Sahin, A.A., Whitman, G., Kroll, S.S., Ames, F.C. and Eva Singletary, S. (2000) Role of Specimen Radiography in Patients Treated with Skin-Sparing Mastectomy for Ductal Carcinoma in Situ of the Breast. Annals of Surgical Oncology, 7, 544-548. http://dx.doi.org/10.1007/s10434-000-0544-5
- Chan, L.W., Rabban, J., Hwang, E.S., Bevan, A., Alvarado, M., Ewing, C., Esserman, L. and Fowble, B. (2011) Is Radiation Indicated in Patients with Ductal Carcinoma in Situ and Close or Positive Mastectomy Margins? International Journal of Radiation Oncology, Biology, Physics, 80, 25-30. http://dx.doi.org/10.1016/j.ijrobp.2010.01.044
- Spiegel, A.J. and Butler, C.E. (2003) Recurrence Following Treatment of Ductal Carcinoma in Situ with Skin-Sparing Mastectomy and Immediate Breast Reconstruction. Plastic & Reconstructive Surgery, 111, 706-711. http://dx.doi.org/10.1097/01.PRS.0000041440.12442.05
- Laronga, C., Kemp, B., Johnston, D., Robb, G.L. and Eva Singletary, S. (1999) The Incidence of Occult Nipple-Areola Complex Involvement in Breast Cancer Patients Receiving a Skin-Sparing Mastectomy. Annals of Surgical Oncology, 6, 609-613. http://dx.doi.org/10.1007/s10434-999-0609-z
- Vaidya, J.S. (1998) Prediction of Nipple and Areola Involvement in Breast Cancer. European Journal of Surgical Oncology, 24, 15-16. http://dx.doi.org/10.1016/S0748-7983(98)80117-0
- Simmons, R.M., Brennan, M., Christos, P., King, V. and Osborne, M. (2002) Analysis of Nipple/Areolar Involvement with Mastectomy: Can the Areola Be Preserved? Annals of Surgical Oncology, 9, 165-168. http://dx.doi.org/10.1007/BF02557369
- Gerber, B., Krause, A., Reimer, T., Muller, H., Kuchenmeister, I., Makovitzky, J., Kundt, G. and Friese, K. (2003) Skin-Sparing Mastectomy with Conservation of the Nipple-Areola Complex and Autologous Reconstruction Is an Oncologically Safe Procedure. Annals of Surgery, 238, 120-127. http://dx.doi.org/10.1097/01.SLA.0000077922.38307.cd
- Banerjee, A., Gupta, S. and Bhattacharya, N. (2008) Preservation of Nipple-Areolacomplex in Breastcancer—A Clinicopathological Assessment. Journal of Plastic, Reconstructive & Aesthetic Surgery, 61, 1195-1198. http://dx.doi.org/10.1016/j.bjps.2007.08.005
- Schecter, A.K., Freeman, M.B., Giri, D., Sabo, E. and Weinzweig, J. (2006) Applicability of the Nipple-Areola Complex-Sparing Mastectomy: A Prediction Model Using Mammography to Estimate Risk of Nipple-Areola Complex Involvement in Breast Cancer Patients. Annals of Plastic Surgery, 56, 498-504. http://dx.doi.org/10.1097/01.sap.0000216946.83252.e4
- Vlajcic, Z., Zic, R., Stanec, S., Lambasa, S., Petrovecki, M. and Stanec, Z. (2005) Nipple-Areola Complex Preservation: Predictive Factors of Neoplastic Nipple-Areola Complex Invasion. Annals of Plastic Surgery, 55, 240-244. http://dx.doi.org/10.1097/01.sap.0000171680.49971.85
- Chen, C.M., Disa, J.J., Sacchini, V., Pusic, A.L., Mehrara, B.J., Garcia-Etienne, C.A. and Cordeiro, P.G. (2009) NippleSparing Mastectomy and Immediate Tissue Expander/Implant Breast Reconstruction. Plastic & Reconstructive Surgery, 124, 1772-1780. http://dx.doi.org/10.1097/PRS.0b013e3181bd05fd
- Verheyden, C.N. (1998) Nipple-Sparing Total Mastectomy of Large Breasts: The Role of Tissue Expansion. Plastic & Reconstructive Surgery, 101, 1494. http://dx.doi.org/10.1097/00006534-199805000-00010
- Komorowski, A.L., Zanini, V., Regolo, L., Carolei, A., Wysocki, W.M. and Costa, A. (2006) Necrotic Complications after Nippleand Areola-Sparing Mastectomy. World Journal of Surgery, 30, 1410-1413. http://dx.doi.org/10.1007/s00268-005-0650-4
- Crowe Jr., J.P., Kim, J.A., Yetman, R., Banbury, J., Patrick, R.J. and Baynes, D. (2004) Nipple-Sparing Mastectomy: Technique and Results of 54 Procedures. JAMA Surgery, 139, 148-150. http://dx.doi.org/10.1001/archsurg.139.2.148
- Regolo, L., Ballardini, B., Gallarotti, E., Scoccia, E. and Zanini, V. (2008) Nipple Sparing Mastectomy: An Innovative Skin Incision for an Alternative Approach. Breast, 17, 8-11. http://dx.doi.org/10.1016/j.breast.2007.07.040
- Mallon, P., Feron, J.G., Couturaud, B., Fitoussi, A., Lemasurier, P., Guihard, T., Cothier-Savay, I. and Reyal, F. (2013) The Role of Nipple-Sparingmastectomy in Breast Cancer: A Comprehensive Review of the Literature. Plastic & Reconstructive Surgery, 131, 969-984. http://dx.doi.org/10.1097/PRS.0b013e3182865a3c
- Petit, J.Y., Veronesi, U., Orecchia, R., Luini, A., Rey, P., Intra, M., Didier, F., Martella, S., Rietjens, M., Garusi, C., De Lorenzi, F., Gatti, G., Leon, M.E. and Casadio, C. (2006) Nipple-Sparing Mastectomy in Association with Intra Operative Radiotherapy (ELIOT): A New Type of Mastectomy for Breast Cancer Treatment. Breast Cancer Research and Treatment, 96, 47-51. http://dx.doi.org/10.1007/s10549-005-9033-7
- Bistoni, G., Rulli, A., Izzo, L., Noya, G., Alfano, C. and Barberini, F. (2006) Nipple-Sparingmastectomy. Preliminary Results. Journal of Experimental & Clinical Cancer Research, 25, 495-497.
- Simmons, R.M., Hollenbeck, S.T. and Latrenta, G.S. (2004) Two-Year Follow-Up of Areola-Sparing Mastectomy with Immediate Reconstruction. American Journal of Surgery, 188, 403-406. http://dx.doi.org/10.1016/j.amjsurg.2004.07.001
- Recht, A. and Edge, S.B. (2003) Evidence-Based Indications for Postmastectomy Irradiation. Surgical Clinics of North America, 83, 995-1013. http://dx.doi.org/10.1016/S0039-6109(03)00033-1
- Overgaard, M., Nielsen, H.M. and Overgaard, J. (2007) Is the Benefit of Postmastectomy Irradiation Limited to Patients with Four or More Positive Nodes, as Recommended in International Consensus Reports? A Subgroup Analysis of the DBCG 82 b&c Randomized Trials. Radiotherapy & Oncology, 82, 247-253. http://dx.doi.org/10.1016/j.radonc.2007.02.001
- Kunkler, I.H., Canney, P., van Tienhoven, G. and Russell, N.S. on Behalf of the MRC/EORTC (BIG 2-04) SUPREMO Trial Management Group (2008) Elucidating the Role of Chest Wall Irradiation in ‘Intermediate-Risk’ Breast Cancer: The MRC/EORTC SUPREMO Trial. Clinical Oncology, 20, 31-34. http://dx.doi.org/10.1016/j.clon.2007.10.004
- Hussien, M., Salah, B., Malyon, A. and Wieler-Mithoff, E.M. (2004) The Effect of Radiotherapy on the Use of Immediate Breast Reconstruction. European Journal of Surgical Oncology, 30, 490-494. http://dx.doi.org/10.1016/j.ejso.2004.03.005
- Buchholz, T.A., Kronowitz, S.J. and Kuerer, H.M. (2002) Immediate Breast Reconstruction after Skin-Sparing Mastectomy for the Treatment of Advanced Breast Cancer: Radiation Oncology Considerations. Annals of Surgical Oncology, 9, 820-821. http://dx.doi.org/10.1007/BF02574506
- Kronowitz, S.J. and Robb, G.L. (2004) Breast Reconstruction with Postmastectomy Radiation Therapy: Current Issues. Plastic & Reconstructive Surgery, 114, 950-960. http://dx.doi.org/10.1097/01.PRS.0000133200.99826.7F
- Hunt, K.K., Baldwin, B.J., Strom, E.A., Ames, F.C., McNeese, M.D., Kroll, S.S. and Singletary, S.E. (1997) Feasibility of Postmastectomy Radiation Therapy after TRAM Flap Breast Reconstruction. Annals of Surgical Oncology, 4, 377-384. http://dx.doi.org/10.1007/BF02305549
- Tran, N.V., Evans, G.R., Kroll, S.S., et al. (2000) Postoperative Adjuvant Irradiation: Effects on Tranverse Rectus Abdominis Muscle Flap Breast Reconstruction. Plastic & Reconstructive Surgery, 106, 313-317. http://dx.doi.org/10.1097/00006534-200008000-00011
- Evans, G.R., Schusterman, M.A. and Kroll, S.S. (1995) Reconstruction and the Radiated Breast: Is There a Role for Implants? Plastic & Reconstructive Surgery, 96, 1111-1115. http://dx.doi.org/10.1097/00006534-199510000-00016
- Cordeiro, P.G., Pusic, A.L., Disa, J.J., McCormick, B. and VanZee, K. (2004) Irradiation after Immediate Tissue Expander/Implant Breast Reconstruction: Outcomes, Complications, Aesthetic Results, and Satisfaction among 156 Patients. Plastic & Reconstructive Surgery, 113, 877-881. http://dx.doi.org/10.1097/01.PRS.0000105689.84930.E5
- Araco, A., Caruso, R., Araco, F., Overton, J. and Gravante, G. (2009) Capsular Contractures: A Systematic Review. Plastic & Reconstructive Surgery, 124, 1808-1819. http://dx.doi.org/10.1097/PRS.0b013e3181bf7f26
- Hultman, C.S. and Daiza, S. (2003) Skin-Sparing Mastectomy Flap Complications after Breast Reconstruction: Review of Incidence, Management, and Outcome. Annals of Plastic Surgery, 50, 249-255. http://dx.doi.org/10.1097/01.SAP.0000046784.70583.E1
- Chang, E.I., Ly, D.P. and Wey, P.D. (2007) Comparison of Aesthetic Breast Reconstruction after Skin-Sparing or Conventional Mastectomy in Patients Receiving Preoperative Radiation Therapy. Annals of Plastic Surgery, 59, 78-81. http://dx.doi.org/10.1097/01.sap.0000252487.27077.d6
NOTES

*Corresponding author.

