Journal of Cancer Therapy
Vol.4 No.8A(2013), Article ID:36111,8 pages DOI:10.4236/jct.2013.48A006
Advances in Lung Cancer and Treatment Research
![]()
Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China.
Email: *xkyyhan@gmail.com
Copyright © 2013 Xiaofei Wang, Baohui Han. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received June 26th, 2013; revised July 29th, 2013; accepted August 6th, 2013
Keywords: NSCLC; EGFR-Targeting Tyrosine Kinase
ABSTRACT
With the advances in surgery, chemotherapy, and radiotherapy over the last decades, the treatment strategies of lung cancer has been largely changed. In this review, we summarize recent advances in lung cancer and treatment research. We discuss current clinical management, highlight stage-specific therapy approaches, chemotherapy options for advanced-stage of non-small-cell lung cancer (NSCLC) patients, along with new agents such as epidermal growth factor receptor (EGFR)-targeting tyrosine kinase inhibitors erlotinib and gefitinib, and the anaplastic lymphoma kinase (ALK) inhibitor crizotinib. We also give an outlook into NSCLC disease biology, focuse on the importance of EGFR activating mutations and the role of the tumor-microenvironment. Finally we summarize the new recommendations in treating small-cell-lung cancer (SCLC).
1. Introduction
Lung cancer is one of the most frequently diagnosed cancers and the leading causes of cancer death globally [1]. The most common type of lung cancer—accounting for approximately 85% of cases—is non-small cell lung cancer (NSCLC), which comprises two main histological types: adenocarcinoma (AD, ~30% of all NSCLC) and squamous cell carcinoma (SCC, ~50% of NSCLC). AD is derived from alveolar, bronchial or bronchiolar epithelial cells, whereas SCC is often centrally located, associated with smoking history, and originates from bronchial epithelial cells [2]. Over the past 10 years, there have been significant advances in clinical trials and translation research on lung cancer, especially on NSCLC. These advances have changed the clinical practice of lung cancer treatment.
2. NSCLC (New Pathologic Classification of Lung Cancer)
A significant change in pathologic classification of lung cancer occurred with the publication in 2011 of a new lung adenocarcinoma classification under the sponsorship of the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS). The classification outlines many paradigm shifts that affect clinical practice and opens new avenues for research [3]. Because 70% of patients with lung cancer present with advanced stages, a new approach to small biopsies and cytology with specific terminology and criteria focused on the need for distinguishing squamous cell carcinoma from adenocarcinoma and on molecular testing for EGFR mutations and ALK rearrangement. Tumors previously classified as non-small-cell carcinoma, not otherwise specified, because of the lack of clear squamous or adenocarcinoma morphology should be classified further by using a limited immunohistochemical workup to preserve tissue for molecular testing. The terms “bronchioloalveolar carcinoma” and “mixed subtype adenocarcinoma” have been discontinued. For resected adenocarcinomas, new concepts of adenocarcinoma in situ and minimally invasive adenocarcinoma define patients who, if they undergo complete resection, will have 100% disease-free survival. Invasive adenocarcinomas are now classified by predominant pattern after using comprehensive histologic subtyping with lepidic, acinar, papillary, and solid patterns; micropapillary is added as a new histologic subtype with poor prognosis. Former mucinous bronchioloalveolar carcinomas are now called “invasive mucinous adenocarcinoma.” Because the lung cancer field is now rapidly evolving with new advances occurring on a frequent basis, particularly in the molecular arena, this classification provides a much needed standard for pathologic diagnosis not only for patient care but also for clinical trials and TNM classification [4].
2.1. Surgery
Lung resection with video-assisted thoracoscopic access gained wide acceptance in the last decade. The new lung adenocarcinoma classification has profound surgical implications as the role of limited resection is reconsidered for early stage lesions. Surgery is curative in early stage disease. However, the role of surgery in locally advanced NSCLC remains controversial. The principal aim is a complete resection as this will determine long-term prognosis. Intra-operative staging of lung cancer is extremely important to determine the extent of resection according to the tumour and nodal status. Systematic nodal dissection is generally advocated to obtain accurate intra-operative staging and to help decide on adjuvant therapy. Lung resection with video-assisted thoracoscopic access gained wide acceptance [5].
2.2. Radiotherapy
Radiotherapy is an essential modality in the management of lung cancer. It is used as a single modality or in combination with other modalities and aimed at cure or palliation. Recent advances in the simulation techniques or more precise targeting of the tumor made radiotherapy more effective tool in the fight against lung cancer. New irradiation techniques such as image-guided radiotherapy (IGRT), stereotactic ablative radiotherapy (SABR), have rapidly entered routine care for early-stage peripheral non-small cell lung cancer in many countries. In the last 2 years, a growing body of publications has reported on clinical outcomes, acute and late radiological changes after SABR, and sub-acute and late toxicity. The local control rates in many publications have exceeded 90% when tumors of up to 5 cm have been treated, with corresponding regional nodal failure rates of approximately 10%. High-grade toxicity is uncommon when so-called “risk-adapted” fractionation schemes are applied; an approach which involves the use of lower daily doses and more fractions when critical normal organs are in the proximity of the tumor volume [6]. The benefit of postoperative radiation therapy is still discussed but usually accepted in patients with metastatic mediastinal lymph nodes and/or positive surgical margins [7]. Brain metastases are a common cause of morbidity and death in patients with NSCLC. In patients with multiple cerebral metastases, whole brain radiotherapy (WBRT) is generally the treatment of choice, as it addresses both macroscopic and microscopic disease. The Radiation Therapy Oncology Group recently completed a large Phase III trail evaluating this approach in 331 patients with good performance status (Karnofsky performance status >70) and one to three brain metastases [8] This study demonstrated an increase in local brain control from 62% with WBRT alone to 91% when stereotactic radio-surgery was added to WBRT. Although the addition of SRS to WBRT did not demonstrate improved survival over WBRT alone in patients with multiple intracranial metastases, the study did find an increase in median survival time in patients with single metastases: The median survival in patients treated with WBRT alone was 4.9 months, while in patients treated with WBRT and SRS the median survival was increased to 6.5 months.
2.3. Chemotherapy
In patients with resectable stage I-II disease, postoperative chemotherapy for stage II-III disease is now standard of care. The LACE meta-analysis pooled 4584 patients accrued in 5% cisplatin-based adjuvant trials. Adjuvant chemotherapy was associated with a 5.3% improvement of survival at 5 years (p = 0.0043) [9,10]. Disease-free survival was also improved (5.2% at 5 years, p < 0.0001). The risk reduction was 8% for stage IB and 17% for stages II and III. Adjuvant chemotherapy is discussed on a case by case basis in stage IB, especially in patients with a tumor larger than 4 cm, but is not recommended for stage IA where it has been reported to be deleterious. Generally, patients with advanced stage are treated with chemotherapy. Traditionally, platinum-based regimens are used, but many other agents such as taxanes, pemetrexed, and gemcitabine are also common. Maintenance therapy has recently become a treatment paradigm for advanced NSCLC. Maintenance therapy, which is designed to prolong a clinically favorable state after completion of a predefined number of induction chemotherapy cycles, has two principal paradigms. Continuation maintenance therapy entails the ongoing administration of a component of the initial chemotherapy regimen, generally the nonplatinum cytotoxic drug or a molecular targeted agent. With switch maintenance (also known as sequential therapy), a new and potentially non-crossresistant agent is introduced immediately on completion of first-line chemotherapy. Potential rationales for maintenance therapy include increased exposure to effective therapies, decreasing chemotherapy resistance, optimizing efficacy of chemotherapeutic agents, antiangiogenic effects, and altering antitumor immunity. To date, switch maintenance therapy strategies with pemetrexed [11] and erlotinib [12] have demonstrated improved overall survival, resulting in US Food and Drug Administration approval for this indication. Recently, continuation maintenance with pemetrexed was found to prolong overall survival as well [13].
2.4. Personalized Therapy
NSCLC was considered to be a single disease and same treatment has been used for all patients for the past few decades, the median overall survival was persistently less than 12 months [14,15]. The first breakthrough towards personalized treatment took place in 2004 when Lynch et al. [16] and Paez et al. [17] reported the presence of activating mutations in the tyrosine kinase domain of EGFR in patients who had a dramatic response to the EGFR tyrosine kinase inhibitor (TKI), gefitinib. The importance of biomarker selection is further supported by four other randomized studies that selected patients with EGFR mutations, who were randomized to receive either EGFR TKIs or chemotherapy (Table 1) [18-21]. The consistently higher response rates and longer PFS have confirmed EGFR TKIs to be a standard therapy for patients with activating mutations in EGFR. Since then, personalized medicine for lung cancer has become a reality. Molecular targeted treatment, with broadening opportunities, plays an important role in the management of lung cancer patients, which renders molecular mapping of the tumor tissue crucial.
Development of inhibitors of the EML4-ALK fusion gene product took a different path. It was previously established that chromosomal translocation of the ALK gene that fuses with nucleophosmin (NPM) results in the expression of an oncogene that drives tumor growth in lymphoma [22]. Soda et al. [23] reported a similar type of translocation in NSCLC that resulted in aberrant fusion of ALK with EML4 and encoded a cytoplasmic chimeric protein with kinase activity. In an in vivo study, the researchers showed that nude mice injected subcutaneously with 3T3 fibroblasts transformed with either the EML4-ALK or NPM-ALK fusion gene had substantial tumor growth, whereas tumor growth was not observed with transfection of the ALK or EML4 genes alone. This finding suggested that the EML4-ALK fusion gene was a driver gene of tumorigenesis. Kwak et al. [24] then reported the first phase II study of crizotinib, an ALK inhibitor, in patients with the EML4-ALK fusion gene. After screening approximately 1500 patients for ALK rearrangements, the researchers found 82 ALK-positive patients who were eligible for the study, 96% of whom had adenocarcinoma and 76% of whom were nonsmokers and also had adenocarcinoma. The tumor response rate was 57%, and 33% of patients achieved stable disease; PFS was approximately 9.2 months. Similar to EGFR mutations, EML4-ALK has been established as an important molecular target for the management of lung cancer. The major clinical characteristics of patients with the EML4-ALK fusion gene are similar to those of patients with EGFR mutations, namely non-smoker and adenocarcinoma.
Molecular-targeted therapies can be effective, but almost all treated tumors will eventually become resistant and progress. Knowing the mechanisms of resistance will help to manage patients at disease progression, and may prevent or defer the process. The main reasons for primary resistance to EGFR TKIs include the absence of EGFR mutations and presence of KRAS mutations, and the two mutations are essentially mutually exclusive [25]. The two major mechanisms of acquired resistance are the EGFR point mutation T790M in exon 20 and overexpression of the components of the hepatocyte growth factor receptor (HGFR, also known as MET) pathway [26-29]. Wenjun et al. [30] identify a covalent pyrimidine EGFR inhibitor by screening an irreversible kinase inhibitor library specifically against EGFR T790M. These agents are proven to be effective in murine models of lung cancer driven by EGFR T790M.
Overexpression of the MET pathway accounts for about 20% of TKI resistance [31]. Tivantinib is a novel TKI targeted against MET. A randomized phase II study in patients with non-squamous cell histology comparing a combination of tivantinib and erlotinib with erlotinib alone reported an improvement in PFS (HR = 0.61, P <0.05) and overall survival (HR = 0.58, P < 0.05) favoring the combination arm [32]. Biomarkers, including MET expression by FISH and EGFR mutations by PCR, were not predictive, whereas a small number of patients
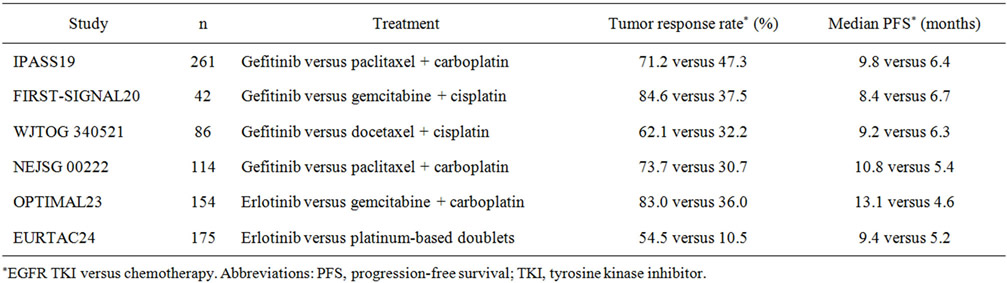
Table 1. Treatment outcome in patients with EGFR mutations after treatment with a EGFR TKI or chemotherapy.
with KRAS-mutant tumors seemed to survive longer with the combination therapy. Targeted therapies inhibit cancer proliferation continuously. In cases in which the tumor progresses slowly in the presence of a TKI, it is better for the patient to continue with the drug, as cessation of inhibition may lead to rapid tumor growth [33]. The concept of personalized medicine should apply to both treatment selection and treatment cessation.
Unlike the “tumor cell-centered” treatment, the malignant microenvironment is now accepted as a key element for cancer growth and spread, and the scientific community appreciates the large contribution of the microenvironment to tumor progression [34]. Recent data suggest that tumor-stromal interactions may drive carcinogenesis and that the tumor compartment is not only for support. As the tumor develops, the surrounding stroma coevolves, providing tumor cells with growth factors and favorable matrix components that foster proliferation, migration and colonization of distant organs. Bone marrow-derived cells like lymphocytes, macrophages, neutrophils and mast cells (MCs) are recruited to the lung in response to lung damage. Together with fibroblasts, endothelial cells and pericytes, they orchestrate to form the tumor microenvironment (TME) [35]. Targeting the TME is a promising approach for tumor management. VEGFR is one of the molecules expressed by cells in the TME that has become a central target for antiangiogenic treatment strategies. Bevacizumab (Avastin, Roche, Basilea, Switzerland) is a monoclonal antibody directed against VEGF that constitutes the only antiangiogenic agent approved by the Food and Drug Administration (FDA) for NSCLC. Because of the possible development of resistance to anti-VEGF therapy, some researchers have suggested that targeting multiple pro-angiogenic pathways, such as PDGF and FGF, may represent a novel therapeutic approach for treatment of NSCLC [36].
2.5. Others
Mesenchymal stem cells (MSCs) are a type of bone marrow-derived stem cell, which can differentiate in vitro into osteoblasts, chondrocytes and adipocytes. It has been widely demonstrated that MSCs home to and infiltrate into areas of new stroma formation possibly forming crucial stromal support [37]. The precise mechanism of homing of MSCs to the tumours is not fully mapped, however, the ability of MSCs to home effectively to tumours makes them an attractive therapeutic option. TRAIL (tumour necrosis factor-related apoptosis-inducing ligand) is the most studied and well-characterised pro-apoptotic agent widely accepted to be ideal as MSC cargo. TRAIL is believed to induce apoptosis in transformed cells with virtually no effect on normal cells [38]. The selective tumour-specific cytotoxicity of TRAIL has led to hailing it as a “silver bullet” for the treatment of cancer. Scientists have engineered MSC to constitutively express TRAIL. This has been demonstrated effective in several models, including including glioma [39], pancreatic cancer [40] and a lung metastasis model [41].
Therapeutic cancer vaccines engaged in non-small-cell lung cancer use different platforms: peptides, cellular vaccines and microbial vectors; they have shown clinical and immunological activity. L-BLP25 is a therapeutic vaccine designed to induce a T-cell-mediated anti-MUC- 1 response to cancer cells expressing the MUC-1 antigen. MUC-1 is a component of the glycocalyx protecting the epithelia but the molecule also has a regulatory activity through its C-terminal intracellular domain. The MUC-1 protein can be found at the apical surface of mucin-secreting epithelial cells in many types of epithelial tissues and frequently overexpressed in cancers of epithelial origin. The expression of MUC-1 in NSCLC has been proven to be associated with a worse prognosis in several studies [42,43]. In a randomized Phase II trial [44], 171 patients received subcutaneous vaccinations of L-BLP25 930 μg weekly for 8 weeks, followed by maintenance vaccinations at 6-week intervals plus best supportive care (BSC) or received BSC alone. Median survival time was longer in patients receiving L-BLP25 plus BSC compared with those receiving BSC alone (17.2 vs 13.0 months, respectively). The 3-year survival rate was 31% in patients who received L-BLP25 plus BSC and 17% in those who received BSC alone. In the subset of patients with stage IIIb locoregional disease, median survival time was longer in patients who received L-BLP25 plus BSC than in those who received BSC alone (30.6 vs 13.3 months, respectively. No major adverse events except some temporary injection site reactions were reported; no autoimmune reactions were noted. The vaccines are used either in monotherapy or in combination with chemotherapy. The most advanced products in development today target different nonoverlapping subpopulations of non-small-cell lung cancer patients.
2.6. SCLC
Small-cell lung cancer (SCLC) comprises approximately 15% of all lung cancer malignancies [45], and is invariably associated with tobacco exposure. The use of PET scanning is likely to improve the accuracy of staging [46,47]. The staging classification should include both the old Veterans Administration staging classification of LS and ES, as well as the new seventh edition American Joint Committee on Cancer/International Union Against Cancer staging by TNM. Comparing with NSCLC, little innovation in the treatment of this disease has been achieved over the past 30 years. Surgery is indicated for carefully selected stage I SCLC. Limited stage (LS) disease should be treated with concurrent chemoradiotherapy in patients with good performance status. Thoracic radiotherapy should be administered early in the course of treatment, preferably beginning with cycle 1 or 2 of chemotherapy. Chemotherapy should consist of four cycles of a platinum agent and etoposide. Extensive stage (ES) disease should be treated primarily with chemotherapy consisting of a platinum agent plus etoposide or irinotecan. Prophylactic cranial irradiation prolongs survival in those individuals with both LS and ES disease who achieve a complete or partial response to initial therapy. With the exception of refinements of the dose and schedule of thoracic radiation therapy for limitedstage disease [48-52] and a better understanding of the role of prophylactic cranial irradiation following completion of first-line therapy [53], the two cytotoxic agents cisplatin/carboplatin and etoposide (developed in the 1960-1970s) remain the backbone of SCLC therapy. Several other platinum-based regimens, particularly platinum plus irinotecan, have been evaluated in phase II and III trials, but none have proven superior to EP, and they frequently carry added toxicity. Many other strategies, including maintenance therapy, alternating regimens, triplet therapy, dose-intense regimens, and dosedense chemotherapy, have failed to demonstrate consistent benefits, and many of these approaches have led to unacceptable toxicity [54]. Thalidomide and Bevacizumab, were tested in was tested in trials as antiangiogenic agents, none has demonstrated clinical benefit [55-57]. In patients with recurrent SCLC, single agent chemotherapy with amrubicin results in higher response rates than does topotecan, however, survival is poor in these patients and despite better response, overall survival does not appear to be improved [58,59]. As the genomic studies continue to evolve, we anticipate that SCLCs will be characterized in greater detail, thus allowing for a personalized, targeted therapy approach for patients with this disease. As an example, Pleasance et al recently completed the first full sequencing of a SCLC cell line (NCI-H209 cells) genome [60]. They identified a total of 22,910 somatic substitutions, 65 indels, 334 copy number segments, and 58 structural variants. Hopefully, with the use of integrative and innovative translational approaches, the oncology research community will be able to streamline resources and rapidly develop more-effective therapies for SCLC.
3. Conclusion
Personalized medicine is now a reality for patients with NSCLC. The successful development of personalized therapy is founded on knowledge of a specific target that drives cancer growth, validation of a clinically applicable biomarker, acceptance of a rational end point, and understanding of the mechanisms of resistance. Therefore, future developments will have to identify novel molecular targets that drive cancer growth in SCLC. It is likely that these targets are relatively uncommon and drug development will be difficult, but this is a challenge worth undertaking.
REFERENCES
- A. Jemal, F. Bray, M. M. Center, J. Ferlay, E. Ward, et al., “Global Cancer Statistics,” CA: A Cancer Journal for Clinicians, Vol. 61, No. 2, 2011, pp. 69-90. doi:10.3322/caac.20107
- C. J. Langer, B. Besse, A. Gualberto, et al., “The Evolving Role of Histology in the Management of Advanced Non-Small-Cell Lung Cancer,” Journal of Clinical Oncology, Vol. 28, No. 36, 2010, pp. 5311-5320. doi:10.1200/JCO.2010.28.8126
- W. D. Travis, E. Brambilla, M. Noguchi, et al., “International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma,” Journal of Thoracic Oncology, Vol. 6, No. 2, 2011, pp. 244-285. doi:10.1097/JTO.0b013e318206a221
- W. D. Travis, E. Brambilla and G. J. Riely, “New Pathologic Classification of Lung Cancer: Relevance for Clinical Practice and Clinical Trials,” Journal of Clinical Oncology, Vol. 31, No. 8, 2013, pp. 992-1001. doi:10.1200/JCO.2012.46.9270
- P. McCloskey, B. Balduyck, P. E. Van Schil, et al., “Radical Treatment of Non-Small Cell Lung Cancer during the Last 5 Years,” European Journal of Cancer, Vol. 49, No. 7, 2013, pp. 1555-1564. doi:10.1016/j.ejca.2012.12.023
- S. Senan, D. A. Palma and F. J. Lagerwaard, “Stereotactic Ablative Radiotherapy for Stage I NSCLC: Recent Advances and Controversies,” Journal of Thorac Disease, Vol. 3, No. 3, 2011, pp. 189-196.
- B. E. Lally, D. Zelterman, J. M. Colasanto, et al., “Postoperative Radiotherapy for Stage II or III Non-Small-Cell Lung Cancer Using the Surveillance, Epidemiology, and End Results Database,” Journal of Clinical Oncology, Vol. 24, No. 19, 2006, pp. 2998-3006. doi:10.1200/JCO.2005.04.6110
- D. W. Andrews, C. B. Scott, P. W. Sperduto, et al., “Whole Brain Radiation Therapy with or without Stereotactic Radiosurgery Boost for Patients with One to Three Brain Metastases: Phase III Results of the RTOG 9508 Randomised Trial,” Lancet, Vol. 363, No. 9422, 2004, pp. 1665-1672. doi:10.1016/S0140-6736(04)16250-8
- J. P. Pignon, H. Tribodet, G. V. Scagliotti, et al., “Lung Adjuvant Cisplatin Evaluation: A Pooled Analysis by the LACE Collaborative Group,” Journal of Clinical Oncology, Vol. 26, No. 21, 2008, pp. 3552-3559. doi:10.1200/JCO.2007.13.9030
- G. M. Strauss, J. E. Herndon II, M. A. Maddaus, et al., “Adjuvant Paclitaxel plus Carboplatin Compared with Observation in Stage IB Non-Small-Cell Lung Cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups,” Journal of Clinical Oncology, Vol. 26, No. 31, 2008, pp. 5043-5051. doi:10.1200/JCO.2008.16.4855
- T. Ciuleanu, T. Brodowicz, C. Zielinski, et al., “Maintenance Pemetrexed plus Best Supportive Care versus Placebo plus Best Supportive Care for Non-Small-Cell Lung Cancer: A Randomised, Double-Blind, Phase 3 Study,” Lancet, Vol. 374, No. 9699, 2009, pp. 1432-1440. doi:10.1016/S0140-6736(09)61497-5
- F. Cappuzzo, T. Ciuleanu, L. Stelmakh, et al., “Erlotinib as Maintenance Treatment in Advanced Non-Small-Cell Lung Cancer: A Multicentre, Randomised, Placebo-Controlled Phase 3 Study,” Lancet Oncology, 11, No. 6, 2010, pp. 521-529. doi:10.1016/S1470-2045(10)70112-1
- L. Paz-Ares, F. de Marinis, M. Dediu, et al., “Maintenance Therapy with Pemetrexed plus Best Supportive Care versus Placebo plus Best Supportive Care after Induction Therapy with Pemetrexed plus Cisplatin for Advanced Non-Squamous Non-Small-Cell Lung Cancer (PARAMOUNT): A Double-Blind, Phase 3, Randomised Controlled Trial,” Lancet Oncology, Vol. 13, No. 3, 2012, pp. 247-255.
- NSCLC Meta-Analyses Collaborative Group, “Chemotherapy in Addition to Supportive Care Improves Survival in Advanced Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis of Individual Patient Data from 16 Randomized Controlled Trials,” Journal of Clinical Oncology, Vol. 26, No. 28, 2008, pp. 4617-4625. doi:10.1200/JCO.2008.17.7162
- C. G. Azzoli, S. Baker Jr., S. Temin, et al., “American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small-Cell Lung Cancer,” Journal of Clinical Oncology, Vol. 27, No. 36, 2009, pp. 6251-6266. doi:10.1200/JCO.2009.23.5622
- T. J. Lynch, D. W. Bell, R. Sordella, et al., “Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non-Small-Cell Lung Cancer to Gefitinib,” New England Journal of Medicine, Vol. 350, 2004, pp. 2129-2139. doi:10.1056/NEJMoa040938
- J. G. Paez, P. A. Jänne, J. C. Lee, et al., “EGFR Mutations in Lung Cancer: Correlation with Clinical Response to Gefitinib Therapy,” Science, Vol. 304, No. 5676, 2004, pp. 1497-1500. doi:10.1126/science.1099314
- T. Mitsudomi, S. Morita, Y. Yatabe, et al., “Gefitinib versus Cisplatin plus Docetaxel in Patients with NonSmall-Cell Lung Cancer Harbouring Mutations of the Epidermal Growth Factor Receptor (WJTOG3405): An Open Label, Randomised Phase 3 Trial,” Lancet Oncology, Vol. 11, No. 2, 2010, pp. 121-128. doi:10.1016/S1470-2045(09)70364-X
- M. Maemondo, A. Inoue, K. Kobayashi, et al., “Gefitinib or Chemotherapy for Non-Small-Cell Lung Cancer with Mutated EGFR,” New England Journal of Medicine, Vol. 362, 2010, pp. 2380-2388. doi:10.1056/NEJMoa0909530
- C. Zhou, Y. L. Wu, G. Chen, et al., “Erlotinib versus Chemotherapy as First-Line Treatment for Patients with Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (OPTIMAL, CTONG-0802): A Multicentre, OpenLabel, Randomised, Phase 3 Study,” Lancet Oncology, Vol. 12, No. 8, 2011, pp. 735-742. doi:10.1016/S1470-2045(11)70184-X
- R. Rosell, E. Carcereny, R. Gervais, et al., “Erlotinib versus Standard Chemotherapy as First-Line Treatment for European Patients with Advanced EGFR MutationPositive Non-Small-Cell Lung Cancer (EURTAC): A Multicentre, Open-Label, Randomised Phase 3 Trial,” Lancet Oncology, Vol. 13, No. 3, 2012, pp. 239-246. doi:10.1016/S1470-2045(11)70393-X
- M. Shiota and S. Mori, “The Clinicopathological Features of Anaplastic Large Cell Lymphomas Expressing p80NPM/ ALK,” Leuk Lymphoma, Vol. 23, 1996, pp. 25-32. doi:10.3109/10428199609054798
- M. Soda, Y. L. Choi, M. Enomoto, et al., “Identification of the Transforming EML4-ALK Fusion Gene in NonSmall-Cell Lung Cancer,” Nature, Vol. 448, No. 7153, 2007, pp. 561-566.
- E. L. Kwak, Y. J. Bang, D. R. Camidge, et al., “Anaplastic Lymphoma Kinase Inhibition in Non-Small-Cell Lung Cancer,” New England Journal of Medicine, Vol. 363, 2010, pp. 1693-1703. doi:10.1056/NEJMoa1006448
- G. M. Stella, R. Scabini, S. Inghilleri, et al., “EGFR and KRAS Mutational Profiling in Fresh Non-Small Cell Lung Cancer (NSCLC) Cells,” Journal of Cancer Research and Clinical Oncology, Vol. 139, No. 8, 2013, pp. pp. 1327-1335. doi:10.1007/s00432-013-1444-y
- S. Kobayashi, T. J. Boggon, T. Dayaram, et al., “EGFR Mutation and Resistance of Non-Small-Cell Lung Cancer to Gefitinib,” New England Journal of Medicine, Vol. 352, 2005, pp. 786-792. doi:10.1056/NEJMoa044238
- W. Pao, V. A. Miller, K. A. Politi, et al., “Acquired Resistance of Lung Adenocarcinomas to Gefitinib or Erlotinib Is Associated with a Second Mutation in the EGFR Kinase Domain,” PLoS Medicine, Vol. 2, No. 3, 2005, p. e73.
- J. A. Engelman, K. Zejnullahu, T. Mitsudomi, et al., “MET Amplification Leads to Gefitinib Resistance in Lung Cancer by Activating ERBB3 Signaling,” Science, Vol. 316, No. 5827, 2007, pp. 1039-1043. doi:10.1126/science.1141478
- J. Bean, C. Brennan, J. Y. Shih, et al., “MET Amplification Occurs with or without T790M Mutations in EGFR Mutant Lung Tumors with Acquired Resistance to Gefitinib or Erlotinib,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 104, No. 52, 2007, pp. 20932-20937. doi:10.1073/pnas.0710370104
- W. Zhou, D. Ercan, L. Chen, et al., “Novel Mutant-Selective EGFR Kinase Inhibitors against EGFR T790M,” Nature, Vol. 462, 2009, pp. 1070-1074. doi:10.1038/nature08622
- J. A. Engelman, K. Zejnullahu, T. Mitsudomi, et al., “MET Amplification Leads to Gefitinib Resistance in Lung Cancer by Activating ERBB3 Signaling,” Science, Vol. 316, No. 5827, 2007, pp. 1039-1043. doi:10.1126/science.1141478
- C. Belani, “The Role of Irreversible EGFR Inhibitors in the Treatment of Non-Small Cell Lung Cancer: Overcoming Resistance to Reversible EGFR Inhibitors,” Cancer Investigation, Vol. 28, No. 4, 2010, pp. 413-423.
- G. J. Riely, M. G. Kris, B. Zhao, et al., “Prospective Assessment of Discontinuation and Reinitiation of Erlotinib or Gefitinib in Patients with Acquired Resistance to Erlotinib or Gefitinib Followed by the Addition of Everolimus,” Clinical Cancer Research, Vol. 13, 2007, pp. 5150-5155. doi:10.1158/1078-0432.CCR-07-0560
- K. Pietras and A. Ostman, “Hallmarks of Cancer: Interactions with the Tumor Stroma,” Experimental Cell Research, Vol. 316, No. 8, 2010, pp. 1324-1331. doi:10.1016/j.yexcr.2010.02.045
- Y. Shaked and E. E. Voest, “Bone Marrow Derived Cells in Tumor Angiogenesis and Growth: Are They the Good, the Bad or the Evil?” Biochimica et Biophysica Acta, Vol. 1796, No. 1, 2009, pp. 1-4.
- O. Casanovas, D. J. Hicklin, G. Bergers and D. Hanahan, “Drug Resistance by Evasion of Antiangiogenic Targeting of VEGF Signaling in Late-Stage Pancreatic Islet Tumors,” Cancer Cell, Vol. 8, No. 4, 2005, pp. 299-309. doi:10.1016/j.ccr.2005.09.005
- D. Bonnet, “Biology of Human Bone Marrow Stem Cells,” Clinical and Experimental Medicine, Vol. 3, No. 3, 2003, pp. 140-149. doi:10.1007/s10238-003-0017-9
- H. N. LeBlanc and A. Ashkenazi, “Apo2L/TRAIL and Its Death and Decoy Receptors,” Cell Death & Differentiation, Vol. 10, No. 1, 2003, pp. 66-75. doi:10.1038/sj.cdd.4401187
- L. G. Menon, K. Kelly, H. W. Yang, et al., “Human Bone Marrow-Derived Mesenchymal Stromal Cells Expressing S-TRAIL as a Cellular Delivery Vehicle for Human Glioma Therapy,” Stem Cells, Vol. 27, No. 9, 2009, pp. 2320-2330. doi:10.1002/stem.136
- A. Mohr, S. M. Albarenque, L. Deedigan, et al., “Targeting of XIAP Combined with Systemic Mesenchymal Stem Cell-Mediated Delivery of sTRAIL Ligand Inhibits Metastatic Growth of Pancreatic Carcinoma Cells,” Stem Cells, Vol. 28, No. 11, 2010, pp. 2109-2120. doi:10.1002/stem.533
- L. Kucerova, V. Altanerova, M. Matuskova, et al., “Adipose Tissue-Derived Human Mesenchymal Stem Cells Mediated Prodrug Cancer Gene Therapy,” Cancer Research, Vol. 67, No. 13, 2007, pp. 6304-6313. doi:10.1158/0008-5472.CAN-06-4024
- F. Guddo, A. Giatromanolaki, M. I. Koukourakis, et al., “MUC1 (Episialin) Expression in Non-Small Cell Lung Cancer Is Independent of EGFR and c-erbB-2 Expression and Correlates with Poor Survival in Node Positive Patients,” Journal of Clinical Pathology, Vol. 51, No. 9, 1998, pp. 667-671. doi:10.1136/jcp.51.9.667
- A. Ohgami, T. Tsuda, T. Osaki, et al., “MUC1 Mucin mRNA Expression in Stage I Lung Adenocarcinoma and Its Association with Early Recurrence,” The Annals of Thoracic Surgery, Vol. 67, No. 3, 1999, pp. 810-814. doi:10.1016/S0003-4975(99)00041-7
- C. Butts, A. Maksymiuk, G. Goss, et al., “Updated Survival Analysis in Patients with Stage IIIB or IV NonSmall-Cell Lung Cancer Receiving BLP25 Liposome Vaccine (L-BLP25): Phase IIB Randomized, Multicenter, Open-Label Trial,” Journal of Cancer Research and Clinical Oncology, Vol. 137, No. 9, 2011, pp. 1337-1342. doi:10.1007/s00432-011-1003-3
- R. Govindan, N. Page, D. Morgensztern, et al., “Changing Epidemiology of Small-Cell Lung Cancer in the United States over the Last 30 Years: Analysis of the Surveillance, Epidemiologic, and End Results Database,” Journal of Clinical Oncology, Vol. 24, No. 28, 2006, pp. 4539-4544. doi:10.1200/JCO.2005.04.4859
- V. Kut, W. Spies, S. Spies, et al., “Staging and Monitoring of Small Cell Lung Cancer Using [18F]Fluoro-2-DeoxyD-Glucose-Positron Emission Tomography (FDG-PET),” American Journal of Clinical Oncology, Vol. 30, No. 1, 2007, pp. 45-50. doi:10.1097/01.coc.0000239095.09662.19
- J. van Loon, D. de Ruysscher, R. Wanders, et al., “Selective Nodal Irradiation on Basis of (18)FDG-PET Scans in Limiteddisease Small-Cell Lung Cancer: A Prospective Study,” International Journal of Radiation Oncology Biology Physics, Vol. 77, No. 2, 2010, pp. 329-336. doi:10.1016/j.ijrobp.2009.04.075
- S. G. Spiro, L. E. James, R. M. Rudd, et al., “Early Compared with Late Radiotherapy in Combined Modality Treatment for Limited Disease Small-Cell Lung Cancer: A London Lung Cancer Group Multicenter Randomized Clinical Trial and Meta-Analysis,” Journal of Clinical Oncology, Vol. 24, No. 24, 2006, pp. 3823-3830. doi:10.1200/JCO.2005.05.3181
- D. B. Fried, D. E. Morris, C. Poole, et al., “Systematic Review Evaluating the Timing of Thoracic Radiation Therapy in Combined Modality Therapy for LimitedStage Small-Cell Lung Cancer,” Journal of Clinical Oncology, Vol. 22, No. 23, 2004, pp. 4837-4845. doi:10.1200/JCO.2004.01.178
- D. de Ruysscher, M. Pijls-Johannesma, S. M. Bentzen, et al., “Time between the First Day of Chemotherapy and the Last Day of Chest Radiation Is the Most Important Predictor of Survival in Limited-Disease Small-Cell Lung Cancer,” Journal of Clinical Oncology, Vol. 24, No. 7, 2006, pp. 1057-1063. doi:10.1200/JCO.2005.02.9793
- D. de Ruysscher, M. Pijls-Johannesma, J. Vansteenkiste, et al., “Systematic Review and Meta-Analysis of Randomised, Controlled Trials of the Timing of Chest Radiotherapy in Patients with Limited-Stage, Small-Cell Lung Cancer,” Annals of Oncology, Vol. 17, No. 4, 2006, pp. 543-552. doi:10.1093/annonc/mdj094
- M. Huncharek, and R. McGarry, “A Meta-Analysis of the Timing of Chest Irradiation in the Combined Modality Treatment of Limited-Stage Small Cell Lung Cancer,” Oncologist, Vol. 9, No. 6, 2004, pp. 665-672. doi:10.1634/theoncologist.9-6-665
- B. Slotman, C. Faivre-Finn, G. Kramer, et al., “Prophylactic Cranial Irradiation in Extensive Small-Cell Lung Cancer,” The New England Journal of Medicine, Vol. 357, 2007, pp. 664-672. doi:10.1056/NEJMoa071780
- J. R. Jett, S. E. Schild, K. A. Kesler and G. P. Kalemkerian, “Treatment of Small Cell Lung Cancer: Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines,” Chest, 2013, Vol. 143, Suppl. 5, pp. e400S-e419S.
- S. M. Lee, P. J. Woll, R. Rudd, et al., “Anti-Angiogenic Therapy Using Thalidomide Combined with Chemotherapy in Small Cell Lung Cancer: A Randomized, Double-Blind, Placebo-Controlled Trial,” Journal of the National Cancer Institute, Vol. 101, No. 15, 2009, pp. 1049-1057. doi:10.1093/jnci/djp200
- N. E. Ready, A. Z. Dudek, H. H. Pang, et al., “Cisplatin, Irinotecan, and Bevacizumab for Untreated ExtensiveStage Small-Cell Lung Cancer: CALGB 30306, a Phase II Study,” Journal of Clinical Oncology, Vol. 29, No. 33, 2011, pp. 4436-4441. doi:10.1200/JCO.2011.35.6923
- D. R. Spigel, F. A. Greco, J. D. Zubkus, et al., “Phase II Trial of Irinotecan, Carboplatin, and Bevacizumab in the Treatment of Patients with Extensive-Stage Small-Cell Lung Cancer,” Journal of Thoracic Oncology, Vol. 4, No. 12, 2009, pp. 1555-1560. doi:10.1097/JTO.0b013e3181bbc540
- A. Inoue, S. Sugawara, K. Yamazaki, et al., “Randomized Phase II Trial Comparing Amrubicin with Topotecan in Patients with Previously Treated Small-Cell Lung Cancer: North Japan Lung Cancer Study Group Trial 0402,” Journal of Clinical Oncology, Vol. 26, No. 33, 2008, pp. 5401-5406. doi:10.1200/JCO.2008.18.1974
- R. Jotte, P. Conkling, C. Reynolds, et al., “Randomized Phase II Trial of Single-Agent Amrubicin or Topotecan as Second-Line Treatment in Patients with Small-Cell Lung Cancer Sensitive to First-Line Platinum-Based Chemotherapy,” Journal of Clinical Oncology, Vol. 29, No. 3, 2011, pp. 287-293. doi:10.1200/JCO.2010.29.8851
- [61] E. D. Pleasance, P. J. Stephens, S. O’Meara, et al., “A Small-Cell Lung Cancer Genome with Complex Signatures of Tobacco Exposure,” Nature, Vol. 463, No. 7278, 2010, pp. 184-190. doi:10.1038/nature08629
NOTES
*Corresponding author.

