Chinese Medicine
Vol. 3 No. 1 (2012) , Article ID: 18052 , 12 pages DOI:10.4236/cm.2012.31006
Inhibitory Activity on Xanthine Oxidase and Antioxidant Properties of Teucrium polium L. Extracts
1Department of Biology, University Bachir El-Ibrahimi, Bordj Bou Arreridj, Algeria
2Laboratory of Applied Biochemistry, Department of Biochemistry, Faculty of Nature and Life Sciences, University Ferhat Abbas, Setif, Algeria
3Laboratory of Chronic Diseases, Department of Biology and Physiology, Faculty of Nature and Life Sciences, University Ferhat Abbas, Setif, Algeria
Email: lekharrar@hotmail.com, baghiani@yahoo.fr
Received January 10, 2012; revised February 2, 2012; accepted February 13, 2012
Keywords: Xanthine Oxidase; Antioxidant; Superoxide Scavenger; Teucrium polium
ABSTRACT
The antioxidative activities of subfractions; methanol (CE), chloroform (CHE) and ethyl acetate (EAE) of Teucrium polium extracts (TPE) were investigated. HPLC analysis of the plant revealed the existence of procyanidins B1 and B2, gallic acid, catechin and epicatechin. All the extracts showed inhibitory properties on xanthine oxidase, with IC50 ranging from 0.80 ± 0.07 to 11.76 ± 0.50 µM/quercetin equivalent. In the cellular system, all the extracts showed a protective effect greater than those of quercetin, rutin and gallic acid against t-BHP induced oxidative damages in human erythrocytes. These results were clearly confirmed by a modified thiobarbituric acid-reactive species (TBARS), and β-carotene/linoleic acid assay which demonstrated that CHE possess an inhibition ratio of the linoleic acid oxidation (83.11%) close to that of BHT (96.77%). In addition, the results showed that the extracts possess a potent DPPH radical scavenging activity and gave a reduction power greater than rutin, quercetin, gallic acid and ascorbic acid in FRAP assay. These results show that Teucrium polium extracts have strong antioxidant effects and may have some clinical benefits.
1. Introduction
Oxidative stress is defined in general as excess formation and/or incomplete removal of highly reactive molecules such as reactive oxygen species (ROS). In vivo, some of these ROS play a positive role such as energy production, phagocytosis, regulation of cell growth and intracellular signaling [1]. On the other hand, ROS are also capable of damaging a wide range of essential biomolecules including proteins, DNA and lipids [2], eventually leading to many chronic diseases, such as atherosclerosis, cancer, diabetes, aging, and other degenerative diseases in humans [3]. One of the very important enzyme that has been reported to increase during oxidative stress is xanthine oxidase (XO), which is conventionally seen as a late enzyme of purine catabolism, catalysing the oxidation of hypoxanthine to xanthine and of xanthine to uric acid [4]. In the context of the inflammatory response, XO is believed to combat infection by generating ROS and can be seen as an agent of innate immunity [5]. Hyperuricemia leads to the accumulation of uric acid in joints and kidneys causing acute arthritis and uric acid nephrolithiasis. One therapeutic approach for gout is the use of XO inhibitors such as allopurinol [6], however, allopurinol use can result in a number of adverse side effects [7], ranging from mild skin allergy to a concerted allopurinol hypersensitivity syndrome [8]. Thus, there is a need to develop compounds with XO inhibitory activities but devoid of the undesirable effects of allopurinol. There is a growing interest in natural phenolic antioxidants, present in medicinal plants that might help attenuate oxidative damage [9]. Epidemiological studies have shown that many of these antioxidant compounds possess anti-inflammatory, antiatherosclerotic, antitumor, antimutagenic, anticarcinogenic, antibacterial, or antiviral activities to a greater or lesser extent [3], and it was found that their antioxidant activity surpasses the effect of known antioxidants, such as the vitamins A and E [9]. The traditional medicinal plant Teucrium polium L. (Labiatae) is a perennial shrub widely distributed in the hills and deserts of Mediterranean countries [10]. This plant is used to prepare herbal tea, which is used as an appetizer especially in children and also as a spice. An infusion of the leaves and flowers of the plant is consumed as a refreshing beverage [11]. Teucrium polium and related species (Teucrium oliverianum and Teucrium mascatense) are commonly used in folk medicine for various types of pathological conditions. Previous studies have demonstrated some of the pharmacological effects of Teucrium polium such as anti-bacterial [12], anti-inflammatory [13, 14], anti-ulcerogenic [15], anti-nociceptive [14,16], antidiabetes [17], antihypertensive [18], hypo-lipidemic [19] and calcium antagonist [20]. The goal of this study was to investigate the antioxidant and radical scavenging effects of the T poluim and its active fractions by applying various established in vitro assays.
2. Material and Methods
2.1. Materials
Teucrium polium was harvested from natural resources from Setif, Algeria in 2008 during the spring (May-June) mainly at flowering stage. Plant parts were dried for 10 - 15 days in shadow at room temperature then powdered and stocked in darkness until use. The authenticated was confirmed by Pr Hocine LAOUAR. A specimen was deposited at the Laboratory of Botany, Faculty of natural and life sciences, University Ferhat Abbas Setif, Algeria. Bovine milk and eggs were obtained from a local farm (Setif). Blood samples were obtained from healthy donors at University Hospital of Setif. All the other reagents were purchased from Sigma chemicals (Germany) and Fluka. Xanthine oxidase is prepared in our laboratory as described by Baghiani et al. (2002).
2.2. Methods
2.2.1. Phenolic Compounds Extraction
The extractions were carried out using various polar and non polar solvents, according to the method of Markham [21]. 100 g of dried Teucrium polium (TP) shoots were ground in warring blender. They were mixed with a 10 - 20 volume of 85% aqueous methanol (MeOH). The slurry was placed on shaker for 24 hours. The extracts were filtered through a Buchner funnel and the MeOH was removed on rotary evaporator to give crude extract (fraction labelled CE). The aqueous solution was extracted with hexane several times to eliminate lipids, and then it was partitioned against chloroform to give chloroform extract (fraction labelled CHE). The remaining aqueous phase was exhausttively extracted with ethyl acetate (EtOAc) until the final EtOAc extract was colorless (fraction labelled EAE), the remaining aqueous extract was labelled AE. All the solvents were removed by evaporation under reduced pressure and the extracts were stored at –20˚C until use.
2.2.2. Determination of Total Polyphénols and Flavonoids
Total polyphenols were measured using Prussian blue assay according to Graham [22]. Phenolics were expressed as gallic acid equivalents (Gal A Eq). Briefly 0.1 mL of TPE are dissolved in methanol, 3 mL distilled water were added and mixed, then 1 mL of K3Fe(CN)6 (0.016 M) was added to each sample, followed by the addition of 1 mL of FeCl3 (0.02 M dissolved in 0.1 M HCl). It was immediately mixed using a vortex, after adding the reagents to the sample, 5 mL stabilizer (30 mL gum arabic (1%), 30 mL H3PO4 (85%) and 90 mL of distilled water) were added to the sample. The absorbance was measured at 700 nm. The amount of total polyphenols in different extracts was determined from a standard curve of gallic acid.
Flavonoids were quantified using aluminium chloride reagent AlCl3 [23]. Flavonoids were measured as quercetin equivalents (Quer-Eq). 1 mL of Tp samples are dissolved in methanol, then 1 ml of AlCl3 (2% in MeOH) was added, after incubation for 10 min, the absorbance was measured at 430 nm.
2.2.3. Determination of Proanthocyanidins
Proanthocyanidins (Flavan-3-olunits) were measured using HCl-acidifieddimethylamino-cinnama-ldehyde (DMACA) [24]. Two gram of DMACA was dissolved in 100 ml of 50% methanol in HCl (6N). To 9 ml of this solution, 16 ml of methanol were added and the resulting solution was used in the assay. Catechin (1 - 25 µg/ml) was used to elaborate the standard curve. This assay was carried out using a multiparameter analyser Hitachi at 660 nm and 37˚C. Diluted sample (20 µl) was added to the reaction tubes followed by 280 µl of distilled water and 300 µl of DMACA solution. After 5 minutes the absorbance of this solution was measured. Results were expressed as mg equivalent catechin per gram dry weight of extract. After the identification of some catechins, their concentrations in TPE were calculated. The concentration of procyanidin B1, procyanidin B2, catechin and epicatechin in were estimated by HPLC-DAD analysis using the internal standard method. Procyanidin B1, procyanidin B2, catechin and epicatechin were injected onto the HPLC system at the same time with sample. The surface under the peak was used to estimate each compound compared with the peak of the standard. The results were expressed as mg/g dry weight.
2.2.4. HPLC Analysis of Phenolic Compounds in Teucrium polium Extract
Plant extract was subjected to HPLC analysis using a Varian 5500 Chromatograph connected to a Waters 990 Diode Array detector. The column was a Merck Superspher 100 RP 18 - 4 µm (250 mm, ID 4 mm) protected with a precolumn of the same material. The flow rate was 0.8 ml/ min the pressure was 108 atmospheres and the oven temperature was set to 23˚C. The elution of the column was performed under gradient conditions. The mobile phase consisted of A: 1% acetic acid; B: 6% acetic acid and C: (5% acetic acid +30% acetonitrile).
The gradient program was as described bellow [25]. The identification of phenolic compounds in the extract was made by comparison of the retention time and the spectral characteristics of the resulting peaks with standards processed under the same conditions.
2.2.5. Effects of Teucrium polium Extracts on the Generation of 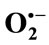 Radicals
Radicals
Anti-radical activity was determined spectrophotometrically according to Robak and Gryglewski [26], by monitoring the effect of TPE on superoxide anion radicals (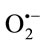 ) produced by xanthine/xanthine oxidase system. These radicals are able to reduce cytochrome c. The reaction mixture contained xanthine (100 µM), horse heart cytochrome c (25 µM), in air-saturated sodium phosphate buffer (50 mM, pH 7.4), supplemented with 0.1 mM EDTA and various concentrations of TPE. The reactions were started by addition of XO. Within 2 min, reduced cytochrome c was spectrometrically determined at 550 nm against enzyme-free mixture using. The cytochrome c activity was calculated using an absorption coefficient of 21.100 M–1·cm–1, and the sensibility of the reaction was determined by using bovine erythrocytes superoxide.
) produced by xanthine/xanthine oxidase system. These radicals are able to reduce cytochrome c. The reaction mixture contained xanthine (100 µM), horse heart cytochrome c (25 µM), in air-saturated sodium phosphate buffer (50 mM, pH 7.4), supplemented with 0.1 mM EDTA and various concentrations of TPE. The reactions were started by addition of XO. Within 2 min, reduced cytochrome c was spectrometrically determined at 550 nm against enzyme-free mixture using. The cytochrome c activity was calculated using an absorption coefficient of 21.100 M–1·cm–1, and the sensibility of the reaction was determined by using bovine erythrocytes superoxide.
2.2.6. Effects of Teucrium polium Extracts on Xanthine Oxidase Activity
The effect of TPE on the xanthine oxidation was examined spectrophotometrically at 295 nm following the production of uric acid using an absorption coefficient of 9600 M–1·cm–1. Assays were performed at room temperature, in presence of final concentration of 100 µM of xanthine, in air saturated sodium phosphate buffer (50 mM, pH 7.4) with various amounts of TPE dissolved in MeOH. Control experiments revealed that solvent didn’t influence the activity of XO at this concentration. The reaction was started by the addition of XO (1176 nmol of urate/min/mg protein) for Enzyme activity of the control sample was set to 100% activity. The percent inhibition was calculated by using the following formula.
Inhibition % = 100(Control OD – Sample OD)/Control OD
2.2.7. Measurement of Superoxide Anion Scavenging Activity
The superoxide scavenging ability of the TPE was assessed by a modified method [27]. Superoxide anions were generated in samples that contained 100 μl each of 1 mM NBT, 3 mM NADH and 0.3 mM PMS and the final volume was adjusted to 1 ml with 0.1 M phosphate buffer (pH 7.8). The reaction mixture (NBT and NADH) was incubated with or without extracts at ambient temperature for 2 min and the reaction was started by adding PMS. The absorbance at 560 nm was measured. Decrease in absorbance in the presence of various plants extracts indicated 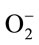 scavenging activity. The percent scavenging activity was calculated by using the following formula.
scavenging activity. The percent scavenging activity was calculated by using the following formula.
S.S.A. (%) = 100(Control OD – Sample OD)/Control OD where S.S.A. = Superoxide scavenging activity.
2.2.8. Evaluation of Red Blood Cells Haemolysis
Blood (10 - 20 ml) was obtained from healthy volunteers. Human erythrocytes from blood were isolated by centrifugation at 3000 g for 10 min and washed four times with rotect e buffer, and then re-suspended using the same buffer to the desired hematocrit level. Cells stored at 4˚C were used within 6 h of sample preparation. In order to induce free-radical chain oxidation in erythrocytes, Human red blood cells (RBC) suspensions (2% hematocrit) were treated with tert-butyl hydroperoxide (t-BHP) (500 µM final concentration) [28]. At the end of 2 h of incubation, the extents of hemolysis were evaluated as described below. To assay the antioxidant rotecttive effect of TPE on RBC from oxidative injury, the cells were pre-treated for 15 min, in the absence or presence of 2 mg/ml of TPE or antioxidant, before the induction of oxidative stress. After the oxidative treatment, samples were centrifuged at 1500 g for 10 min, and the absorption (A) of the supernatant (S1) at 540 nm was measured. The precipitates (packed RBC) were then haemolysed with 40 volumes of ice-cold distilled water and centrifuged at 1500 g for 10 min. The supernatant (S2) was then added to S1 and the absorption (B) of the combined supernatants (S1 + S2) was measured at 540 nm; the percentage of haemolysis was calculated from the ratio of the readings: (A/B) × 100.
2.2.9. Thiobarbituric Acid-Reactive Substances Assay
A modified thiobarbituric acid-reactive species (TBARS) assay [29] was used to measure the lipid peroxide formed, using egg yolk homogenates as lipid rich media. Malondialdehyde (MDA reacts with two molecules of thiobarbituric acid (TBA) yielding a pinkish red chromogen with an absorbance maximum at 532 nm. Egg homogenate (0.5 ml of 10% v/v) and 0.1 ml of various concentration TPE were added to a test tube and made up to 1 ml with distilled water, 0.05 ml of FeSO4 (0.07 M) was added to induce lipid peroxidation and incubated for 30 min, then 1.5 ml of 20% acetic acid (pH adjusted to 3.5 with NaOH) and 1.5 ml of 0.8% (w/v) TBA in 1.1% sodium dodecyl sulphate and 0.05 ml 20% trichloroacetic acid (TCA) were added then heated at 95˚C for 60 min. To eliminate this non-MDA interference, another set of samples was treated in the same way, incubating without TBA, to subtract the absorbance. After cooling, 5.0 ml of butan-1-ol were added to each tube and centrifuged at 3000 rpm for 10 min. The absorbance of the organic upper layer was measured and the inhibition of lipid peroxidation (%) by the extract was calculated according to:
Inhibition of lipid peroxidation (%) = 100(1 – E)/CC: is the absorbance value of the fully oxidised control, E: is (Abs532+TBA – Abs532–TBA)].
2.2.10. β-Caroten-Linoleic Acid Assay
In this assay, antioxidant capacity is determined by measuring the inhibition of the volatile organic compounds and the conjugated diene hydroperoxides arising from linoleic acid oxidation [30]. A stock solution of β-carotene-linoleic acid mixture was prepared as follows: 0.5 mg β-carotene was dissolved in 1 ml of chloroform (HPLC grade) and 25 µl linoleic acid and 200 mg Tween 40 were added. Chloroform was completely evaporated. Then, 100 ml distilled water saturated with oxygen (30 min, 100 ml/min) were added with vigorous shaking. 2500 µL of this reaction mixture were dispensed into test tubes and 350 µl of the TPE, prepared at 2 g/l concentrations, were added and the emulsion system was incubated for 48 h at room temperature. The same procedure was repeated with synthetic antioxidant, BHT, as positive control, and a blank. After this incubation period, absorbances of the mixtures were measured at 490 nm. Antioxidative capacities of the extracts were compared with those of BHT and blank.
2.2.11. DPPH Assay
The hydrogen atom or electron donation abilities of TPE and some pure compounds were measured from the bleaching of the purple-coloured methanol solution of 2,20- diphenylpicrylhydrazyl (DPPH). This spectrophotometric assay uses the stable radical DPPH as a reagent [31]. 50 µl of various concentrations of the extracts in methanol were added to 5 ml of a 0.004% methanol solution of DPPH. After a 30 min incubation period at room temperature, the absorbance was read against a blank at 517 nm. Inhibition of free radical DPPH in percent (I%) was calculated in following way:
I% = 100(Ablank – Asample)/Ablank
where A blank is the absorbance of the control reaction (containing all reagents except the test compound), and Asample is the absorbance of the test compound. Extract concentration providing 50% inhibition (IC50) was calculated form the plot of inhibition percentage against extract concentration. Tests were carried out in triplicate.
2.2.12. Ferrous Ion Chelating Activity
Ferrous ion chelating activity was measured by inhibition of the formation of iron (II)-ferrozine complex after treatment of various concentration of TPE with Fe2+, following the method of Decker and Welch [32]. The reaction mixture (1.50 ml) contained 500 µl test material or EDTA, 100 µl FeCl2 (0.6 mM in water) and 900 µl MeOH. The control contained all the reaction reagents except the extract and EDTA. The mixture was shaken well and allowed to react at room temperature for 5 min; 100 µL of ferrozine (5 mM in methanol) was then added. The absorbance of the Fe2+-ferrozine complex was measured at 562 nm. The chelating effect was calculated as a percentage, using the equation below, an IC50 value defined as the effective concentration of test material which produces 50% of maximal scavenging effect.
Chelating effect (%) = 100(1 – Asample/Acontrol)
2.2.13. Ferric Reducing Ability of Plasma Assay (FRAP)
The antioxidant capacity of each sample was estimated according to Pulido et al. [33]. Briefly, 900 µL of FRAP reagent, prepared freshly and warmed at 37˚C, was mixed with 90 µL of distilled water and 30 µL of test sample. Readings at the absorption maximum (593 nm were taken every 15 s, the reaction monitored for up to 30 min. Methanolic solutions of known Fe(II) concentrations in the range of 100 - 2000 µmol/L (FeSO4·7H2O) were used for calibration.
2.2.14. Statical Analysis
All determinations were conducted in triplicate or more and all results were calculated as mean ± standard deviation (SD). In this study, Statistical analysis was performed using Student’s t-test for significance and analysis of variance (ANOVA) followed by Dunnet’s test were done for the multiple effects comparison of the different extracts. The p values less than 0.05 were considered statistically significant.
3. Results and Discussion
3.1. Extraction and Determination of Total Polyphenol Contents
Plants extracts preparations of Teucrium polium were carried out using various polar and non polar solvents [21]. The estimate of yields in relation to the total powder weight (100 g) of plant shows that the CE has the high yield 20.077% ± 1.034% followed by AE with 10.04% ± 0.495% while the other extracts displayed the lower yields (Table 1).
Total phenolicand flavonoids contents were expressed as mg gallic acid equivalents per gram dry weight (mg GA-Eq/g) and mg quercetin equivalents per gram dry weight (mg Q-Eq/g) (Table 1: A,B), respectively. There was a wide range of phenol concentration in different extracts. The highest level of polyphenols was recorded in EAE followed by CHE (Table 1: A).
Flavonoids were quantified using AlCl3 method described by Bahorun et al. [23]. Quercetin (Quer) and Rutin (Rut) are used as standards. Results showed that EAE gave the greatest level (Table 1: B). In this study, an attempt was made to quantify and identify polyphenols in Teucruim polium. This plant contains polyphenols and flavonoids mainley procyanidins as determined by HPLC
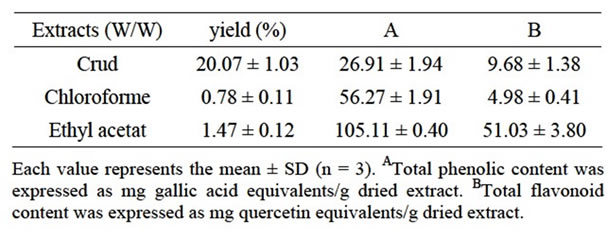
Table 1. The yield, total phenolicA and flavonoid contentB in Teucrium polium L extracts.
techniques (Figure 1). Procyanidins were quantified in the crude extract showing 12 mg/g dry weight of total procyanidins containing procyanidin B1 (0.4 mg/g), procyanidin B2 (0.005 mg/g), Catechin (0.003 mg/g) and epicatechin (0.92 mg/g).
3.2. Effects of TPE on the Generation of 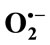 by the Xanthine/Xanthine Oxidase System
by the Xanthine/Xanthine Oxidase System
Cytochrome c3+ has been extensively used for the 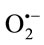 detection produced in biological systems due to its fast superoxide-mediated reduction to cytochrome c2+ [34]. The effect of TPE at different concentrations were studied for their ability to scavenge
detection produced in biological systems due to its fast superoxide-mediated reduction to cytochrome c2+ [34]. The effect of TPE at different concentrations were studied for their ability to scavenge 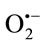 generated by the xanthine/xanthine oxidase system. The XOR activity was specrophotometrically determined by measuring the production of uric acid from xanthine (100 µM, final concentration) at 295 nm using an absorption coefficient of 9600 M–1·cm–1 [35]. Assays were performed at room temperature in air-saturated 50 mM phosphate buffer, pH 7.4, supplemented with 0.1 mM EDTA. The amount of
generated by the xanthine/xanthine oxidase system. The XOR activity was specrophotometrically determined by measuring the production of uric acid from xanthine (100 µM, final concentration) at 295 nm using an absorption coefficient of 9600 M–1·cm–1 [35]. Assays were performed at room temperature in air-saturated 50 mM phosphate buffer, pH 7.4, supplemented with 0.1 mM EDTA. The amount of 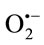 generated was determined by measuring the reduction of cytochrome c. Under our experimental conditions, the activity of cytochrome c in the absence of extracts was (2135.91 nmols/min/mg protein) reduced by
generated was determined by measuring the reduction of cytochrome c. Under our experimental conditions, the activity of cytochrome c in the absence of extracts was (2135.91 nmols/min/mg protein) reduced by 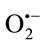 generated from XO. The reduction of cytochrome c3+ was almost totally inhibited by SOD (330 U/mL). Results showed that all the extracts were able to inhibit cytochrome c3+. The superoxide scavenging effect was found to increase with increasing concentration of TPE. The CHE was the most potent scavenger of
generated from XO. The reduction of cytochrome c3+ was almost totally inhibited by SOD (330 U/mL). Results showed that all the extracts were able to inhibit cytochrome c3+. The superoxide scavenging effect was found to increase with increasing concentration of TPE. The CHE was the most potent scavenger of 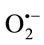 with IC50 (µM/quercetin equivalent) of 0.756 ± 0.0514 followed by EAE then CE (Figure 1).
with IC50 (µM/quercetin equivalent) of 0.756 ± 0.0514 followed by EAE then CE (Figure 1).
3.3. Effects of TPE on XO Activity
To study the possibility that the extracts suppressed the rate of xanthine conversion to uric acid to account for this 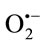 scavenging ability, the effect of TPE on the XO activity were checked. At the identical concentrations of TPE we observed significant inhibition of XO activity in dose dependent manner. The inhibitory effects of TPE were compared with allopurinol, clinically used as a specific inhibitor for XO. The results demonstrated that CHE possessed high XO inhibitory activity with IC50
scavenging ability, the effect of TPE on the XO activity were checked. At the identical concentrations of TPE we observed significant inhibition of XO activity in dose dependent manner. The inhibitory effects of TPE were compared with allopurinol, clinically used as a specific inhibitor for XO. The results demonstrated that CHE possessed high XO inhibitory activity with IC50
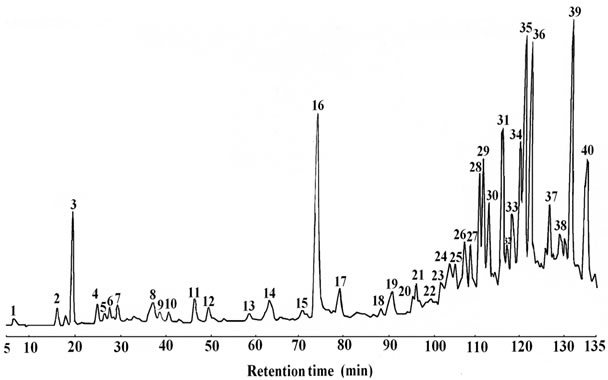
Figure 1. HPLC profile of phenolic compounds in the crude extract of Teucrium polium. 3: Gallic acid; 4, 12, 2: Flavones; 8: Procyanidin B1; 11: Catechin; 13: Procyanidin B2; 16: Epicatechin; 24, 26, 29, 33, 37, 40: Flavonols; 14, 18, 27, 31: Flavanones.
(µM/quercetin equivalent) of 0.79 ± 0.07 followed by CE (10.59 ± 0.58) then EAE (11.75 ± 0.49), respectively, meanwhile, the specific inhibitor of XO, Allopurinol gave a IC50 of 57.114 ± 1.093 µM higher than the TPE (P ≤ 0.01) which indicat the potent inhibitory effects of TPE on XO. Several data has been reported that phenolic compounds are considered as antioxidants not only because they act as free radical scavengers, but also because of their ability to inhibit XO [36,37].
3.4. Measurement of 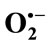 Scavenging Activity
Scavenging Activity
The inhibition of cytochrome c reduction is due to dual effect of extracts as demonstrated above. Firstly, these compounds inhibit the XO activity and secondly, some of them scavenge 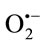 approximately at the same concentrations. To give a clear cut that TPE have
approximately at the same concentrations. To give a clear cut that TPE have 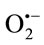 scavenging activity or not the PMS-NADH-NBT system was used as a nonenzymatic method to measure
scavenging activity or not the PMS-NADH-NBT system was used as a nonenzymatic method to measure 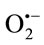 scavenging activity.
scavenging activity. 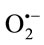 derived from the dissolved oxygen by PMS/ NADH coupling reaction reduces NBT. The decrease in the absorbance at 560 nm with antioxidants indicates the consumption of the generated superoxide anion in the reaction mixture. The results demonstrated that TPE had a concentration-dependent scavenging activity by neutralizing superoxide radicals in the same order as shown in the results obtained by using enzymatic method (Cyt c). The most potent scavenger of
derived from the dissolved oxygen by PMS/ NADH coupling reaction reduces NBT. The decrease in the absorbance at 560 nm with antioxidants indicates the consumption of the generated superoxide anion in the reaction mixture. The results demonstrated that TPE had a concentration-dependent scavenging activity by neutralizing superoxide radicals in the same order as shown in the results obtained by using enzymatic method (Cyt c). The most potent scavenger of 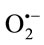 observed was CHE with IC50 of 6. 209 ± 0.0174 (µM/quercetin equivalent), which was found better than quercetin (IC50 = 1.3 × 103 ± 0.053 µM) (P ≤ 0.01) followed by the EAE (27.441 ± 0.618) then CE (25.175 ± 0.496). TPE scavenging effects were found to be higher than gallic acid, which have an antioxidant capacity, determined by both ABTS and DPPH assays, more than that of vitamin C and other phenolic constituents such as quercetin, epicatechin, catechin, rutin and chlorogenic acid [38]. The results clearly indicated that TPE are potent scavengers of
observed was CHE with IC50 of 6. 209 ± 0.0174 (µM/quercetin equivalent), which was found better than quercetin (IC50 = 1.3 × 103 ± 0.053 µM) (P ≤ 0.01) followed by the EAE (27.441 ± 0.618) then CE (25.175 ± 0.496). TPE scavenging effects were found to be higher than gallic acid, which have an antioxidant capacity, determined by both ABTS and DPPH assays, more than that of vitamin C and other phenolic constituents such as quercetin, epicatechin, catechin, rutin and chlorogenic acid [38]. The results clearly indicated that TPE are potent scavengers of 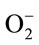 This can prevent the formation of ROS.
This can prevent the formation of ROS.
3.5. Evaluation of RBC Haemolysis
Oxidative damage of erythrocyte membrane (lipid/protein) may be implicated in haemolysis [39]. Therefore this cellular system could be very useful to study oxidative stress. The antioxidant activity of the TPE was confirmed in Human erythrocytes (RBC) exposed to 500 µM t-BHP, by measuring the erythrocyte membrane resistance to free radical-induced hemolysis. When control RBC were incubated with extracts (at final concentration; 2 µg/ml), no significant hemolysis was observed within 2 h, thus to exclude any membrane-perturbing effect of TPE. In RBC exposed to t-BHP hemolysis started after 60 min incubation and plateaued between 90 and 120 min (27% hemolysis was considered as 100%). At 120 min hemolysis was inhibited by CE, CHE, and EAE with 84.66%, 74.62% and 65.14%, respectively (p ≤ 0.01). Although EAE was less effective than the other extracts, this extract has exhibited the same effect as Quercetin (65.46%) and it was more potent than rutin (59.62%) and gallic acid with 47.73% (p ≤ 0.05).
The protective effects of TPE may be due to: 1) their kind of phenolic content, because there is no significatif correlation between antihymolitic effect of extracts and their phenolic compound content, 2) and /or the difference in the degree of the flavonoid molecules penetrations in intact erythrocytes. It was demonstrate that binding of the flavonoids to the RBC membranes significantly inhibits lipid peroxidation, and at the same time enhances their integrity against lysis [40] confirmed this hypothesis; although phenolic contents in CHE are lower than EAE, the CHE (which contain apolar compound so more soluble in RBC membran) exhibits an anti hemolytic action stronger than that of EAE (less apoler than CHE), however the crud extract (more polar) was more efficiency than chloroform extract, this may due to the synergetic effect of different compounds present in [41]. The results of present study have shown that TPE can effectively protect erythrocytes against haemolytic injury induced t-BHP, TPE is a powerful scavenger as previously shown; it could have provided a defence against haemolytic injury by suppressing t-BHP related fall in reduce glutathione. Studies involving plasma have indicated that flavonoids have the ability to delay the consumption of some endogenously present antioxidants in the human body [42]. It has been reported that a methanolic extract of T. polium protected red blood cells (RBCs) against lipid peroxidation induced by 10 mM hydrogen peroxide [43]. It was showed that the aqueous extracts prepared from the foliage of T. polium suppressed iron (Fe2)-induced lipid peroxidation in rat liver homogenates to the same extent as Trolox [44]. Additionally, it showed that the extract was not cytotoxic because it did not adversely affect cell membrane integrity or suppress mitochondrial respiration of cultured Hep G2 and PC12 cells following 24 h exposure [44]. Total antioxidant activities of the plant extracts can not be evaluated by any single method, due to the complex nature of phytochemicals [45,46]. In this regard and/or to confirm previous results two supplementary essays were performed, lipid Peroxidation by using MDA as a marker of lipid peroxidation and β-carotene/linoleic acid essays with linoleic acid as a model of lipid peroxidation and as a radicals source.
3.6. Thiobarbituric Acid-Reactive Substances Assay
To confirm the effects of TPE against lipid peroxidation as a direct antioxidant, Thiobarbituric acid-reactive substance (TBARS) assay was used. The extent of lipid peroxidation was estimated by the levels of MDA measured using the thiobarbituric acid. FeSO4 was added to egg yolk homogenates, as lipid rich media, to induce lipid peroxidation. MDA a secondary end product of polyunsaturated fatty acids oxidation, reacts with two molecules of thiobarbituric acid (TBA) yielding a pinkish red chromogen with an absorbance maximum at 532 nm. The result showed that the phospholipid peroxidation induced by FeSO4 was significantly counteracted by TPE in dose dependent manner.The CHE extract exhibited the highest inhibitory potency with IC50 (µM/quercetin equivalent) of 2.659 ± 0.19 fellowed by CE then EAE with 2.2 and 3.7 folds respectively. In general all the extracts showed a remarkable effect in suppressing lipo-peroxidation, which were more active than reference substances; quercetin, rutin, gallic acid and ascorbic acid (p ≤ 0.01) (Figure 2).
The results showed a feeble correlation between antioxidant potent and TPE phenolic content (r2 = 0.149, p > 0.5) (Table 1), similar to the results of Agbor et al. [47] working on some herbs/spices from Cameroon and the results of Demiray et al. [48] working on Turkish medicinal plants: Tilia argentea, Crataegi folium leaves and
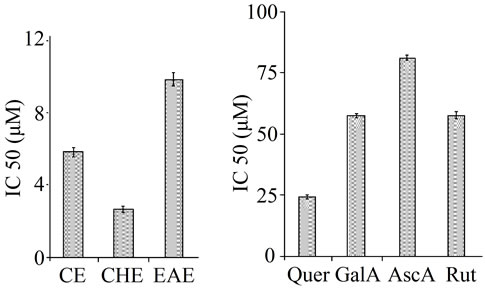
Figure 2. Antilipoperoxidative effects of the TPE and standards at IC50 concentration against FeSO4 (0.07 M) induced lipid peroxidation in egg yolk homogenates. After incubating the yolk homogenates with FeSO4 (0.07 M) and/or extracts/ standards the lipid peroxidation measured by TBARS assay. Values are mean ± SD, n = 3.
Polygonum bistorta roots, therefore the potent effect of CHE may due to other products and/or the type of phenolic compound. Hall and Cuppett [49] showed that plant tissues contain a wide variety of compounds with antioxidant activity, such as phenolic compounds, nitrogen compounds, carotenoids, lignans and terpenes.
3.7. β-Carotene/Linoleic Acid Assay
According to several authors, the test of linoleic acid oxidation inhibition coupled with β-carotene, appears very useful as a mimetic model of lipid peroxidation in biological membranes [50]. β-carotene/linoleic acid assay determines the inhibition ratios of linoleic acid oxidation as further methods to confirm the antilipoperoxidation effects of TPE. Lower absorbance indicates a higher level of antioxidant activity. The changes in the percentage of the inhibition ratios of linoleic acid oxidation under the influence of TPE (2 mg/ml), was compared with BHT at the same concentration (2 mg/ml) as positive control during 24 h. The inhibition extent of lipid oxidation by TPE when compared to BHT showed a marked activity effects (p ≤ 0.01) (Figure 3). The highest inhibition ratios of linoleic acid oxidation were showed for CHE (83.11% ± 0.816%), not far from that of BHT (95.77%) followed by that of EAE (78.15% ± 0.772%) (p ≤ 0.01), then CE (75.426% ± 0.594%).
In conclusion the activity of tested extracts in terms of polarity activity the apolar extracts (CHE) are more active than the polar extract EAE. Frankel and Meyer [51] have suggested that antioxidants which exhibit apolar properties are most important because they are concentrated in the lipid-water interface, thereby preventing the formation of lipid radicals and β-carotene oxidation. It was reported that sample that inhibits or retards the bleaching β-carotene can be described as a scavenger of free radicals and as a primary antioxidant [52]. Our study are in agreement
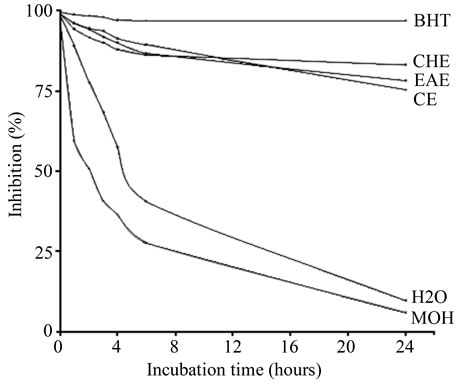
Figure 3. The changes in the percentage of the linoleic acid oxidation inhibition ratios under the influence of Teucrium poluim extracts (2 mg/ml), compared to BHT as a positive control during 24 h. For all values statistically different (p ≤ 0.01).
with the work of Kadifkova-Panovska et al. [53] demonstrating that extracts of TPE prepared using different organic solvents were effective inhibitors of β-carotene oxidation. In order to correlate and understand the mechanism of the observed antioxidant activity of TPE, we have applied DPPH, Ferrous ion chelating, and FRAP assays for testing scavenging (hydrogen donating ability), chelating and reducing proprieties respectively.
3.8. DPPH Assay
The DPPH radical is a stable organic free radical with an absorption maximum band around 515 - 528 nm and thus, it is a useful reagent for evaluation of antioxidant activity of compounds [54]. In the DPPH test, the antioxidants reduce the DPPH radical to a yellow-colored compound, diphenylpicrylhydrazine, and the extent of the reaction will depend on the hydrogen donating ability of the antioxidants [55]. The TPE demonstrated a concentrationdependent scavenging activity by quenching DPPH radicals. Extracts are a good free radical scavenger and contributes significantly to the antioxidant capacity of TPE with IC50 of; 2.012% ± 0.190%, 2.371% ± 0.284%, 3.713% ± 0.302% for CHE, CE, EAE, respectively, which were more effective than used standards (p ≤ 0.01); quercetin gallic acid and rutin, with IC50 of 12.787% ± 0.3659%, 12.785% ± 0.294%, 14.431% ± 0.4154% respectively. The results revealed a feeble correlation between antiradical potent and phenolic content. In general the most effective extracts were CHE (aglycone flavonoids), in agreement with the results described Rice-Evans et al. [56] reporting that the phenolic acids and flavonoids especially aglycone flavonoids, are effective hydrogen donors, which make them good antioxidant.
3.9. Ferrous Ion Chelating Activity
In this study, both TPE and EDTA showed chelating activity as demonstrated by their effectiveness in inhibiting the formation of Fe2+-ferrozine complex, the absorbance of this complex was dose dependently decreased. The results showed that the iron chelating ability of TPE was not far from that of EDTA. The CE showed an excellent chelating with IC50 of 8.438 ± 0.953 µM/quercetin equivalent, approximately 15 folds lower than that of EDTA, followed by that of CHE (IC50 = 17.79 ± 2.11 µM/ quercetin equivalent), while the EAE (IC50 = 168.673 ± 3.11 µM/ quercetin equivalent do not showed a remarkable chelating effect. It was showed that the aqueous extracts prepared from the foliage of T. polium suppressed iron (Fe2)- induced lipid peroxidation in rat liver homogenates to the same extent as Trolox [44].
3.10. Ferric Reducing Ability of Plasma Assay (FRAP)
The FRAP values calculated using the respective Fe(II) calibration curves. Gallic acid, Quercetin, Rutin and Ascorbic acid were used for comparison of ferric reducing ability of extracts used in the present study. Conditions for the determination of the ferric reducing ability of antioxidants in the original method established at 4 min interval as suitable for such measurements, since the absorbance of the reduced ferrous-TPTZ complex was stable at this time [57]. When these conditions were used in the present study, we observed that the reduction of the ferric-TPTZ complex was not established after 4 min reaction time and most of the compound shows a steady increase in the absorbance with time, therefore we prolonged the reaction time for several hours and the continuous increment of absorbance at 595 nm was determined in interval time. However 30 min was maintained as one of the best time showing the antioxidant efficiency of the studied samples, this was in agreement with Pulido et al. [33] studding the anti oxidant effect of many pure polyphenols. The 4 min absorbance recording established in the original method was also kept [57].
As showed in Figure 4, some antioxidants even doubled their initial absorbance after 30 min of reaction, as was the case with the most standards and samples, nevertheless, these increments in the absorbance readings were not caused by alterations of the reaction mixture with time, since blank samples showed no significant modification of their initial absorbance.
The equivalent concentration 1 or EC1, is the concentration of antioxidant having a ferric-TPTZ reducing ability equivalent to that of a 1 mmol/L concentration of FeSO4·7H2O was measured for comparison. The higher EC1 value, expressed as µM indicat the lower antioxidant activity. Figure 4 shows the EC1 values at 4 and 30 min of TPE and standards. EC1 values, and therefore ferric reducing ability, were lower at longer reaction times as expected from the kinetic behaviour of the compounds at 4 min. At 30 min the results indicated that TPE stronger
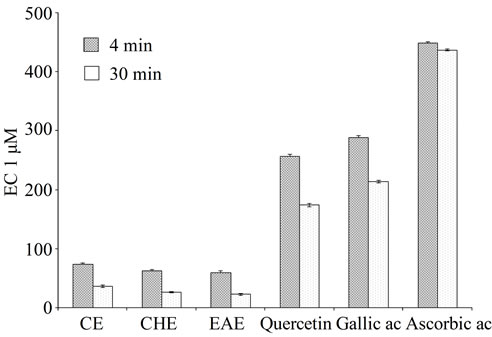
Figure 4. Comparison the EC1 values of Teucrium polium L. extracts with standards gallic acid rutin ascorbic acid and quercetin. Data are the Mean values ± SD, n = 3; EC1: concentration of antioxidant having a ferric reducing ability equivalent to that of 1 mM/L FeSO4·7H2O; CE: crud extract; EAE: ethyl acetate extract; CHE: chloroform extract.
than all the standards. Our results revealed a proportionall incresing the antioxidant activity with the polyphenol content (Figure 4, Table 1) agreeing with results obtained by Maksimović et al. [58] working on various polyphenol classes in the silks of fifteen maize hybrids. In this study quercetin showed the lowest EC1 values, followed by gallic, however Ascorbic acid had a ferric reducing ability lower than those of most polyphenols, these results are in agreement with the results of Pulido et al. [33], in different classification in terms of antioxidant efficiency of the studied compounds at short versus long reaction times, the quercetin showed the lowest EC1 values, followed by gallic and ascorbic acids, however, ascorbic acid had a ferric reducing ability lower than those of most polyphenols. In general, the classification of antioxidants according to their ferric reducing ability reported in this study agreed with results reported by other authors using different methods to estimate antioxidant power. Thus, Wang and Goodman [59] reported a decreased effect of polyphenols inhibiting peroxidation of LDL in the order quercetin > rutin > gallic acid. In other study, they reported that adding fertilizer caused a significant concentrationdependent increase in antioxidant activity of the cultivated T. polium compared with the wild-type [60]. Data clearly established that the antioxidant activity of TPE was measured using ferric thiocyanate test indicated that the extracts had the highest antioxidant capacity [61].
In vivo studies have shown grape seed proanthocyanidin extract is a better free radical scavenger and inhibittor of oxidative tissue damage than vitamin C, vitamin E succinate, vitamin C and vitamin E succinate combined, and β-carotene [62]. Moreover, in vitro experimental results have demonstrated that proanthocyanidins have a specificity for the hydroxyl radical [63,64], in addition to having the ability to non-competitively inhibit of the xanthine oxidase activity [63,65] elastase, collagenase, hyaluronidase, and beta-glucuronidase [65]. Structure activity relationship of some phenolic compounds (e.g. flavonoids, phenolic acids, tannins) has been studied [56,66]. In general, free radical scavenging and antioxidant activity of phenolics (e.g. flavonoids, phenolic acids) mainly depends on the number and position of hydrogen-donating hydroxyl groups on the aromatic ring of the phenolic molecules, and is also affected by other factors, such as glycosylation of aglycones, other H-donating groups (-NH, -SH), etc. For example, flavonol aglycones such as quercetin, myricetin, and kaemperol, containing multiple hydroxyl groups, had higher antioxidant activity than their glycosides such as rutin, myricitrin, astragalin. There were many reports on antioxidant components, generally focusing on flavonoids and phenolic acids [56,67].
4. Conclusion
The results suggest that the various antioxidant mechanisms of TPE may be attributed to xanthine oxidase inhibition, metal chelating ability, hydrogen donating ability, reducing power and their effectiveness as strong scavengers of superoxide and other free radicals. The enzymatic method had not gave a clear cut that the extracts have a real scavenger effects on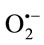 , because they exhibited a high scavenging effects on
, because they exhibited a high scavenging effects on 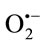 generated by XO and a strong inhibitory effects on XO. Therefore, a nonenzymatic method for scavenging effects on
generated by XO and a strong inhibitory effects on XO. Therefore, a nonenzymatic method for scavenging effects on 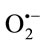 was realized which showed that TPE are a good superoxide radical scavengers. Data obtained in this study proved a relationship between the inhibition percentage of hemolysis and chelating power of TPE suggesting that their action as anti hemolytic may be related to their iron-binding capacity. Further, to confirm the possibility that extracts can act as direct antioxidant by possessing antilipoperoxidative mechanism, which may prevent RBC hemolysis, tow other assay were applied; TBARS assay and β- carotene/linoleic acid which displayed an antiradical effect approximatel in the same array, which indicate that TPE possesses a substantial protective effect and free radical scavenging mechanism against lipid peroxidation. Since the compounds with reducing power indicate that they are electron/or hydrogen donors can reduce the oxidized intermediates of lipid peroxidation, therefore the DPPH and FRAP assay were realised. Results obtained from this study demonstrated that TPE possess a good reducing power in the same order and that can prevent lipide peoxidation then prevent many oxidative damage. The various antioxidant mechanisms action of the extracts in their antioxidant activity appeared to be identical, throughout the good correlation observed between different applied techniques however, the magnitude of antioxidative potency varies with the type of extracts, which being related to the content in phenolic compounds and other yet to be discovered antioxidant compounds. Based on our results and these reports, there can’t be little doubt that TPE, irrespective of the preparation mode, can inhibit oxidative processes leading us to conclude that the extracts have substantial antioxidant activity in vitro. The antioxidative activity of phenolic compounds is mainly due to their redox properties, which can play an important role in neutralizing free radicals, quenching singlet and triplet oxygen species or decomposing peroxides. These results are preliminary, it would be interesting to test the activity of high purified fractions and isolate the responsible molecules underlie the various detected activities in different extracts by more efficient methods. In any case it is important to highlight that the majority of the test were performed in vitro. It is thus mandatory to confirm these findings by in vivo studies so as to obtain useful information for eventual therapeutic or dietary interventions.
was realized which showed that TPE are a good superoxide radical scavengers. Data obtained in this study proved a relationship between the inhibition percentage of hemolysis and chelating power of TPE suggesting that their action as anti hemolytic may be related to their iron-binding capacity. Further, to confirm the possibility that extracts can act as direct antioxidant by possessing antilipoperoxidative mechanism, which may prevent RBC hemolysis, tow other assay were applied; TBARS assay and β- carotene/linoleic acid which displayed an antiradical effect approximatel in the same array, which indicate that TPE possesses a substantial protective effect and free radical scavenging mechanism against lipid peroxidation. Since the compounds with reducing power indicate that they are electron/or hydrogen donors can reduce the oxidized intermediates of lipid peroxidation, therefore the DPPH and FRAP assay were realised. Results obtained from this study demonstrated that TPE possess a good reducing power in the same order and that can prevent lipide peoxidation then prevent many oxidative damage. The various antioxidant mechanisms action of the extracts in their antioxidant activity appeared to be identical, throughout the good correlation observed between different applied techniques however, the magnitude of antioxidative potency varies with the type of extracts, which being related to the content in phenolic compounds and other yet to be discovered antioxidant compounds. Based on our results and these reports, there can’t be little doubt that TPE, irrespective of the preparation mode, can inhibit oxidative processes leading us to conclude that the extracts have substantial antioxidant activity in vitro. The antioxidative activity of phenolic compounds is mainly due to their redox properties, which can play an important role in neutralizing free radicals, quenching singlet and triplet oxygen species or decomposing peroxides. These results are preliminary, it would be interesting to test the activity of high purified fractions and isolate the responsible molecules underlie the various detected activities in different extracts by more efficient methods. In any case it is important to highlight that the majority of the test were performed in vitro. It is thus mandatory to confirm these findings by in vivo studies so as to obtain useful information for eventual therapeutic or dietary interventions.
5. Acknowledgements
This work was supported by the Algerian Ministry of Higher Education and Scientific Research (MERS) and by the Algerian Agency for the Development of Research in Health (ANDRS).
REFERENCES
- B. Halliwell and J. M. C. Gutteridge, “Free Radicals in Biology and Medicine,” 3rd Edition, Oxford University Press, London, 1999.
- J. L. Farber, “Mechanisms of Cell Injury by Activated Oxygen Species,” Environmental Health Perspectives, Vol. 102, 1994, pp. 17-24.
- B. Halliwell, “Free Radicals, Antioxidants, and Human Disease: Curiosity, Cause, or Consequence?” Lancet, Vol. 344, No. 8924, 1994, pp. 721-724. doi:10.1016/S0140-6736(94)92211-X
- R. Harrison, “Physiological Roles of Xanthine Oxidoreductase,” Drug Metabolism Reviews, Vol. 36, 2004, pp. 363-375. doi:10.1081/DMR-120037569
- H. M. Martin, J. T. Hancock, V. Salisbury and R. Harrison, “Role of Xanthine Oxidoreductase as an Antimicrobial Agent,” Infection and Immunity, Vol. 72, No. 9, 2004, pp. 4933-4939. doi:10.1128/IAI.72.9.4933-4939.2004
- B. T. Emmerson, “The Management of Gout,” The New England Journal of Medicine, Vol. 334, 1996, pp. 445- 451. doi:10.1056/NEJM199602153340707
- S. L. Wallach, “The Side Effects of Allopurinol,” Hospital Practice, Vol. 33, 1998, p. 22.
- A. Bouloc, P. Reygagne, P. Lecoz and L. Dubertret, “Perforating Foot Ulceration with Allopurinol Therapy,” Clinical and Experimental Dermatology, Vol. 21, No. 5, 1996, pp. 351-352. doi:10.1111/j.1365-2230.1996.tb00121.x
- C. A. Rice-Evans, N. J. Miller and G. Paganga, “Antioxidants Properties of Phenolic Compounds,” Trends in Plant Science, Vol. 2, No. 4, 1997, pp. 152-159. doi:10.1016/S1360-1385(97)01018-2
- U. Plitmann, C. Heyn, A. Daniu and A. Shmida, “Pictorial Flora of Israel,” Massada, Israel, 1982.
- S. Facciola, “Cornucopia—A Source Book of Edible Plants,” Kampong Publications, Vista, 1990.
- G. Autore, F. Capasso, R. De Fusco, M. P. Fasulo, M. Lembo, N. Mascolo and A. Menghini, “Antipyretic and Antibacterial Actions of Teucrium polium,” Pharmacological Research Communications, Vol. 16, 1984, pp. 21- 29. doi:10.1016/S0031-6989(84)80101-0
- F. Capasso, R. Cerri, P. Morrica and F. Senatore, “Chemical Composition and Anti-Inflammatory Activity of an Alcoholic Extract of Teucrium polium,” Bollettino-Societa Italiana Biologia Sperimentale, Vol. 59, No. 11, 1983, pp. 1639-1643.
- M. Abdollahi, H. Karimpour and H. R. Monsef-Esfehani, “Antinociceptive Effects of Teucrium polium L. Total Extract and Essential Oil in Mouse Writhing Test,” Pharmacology Research, Vol. 48, 2003, pp. 31-35.
- A. Alkofahi and A. H. Atta, “Pharmacological Screening of the Anti-Ulcerogenic Effects of Some Jordanian Medicinal Plants in Rats,” Journal of Ethnopharmacology, Vol. 67, No. 3, 1999, pp. 341-345. doi:10.1016/S0378-8741(98)00126-3
- T. Baluchnejadmojarad, M. Roghani and F. RoghaniDehkordi, “Antinociceptive Effects of Teucrium polium Leaf Extract in the Diabetic Rat Formalin Test,” Journal of Ethnopharmacology, Vol. 97, 2005, pp. 207-210. doi:10.1016/j.jep.2004.10.030
- A. M. Esmaeili and R. Yazdanparast, “Hypoglycaemic Effects of Teucrium polium: Studies with Rat Pancreatic Islets,” Journal of Ethnopharmacology, Vol. 95, No. 1, 2004, pp. 27-30. doi:10.1016/j.jep.2004.06.023
- M. S. Suleiman, A. S. Abdul-Ghani, S. Al-Khalil and R. Amir, “Effect of Teucrium polium Boiled Leaf Extract on Intestinal Motility and Blood Pressure,” Journal of Ethnopharmacology, Vol. 22, No. 1, 1988, pp. 111-116. doi:10.1016/0378-8741(88)90236-X
- H. R. Rasekh, M. J. Khoshnood-Mansourkhani and M. Kamalinejad, “Hypolipidemic Effects of Teucrium polium in Rats,” Fitohterapia, Vol. 72, No. 8, 2001, pp. 937-939. doi:10.1016/S0367-326X(01)00348-3
- M. B. Aqel, M. N. Garaibeh and A. S. Salhab, “The Calcium Antagonistic Effect of the Volatile Oil of Teucrium polium,” International Journal of Crude Drugs Research, Vol. 28, No. 3, 1990, pp. 201-207.
- K. R. Markham, “Techniques of Flavonoid Identification,” London Academic Press, London, 1982.
- H. D. Graham, “Modified Prussian Blue Assay for Total Phenols,” Journal of Agricultural Food Chemistry, Vol. 40, No. 5, 1992, pp. 801-805. doi:10.1021/jf00017a018
- T. Bahorun, B. Gressier, F. Trotin, C. Brunet, T. Din, J. Vasseur, J. C. Gazin, M. Pinkas, M. Luyckx and M. Gazin, “Oxygen Species Scavenging Activity of Phenolic Extract from Howthorn Fresh Plant Organs and Pharmaceutical Preparation,” Arzneimittelforschung/Drug Research, Vol. 46, No. 11, 1996, pp. 1086-1089.
- Y. Li, G. Tanner and P. Larkin, “The DMACA-HCl Protocol and the Threshold Proanthocyanidin Content for Bloat Safety in Forage Legumes,” Journal of Sciences of Food Agriculture, Vol. 70, No. 1, 1996, pp. 89-101. doi:10.1002/(SICI)1097-0010(199601)70:1<89::AID-JSFA470>3.0.CO;2-N
- A. Scalbert, B. Monties and G. Janin, “Tannins in Wood: Comparison of Different Estimation Methods,” Journal of Agricultural Food Chemistry, Vol. 37, No. 5, 1989, pp. 1324-1329. doi:10.1021/jf00089a026
- J. Robak and R. J. Gryglewski, “Flavonoids Are Scavengers of Superoxide Anions,” Biochemistry and Pharmacology, Vol. 37, No. 5, 1988, pp. 837-841. doi:10.1016/0006-2952(88)90169-4
- M. Nishikimi, N. A. Rao and K. Yagi, “The Occurrence of Superoxide Anion in the Reaction of Reduced Phenazine Methosulfate and Molecular Oxygen,” Biochemical and Biophysical Research Communications, Vol. 46, No. 2, 1972, pp. 849-854. doi:10.1016/S0006-291X(72)80218-3
- V. Manna, P. Galletti, V. Cucciolla, G. Montedoro and V. Zappia, “Olive Oil Hydroxytyrosol Protects Human Erythrocytes against Oxidative Damages,” The Journal of Nutritional Biochemistry, Vol. 10, No. 3, 1999, pp. 159- 165. doi:10.1016/S0955-2863(98)00085-0
- H. Ohkowa, N. Ohisi and K. Yagi, “Assay for Lipid Peroxides in Animal Tissue by Thiobarbituric Acid Reaction,” Analytical Biochemistry, Vol. 95, No. 2, 1979, pp. 351-358. doi:10.1016/0003-2697(79)90738-3
- A. Dapkevicius, R. Venskutonis, T. A. Van Beek and P. H. Linssen, “Antioxidant Activity of Extracts Obtained by Different Isolation Procedures from Some Aromatic Herbs Grown in Lithuania,” Journal of the Science of Food and Agriculture, Vol. 77, No. 1, 1998, pp. 140-146. doi:10.1002/(SICI)1097-0010(199805)77:1<140::AID-JSFA18>3.0.CO;2-K
- M. Burits and F. Bucar, “Antioxidant Activity of Nigella Sativa Essential Oil,” Phytotheraphy Research, Vol. 14, No. 5, 2000, pp. 323-328. doi:10.1002/1099-1573(200008)14:5<323::AID-PTR621>3.0.CO;2-Q
- E. A. Decker and B. Welch, “Role of Feritin as Lipid Oxidation Catalyst in Muscle Food,” Journal of Agricultural and Food Chemistry, Vol. 36, No. 3, 1990, pp. 674- 677. doi:10.1021/jf00093a019
- R. Pulido, L. Bravo and F. Saura-Calixto, “Antioxidant Activity of Dietary Polyphenols as Determined Modified Ferric Reducing/Antioxidant Power Assay,” Journal of Agricultural Food Chemistry, Vol. 48, No. 8, 2000, pp. 3396-3402. doi:10.1021/jf9913458
- J. M. McCord and I. Fridovich, “The Reduction of Cytochrome C by Milk Xanthine Oxidase,” Journal of Biological Chemistry, Vol. 243, 1968, pp. 5753-5760.
- P. G. Avis, F. Bergel, R. C. Bray, D. W. F. James and K. V. Shooter, “Cellular Constituent, the Chemistry of Xanthine Oxidase. Part II: The Homogeneity of Crystalline Metalloflavoproteine Fraction,” Journal of Chemical Society, 1956, pp. 1212-1219. doi:10.1039/jr9560001212
- S. Boumerfeg, A. Baghiani, D. Messaoudi, S. Khennouf and L. Arrar, “Antioxidant Properties and Xanthine Oxidase Inhibitory Effects of Tamus communis L. Root Extracts,” Phytotherapy Research, Vol. 23, No. 2, 2009, pp. 283-288. doi:10.1002/ptr.2621
- A. Baghiani, S. Boumerfeg, F. Belkhiri, S. Khennouf, N. Charef, D. Harzallah, L. Arrar and M. Abdel-Wahhab, “Antioxidant and Radical Scavenging Properties of Carthamus caeruleus L. Extracts Grow Wild in the Algeria Flora,” Comunicata Scientiae, Vol. 1, No. 2, 2010, pp. 128-136.
- D. O. Kim, K. W. Lee, H. J. Lee and C. Y. Lee, “Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Phenolic Phytochemicals,” Journal of Agricultural and Food Chemistry, Vol. 50, No. 13, 2002, pp. 3713-3717. doi:10.1021/jf020071c
- F. N. Ko, G. Hsiao and Y. H. Kuo, “Protection of Oxidative Hemolysis by Demethydiisoeugenol in Normal and Beta-Thalassemic Red Blood Cells,” Free Radical Biology and Medicine, Vol. 22, No. 1-2, 1997, pp. 215-222. doi:10.1016/S0891-5849(96)00295-X
- H. Kitagawa, H. Sakamoto and Y. Tano, “Inhibitory Effects of Flavonoids on Free Radical-Induced Hemolysis and Their Oxidative Effects on Hemoglobin,” Chemical and Pharmaceutical Bulletin, Vol. 52, 2004, pp. 999- 1001. doi:10.1248/cpb.52.999
- J. Zhishen, T. Mengcheng and W. Jianming, “The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals,” Food Chemistry, Vol. 64, No. 4, 1999, pp. 555-559. doi:10.1016/S0308-8146(98)00102-2
- S. B. Lotitio and C. G. Fraga, “Catechin Prevents Human Plasma Oxidation,” Free Radical Biology and Medicine, Vol. 24, 1998, pp. 435-441. doi:10.1016/S0891-5849(97)00276-1
- S. M. Suboh, Y. Y. Bilto and T. A. Aburjai, “Protective Effects of Selected Medicinal Plants against Protein Degradation, Lipid Peroxidation and Deformability Loss of Oxidatively Stressed Human Erythrocytes,” Phytotherapy Research, Vol. 18, No. 4, 2004, pp. 280-284. doi:10.1002/ptr.1380
- P. Ljubuncic, H. Azaizeh, I. Portnaya, U. Cogan, O. Said, K. A. Saleh, K. Abu Saleh and A. Bomzon, “Antioxidant Activity and Cytotoxicity of Eight Plants Used in Traditional Arab Medicine in Israel,” Journal of Ethnopharmacology, Vol. 99, No. 1, 2005, pp. 43-47. doi:10.1016/j.jep.2005.01.060
- Y. H. Chu, C. L. Chang and H. F. Hsu, “Flavonoid Content of Several Vegetables and Their Antioxidant Activity,” Journal of the Science of Food and Agriculture, Vol. 80, No. 5, 2000, pp. 561-566. doi:10.1002/(SICI)1097-0010(200004)80:5<561::AID-JSFA574>3.0.CO;2-#
- A. M. Nuutila, R. Puupponen-Pimia, M. Aarni and K. M. Oksman-Caldentey, “Comparison of Antioxidant Activities of Onion and Garlic Extracts by Inhibition of Lipid Peroxidation and Radical Scavenging Activity,” Food Chemistry, Vol. 81, No. 4, 2003, pp. 485-493. doi:10.1016/S0308-8146(02)00476-4
- G. A. Agbor, J. E. Oben, J. Y. Ngogang, C. Xin and J. A. Vinson, “Antioxidant Capacity of Some Herbs/Spices from Cameroon: A Comparative Study of Two Methods,” Journal of Agricultural and Food Chemistry, Vol. 53, No. 17, 2005, pp. 6819-6824. doi:10.1021/jf050445c
- S. Demiray, M. E. Pintado and P. M. L. Castro, “Evaluation of Phenolic Profiles and Antioxidant Activities of Turkish Medicinal Plants: Tilia Argentea, Crataegi Folium Leaves and Polygonum Bistorta Roots,” World Academy of Science, Engineering and Technology, Vol. 54, 2009, pp. 312-317.
- C. A. Hall and S. L. Cuppett, “Activities of Natural Antioxidants,” In: O. I. Aruoma and S. L. Cuppett, Eds., Antioxidant Methodology in Vivo and in Vitro Concepts, AOCS Press, Champaign, 1997, pp. 2-29.
- D. Ferreira, D. Slade and J. P. J. Marais, “Flavans and Proanthocyanidins,” In: O. M. Anderson and K. R. Markham, Eds., Flavonoids: Chemistry, Biochemistry and Applications, CRC Press, Boca Raton, 2006, pp. 553-616.
- E. N. Frankel and A. S. Meyer, “The Problems of Using One-Dimensional Methods to Evaluate Multifunctional Food and Biological Antioxidants,” Journal of the Science of Food and Agriculture, Vol. 80, No. 13, 2000, pp. 1925-1940. doi:10.1002/1097-0010(200010)80:13<1925::AID-JSFA714>3.0.CO;2-4
- C. M. Liyana-Pathirana and F. Shahidi, “Antioxydant Propreties of Commercial Soft and Hard Winter Wheats (Triticum aestivium L.) and Their Milling Fractions,” Journal of the Science of Food and Agriculture, Vol. 86, No. 3, 2006, pp. 477-485. doi:10.1002/jsfa.2374
- T. Kadifkova-Panovska, S. Kulevanova and M. Stefova, “In Vitro Antioxidant Activity of Some Teucrium Species (Lamiaceae),” Acta Pharmaceutica, Vol. 55, 2005, pp. 207-214.
- C. Sánchez-Moreno, “Methods Used to Evaluate the Free Radical Scavenging Activity in Foods and Biological Systems,” Food Science and Technology International, Vol. 8, 2002, pp. 121-137.
- V. Bondent, W. Brand-Williams and C. Bereset, “Kinetic and Mechanism of Antioxidant Activity Using the DPPH Free Radical Methods,” Lebensmittel Wissenschaft and Technologie, Vol. 30, No. 6, 1997, pp. 609-615. doi:10.1006/fstl.1997.0240
- C. A. Rice-Evans, N. J. Miller and G. Paganga, “Structure, Antioxidant Activity Relationship of Flavonoids and Phenolic Acids,” Free Radical Biology and Medicine, Vol. 20, No. 7, 1996, pp. 933-956. doi:10.1016/0891-5849(95)02227-9
- I. F. Benzie and J. J. Strain, “The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ‘Antioxidant Power’: The FRAP Assay,” Analytical and Bioanalytical Chemistry, Vol. 239, 1996, pp. 70-76.
- Z. Maksimović, D. Malenčić and N. Kovačević, “Polyphenol Contents and Antioxidant Activity of Maydis Stigma Extracts,” Bioresource Technology, Vol. 96, No. 8, 2005, pp. 873-877. doi:10.1016/j.biortech.2004.09.006
- W. Wang and M. T. Goodman, “Antioxidant Property of Dietary Phenolic Agents in a Human LDL-Oxidation ex Vivo Model: Interaction of Protein Binding Activity,” Nutrition Research, Vol. 19, No. 2, 1999, pp. 191-202. doi:10.1016/S0271-5317(98)00183-3
- H. Azaizeh, P. Ljubuncic, I. Portnaya, O. Said, U. Cogan and A. Bomzon, “Fertilization-Induced Changes in Growth Parameters and Antioxidant Activity of Medicinal Plants Used in Traditional Arab Medicine,” Evidence-Based Complementary and Alternative Medicine, Vol. 2, 2005, pp. 549-556. doi:10.1093/ecam/neh131
- A. Ardestani and R. Yazdanparast, “Inhibitory Effects of Ethyl Acetate Extract of Teucrium polium on in Vitro Protein Glycoxidation,” Food and Chemical Toxicology, Vol. 45, No. 12, 2007, pp. 2402-2411. doi:10.1016/j.fct.2007.06.020
- D. Bagchi, A. Garg, R. L. Krohn , M. Bagchi, J. Balmoori and S. J. Stohs, “Protective Effects of Grape Seed Proanthocyanidins and Selected Antioxidants against TPA-Induced Hepatic and Brain Lipid Peroxidation and DNA Fragmentation, and Peritoneal Macrophage Activation in Mice,” General Pharmacology, Vol. 30, No. 6, 1998, pp. 771-776. doi:10.1016/S0306-3623(97)00332-7
- M. Murray and J. Pizzorno, “Procyanidolic Oligomers,” In: M. Murray and J. Pizzorno, Eds., The Textbook of Natural Medicine, 2nd Edition, Churchill Livingston, London, 1999, pp. 899-902.
- D. Bagchi, R. L. Krohn, M. Bagchi, M. X. Tran and S. J. Stohs, “Oxygen Free Radical Scavenging Abilities of Vitamins C and E, and a Grape Seed Proanthocyanidin Extract in Vitro,” Research Communications in Molecular Pathology & Pharmacology, Vol. 95, 1997, pp. 179-189.
- E. Bombardelli, P. Morazzoni, M. Carini, G. Aldini and F. R. Maffei, “Biological Activity of Procyanidins from Vitis vinifera L.,” BioFactors, Vol. 6, No. 4, 1997, pp. 429- 431. doi:10.1002/biof.5520060411
- S. Son and B. A. Lewis, “Free Radical Scavenging and Antioxidant Activity of Caffeic Acid Amide and Ester Analogues: Structure-Activity Relationship,” Journal of Agricultural and Food Chemistry, Vol. 50, No. 3, 2002, pp. 468-472. doi:10.1021/jf010830b
- W. Zheng and S. Y. Wang, “Antioxidant Activity and Phenolic Compounds in Selected Herbs,” Journal of Agricultural and Food Chemistry, Vol. 49, No. 11, 2001, pp. 5165-5170. doi:10.1021/jf010697n

