Engineering
Vol. 2 No. 12 (2010) , Article ID: 3608 , 4 pages DOI:10.4236/eng.2010.212123
Influence of Heat Treatment Temperature and of Sb2O3 addition on Photoluminescence Properties of ZnO Ceramics Prepared by Sol-Gel Technique
Division of Chemistry for Materials, Faculty of Engineering, Mie University, Tsu, Japan
E-mail: nasu@chem.mie-u.ac.jp
Preparation
Received July 26, 2010; revised September 21, 2010; accepted September 30, 2010
Keywords: Zno Ceramics, Sb2O3 Doping, Photoluminescence, Heat Treatment Temperature, Sol-Gel
ABSTRACT
To explore thin transparent electroluminescence and electric conductive films by sol-gel technique, Sb2O3 doped n-type ZnO ceramics powders were prepared by sol-gel technique and photoluminescence properties were measured. Then, the influences of composition and heat treatment temperature on photoluminescence properties were investigated in detail. With respect to the dopant concentration, about 1 mol% addition of Sb2O3 was effective to increase photoluminescence intensity. With respect to heat treatment temperature, 800○С was appropriate, and rather higher heat treatment temperature resulted in the formation of Zn7Sb2O12 and decrease the intensity. The excited ultraviolet wavelength of 200 nm was proper to intense photoluminescence.
1. Introduction
ZnO is aⅡ-Ⅵ type semiconductor with direct transition of 3.37 eV. Further, the exciton binding energy is 60 meV at room temperature, while which of GaN currently used for blue light emitting diode (LED) is 25 meV. Moreover, GaN is a point contact LED, which means that it cannot be used as plane display, although ZnO ceramics can be considered to be used as plane LED. That is significant advance in display application. Recently, the successful preparation of ZnO diode which has 10 times larger emission efficiency than GaN LED was reported [1]. Thus, ZnO is a promising material for the future display device.
For the practical use of ZnO ceramics, it should have electroluminescence property. Al or Ga was successfully doped in ZnO and make it electrically conductive [2-4]. Similarly, the addition of Sb2O3 is expected n-doped ZnO making electric conductive. However, no report has been concerned with the influence of Sb2O3 doping into ZnO ceramics to the author’s knowledge.
To develop electroluminescence materials, photoluminescence property is important since the relaxation process is quite similar. The difference between those properties is only the difference in electron excitation process. The oxygen defects can be considered to cause emission process [4,5]. Thus, emission process and electrically conducting process are pointed out to be relating oxygen vacancies [6,7]
The sol-gel preparation technique is quite useful and easy technique to prepare thin oxide films because of the technique using solution state, and it is applicable to prepare ZnO ceramics on transparent glass substrate [8-10]. The advantages of the sol-gel technique for film preparation is the possible coating on the complicate shapes of the surfaces, the preparation under the ambient atmosphere at room temperature and the high homogeneity of the resultant products.
Therefore, this paper reports sol-gel preparation of Sb2O3 doped ZnO polycrystalline ceramics and these photoluminescence properties.
2. Experimental Procedure
Figure 1 indicates the flowchart of the sample preparation. The raw chemicals were commercially available, and analytical grade Zn(CH3COO)2・2H2O, SbCl3, NH2CH2CH2OH(MEA) and CH3OC2H4OH(MEH)

Figure 1. Flowchart of the sample preparation.
(Nakalai Tesque Co. Ltd.)
The precursor liquids were prepared by the addition of .Zn(CH3COO)2・2H2O and SbCl3 into mixed MEA and MEH stirred for 30 min at 60○С. The composition of the precursor liquid is tabulated in Table 1.
The liquid was stirred well at 60○С and gel was derived after 3 days. The liquid was turned to be viscous since water and some parts of organics may be evaporated. The liquid was hold in glass wares and exposed to air without any cap.
The gels were derived from the precursors after heat treated for 3 days at 60○С. Then, gels were fired to 400○С for 5○С/min and at 400~1,150○С for 1h in air.
X-ray diffraction (XRD) patterns of the obtained powders were detected using Ni-filtered Cu-Kα radiation (Rigaku Ultima IV) as X-ray source.
Photoluminescence spectra were measured UV-Vis spectrophotometer using Xe light at 200, 220 and 250 nm for the derived powders.
3. Results and Discussion
Figure 2 depicts the dependence of photoluminescence spectra on the excited different UV light wavelengths, in specific, 200, 220 and 250 nm. Since the power of the excited light is the same and second-order emitting light intensity should be similar, it can be elucidated that the intensity is strongest by 200 nm excitation, and decreases as longer excitation wavelength by comparing with the intensity of second-order emission of Xe lump. In specific, the intensity of 470 nm luminescence by 200 nm
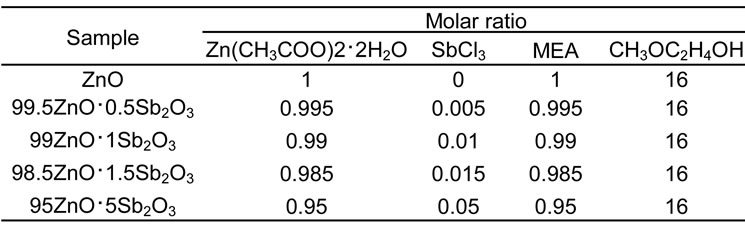
Table1. Composition of the starting liquids.

Figure 2. Influence of excited light wavelength on photoluminescence.
excitation is so intense to be comparable with that of 400 nm second-order emission peak of Xe lump. Similar tendency can be seen for all samples. Therefore, we focus on the luminescence by excitation of 200 nm hereafter. This phenomenon implies the bandgap of oxygen defects or exciton is over 6.2 eV.
The photoluminescence spectra heated at 600○С for various Sb2O3 concentration are shown in Figure 3. To compare them with each other, the second-order emission intensity is determined as the standard. Hereafter, all spectra are normalized by the second-order emission of Xe light source which is 400 nm. The strongest photoluminescence peaks are located near 470 nm (3.1 eV) for all samples, and that increases to near 1.0 mol% Sb2O3 addition and then decreases as increasing the dopant concentration.
Figure 4 depicts the powders heated at 800○С for the various Sb2O3 concentration. Similarly, the strongest peaks appear at near 470 nm for all samples, and about 1 mol% addition is most effective to increase photoluminescence spectra.
The influence of heat treatment temperature on the intensity is shown in Figure 5 for 1 mol% Sb2O3 concentration powder. Except the sample heated at 400○С, all other samples show the strongest peak at near 470 nm.

Figure 3. Influence of Sb2O3 concentration after heated at 600○С on photoluminescence.
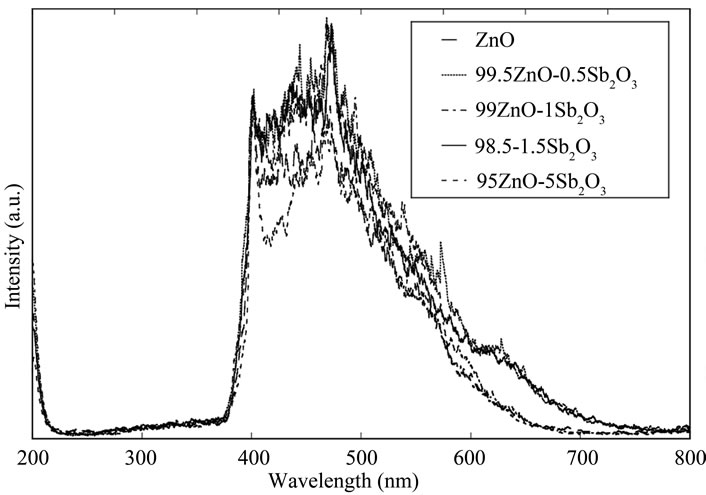
Figure 4. Influence of Sb2O3 concentration after heated at 800○С on photoluminescence.

Figure 5. Influence of heat treatment temperature for ZnO powder containing 1 mol% Sb2O3.
The intensity increases to 800○С as heat treatment temperature increases, but higher heat treatment lowers the peak intensity.
Figure 6 summarizes the influences of the dopant concentration and heat treatment temperature on the peak at near 470 nm. From this figure, it can be said that about 1 mol% addition of Sb2O3 is the most efficient and 800○С is also efficient to enhance 470 nm photoluminescence intensity.
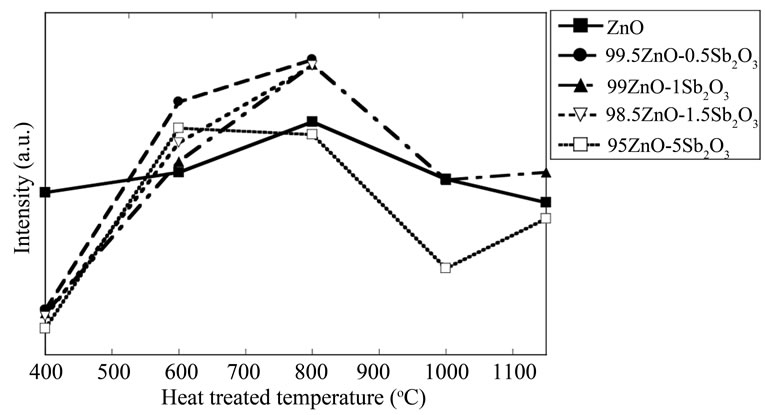
Figure 6. Influences of Sb2O3 concentration and heat treatment temperature on photoluminescence.
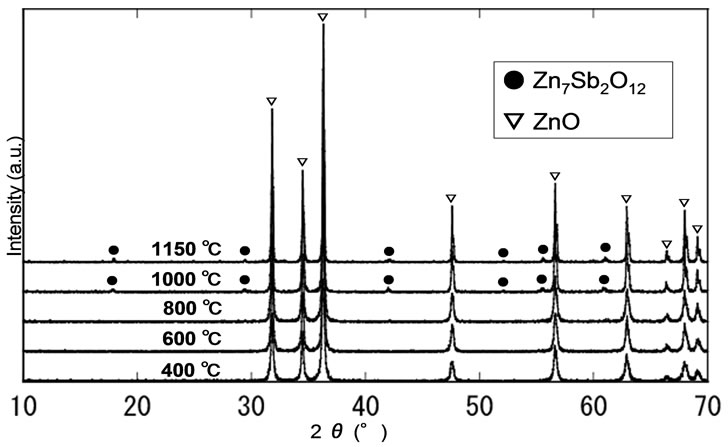
Figure 7. XRD patterns of the ZnO powder containing 1 mol% Sb2O3 after heat treated at various temperatures.
The XRD patterns obtained for 1 mol% Sb2O3 doped ZnO polycrystalline powders at the various heat treatment temperatures are shown in Figure 7. One can only see ZnO crystalline peaks in the patterns below 800○С heat treatment. It implies the Sb3+ ions are uniformly dispersed in ZnO matrix and crystallinity is improved as heat treatment temperature increases since the full width at half maximum of ZnO (101) monotonically decreases with increasing heat treatment temperature.
Careful observation of the peaks from the powder heat treated above 1000○С reveals the formation of Zn7Sb2O12 crystal. Thus, it is elucidated that the formation of the complex ceramic crystal lowers the photoluminescence intensity.
4. Conclusions
Sb2O3 doped ZnO polycrystalline powders were successfully prepared by sol-gel technique, which will be used for future dip coating films preparation.
With respect to the influence of dopant concentration, about 1 mol% addition of Sb2O3 was effective to enhance photoluminescence intensity.
With respect to heat treatment temperature, 800○С was good for increasing photoluminescence intensity. Further higher temperature was good in sense of crystallinity, but formation of complex crystal lowered the intensity.
5. REFERENCES
- A. Tsukazaki, M. Kubota, A. Ohotomo, T. Onuma, K. Ohtani, H. Ohono, S. F. Chichibu and M. Kawasaki, “Blue Light-Emitting Diode Based on ZnO,” Japanese Journal of Applied Physics, Vol. 44, 2005, pp. 643-645.
- H. Sato, T. Minami, S. Takata, T. Mouri and N. Ogawa, “Highly Conductive and Transparent ZnO: Al Thin Films Prepared on High-Temperature Substrates by d.c. Magnetron Sputtering,” Thin Solid Films, Vol. 220, 1992, pp. 327-332.
- T. Minami, T. Kakumu, K. Shimokawa and S. Takata, “New Transparent Conducting Zno-In2O3-Sno2 Thin Films Prepared by Magnetron Sputtering,” Thin Solid Film, Vol. 317, No. 1-2, April 1998, pp. 318-321.
- M. Lippmas, T. Koida, H. Minami, Z. W. Jin, M. Kawasaki and H. Koinuma, “Design of Compact Pulsed Laser Deposition Chambers for the Growth of Combinatorial Oxide Thin Film Libralies,” Applied Surface Science, Vol. 189, No. 3-4, April 2002, pp. 205-209 .
- K. Vanheusden, W. L. Warren, C. H. Seager, D. R. Tallant, J. A. Voigt and B. E. Gnade, “Mechanisms behind Green Photoluminescence in Zno Phosphor Powders,” Japanese Journal of Applied Physics, Vol. 79, No. 10, June 1996, pp. 7983-7990.
- M. A. Reshchikov, H. Morkoc, B. Nemeth, J. Nause, J. Xie, BHertog and A. Osinsky, “Luminescence Properties of Defects in Zno,” Physica B: Condensed Matter, Vol. 401-402, December 2007, 358-361.
- G. H. Ning, X. P. Zhao, J. Li and C. Q. Zhang, “Hugely Enhanced Electroluminescence From Mesoporous Zno Particles,” Optical Materials, Vol. 28, No. 4, March 2006, pp. 385-390.
- C. M. Yan, Z. Chen and X. P. Zhao, “Enhanced Electroluminescence of Zno Nanocrystalline Annealing from Mesoporous Precursors,” Solid State Communications, Vol. 140, No. 1, October 2006, pp. 18-22.
- E. A. Meulenkamp, “Synthesis and Growth of ZnO Nanoparticles,” The Journal of Physical and Chemistry B, Vol. 102, No. 29, 1998, pp. 5566-5572.
- M. Ohyama, H. Kozuka, T. Yoko and S. Sakka, “Preparation of Zno Films with Preferential Orientation by Sol-Gel Method,” Journal of the Ceramic Society of Japan, Vol. 104, 1996, pp. 296-300.

