Materials Sciences and Applications
Vol.07 No.11(2016), Article ID:72324,18 pages
10.4236/msa.2016.711059
Effect of Aqueous Extracts of Salvadora persica “Miswak” on the Acid Eroded Enamel Surface at Nano-Mechanical Scale
Tahani M. Bawazeer1*, Mohammad S. Alsoufi2, Dina Katowah1, Waad S. Alharbi3
1Chemistry Department, College of Science, Umm Al-Qura University, Makkah, KSA
2Mechanical Engineering Department, College of Engineering and Islamic Architecture, Umm Al-Qura University, Makkah, KSA
3Chemistry Department, Science College, University of Jeddah, Jeddah, KSA

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: October 22, 2016; Accepted: November 26, 2016; Published: November 29, 2016
ABSTRACT
Understanding the effects of enamel loss and surface softening caused by acidic soft drinks consumption would help in the clinical treatment of tooth wear and aid in the development of novel dental restorative materials. Hence, this investigation was carried out to examine the effects of “Miswak” on the acid eroded enamel surface at nano-mechanical scale. The first use of a stylus-based inductive gauge is introduced as means of measurement of acid attack on enamel surfaces and the effectiveness of aqueous extracts of Salvadora persica as dissolution inhibitors for acid eroded enamel surfaces. For pre-molar protective, after being exposed to aqueous extracts of Salvadora persica solution, the performance of the enamel surface was improved by Ra ≈ 0.82 µm. Whereas, the surface was damaged after immersing in citric acid solution by Ra ≈ 0.63 µm. For pre-molar restoring, the enamel surface was degraded by Ra ≈ 0.34 µm when exposed to citric acid solution. While, the surface roughness was improved by Ra ≈ 0.95 µm when aqueous extracts of Salvadora persica solution was introduced. So, this study concludes that the aqueous extracts of Salvadora persica treatment work effectively on the eroded enamel surface.
Keywords:
Enamel, Salvadora persica, Miswak, Erosion, Citric Acid, Surface Roughness

1. Introduction
Tooth enamel is the hardest and most highly mineralized material of the human body. It is one of the three main tissues, which form the tooth structure, along with dentine and pulp. It is visible and supported by embedded dentine. Dental enamel has a prismatic macro-structure arising from its production from ameloblast cells. Enamel is essentially composed of 95 wt% impure calcium hydroxyapatite (HAP) with the remaining being organic material and water (H2O) [1] [2] . This semi-clear, external layer protects human teeth from the daily wear and tear of chewing and biting as well as foods and drinks of temperatures ranging from hot to cold. Enamel also shields the teeth against the effects of acid and chemical attacks. When this shell erodes, the teeth are more likely to become subject to cavities and decay and start to respond more to cold or hot foods, sweets and drinks, as these can get through the dentine pores to the nerves inside causing dentine sensitivity (DS) [3] [4] .
1.1. Enamel Erosion
The potential of enamel erosion has been documented over a great many years. Darby provided early report results of enamel erosion in 1892 [5] and Miller in 1907 [6] . Recently, enamel acid-induced dissolution has been of increased interest due to its wide implication in oral science, from caries to acid erosion. The carboxylic acids in the acidic medium chemically adsorb onto the enamel surface and dissolve Ca2+ ions out of the HAP as per Equation (1), which is usually defined as surface demineralization [7] .
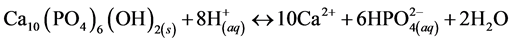 (1)
(1)
Enamel dissolution can be defined as the localized loss of dental hard tissue [8] from the tooth surface due to the chemical influence of extrinsic acids and is irreversible [9] . These extrinsic acids can include beverages, acidic substances, medication, foods and environmental exposure to acidic agents Intrinsic acids [9] meanwhile are produced by frequent vomiting due to the eating disorder bulimia nervosa or by the regurgitation of the gastric substances) without the involvement of microorganisms [9] [10] . Frequent consumption of acid-containing drinks/foods is an important factor in enamel erosion, with many soft drinks being in the pH range between 2 and 3 [11] and being the most commonly consumed erosive acids [10] . Dental erosion does not only affect enamel but can also reach dentine, causing hypersensitivity, or in severe cases, pulp exposure and even tooth fracture. Investigating the topic of dental erosion would aid in the medical treatment of tooth wear and also help in the development of advanced dental restorative materials [12] . It would also aid in providing broader insights into the chemical process of dental erosion, and into the means by which erosion can be reduced, modified or prevented.
An interesting property of HAP is the ability to exchange calcium ions by sodium, magnesium, zinc, strontium zinc, or lead ions. Additionally, hydroxyl and phosphate groups can be substituted by chloride, bicarbonate and fluoride ions at the apatite surfaces and incorporate in the surface layer in the form of zinc apatites, fluorapatite, etc. [13] [14] . Since the resistance of the enamel surface to acids depends largely on its chemical composition, it is clearly of interest to alter the composition that supports or prevents the process of erosion. Fluoride ions are known to be a good inhibitor of enamel dissolution as it is thought to incorporate into the enamel and increase its hardness and resistance to acid erosion. This incorporation may involve loosely or firmly bound fluoride and the extent to which the fluoride penetrates the enamel is a subject of much debate [15] [16] [17] [18] [19] . Strengthening the acid resistance of the enamel surface by forming organic or inorganic protective layers on the tooth surface is another approach in altering the chemical composition of the outer enamel layer. These materials have been used as inhibitors in the erosive medium or experimental rinses, exhibiting reduction values of the erosive deterioration and their potential to inhibit or decrease the dissolution rate of Ca2+ and 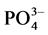 ions from the HAP [20] . Generally, there is limited data concerning the role of the incorporation of these polymers in the protective layers.
ions from the HAP [20] . Generally, there is limited data concerning the role of the incorporation of these polymers in the protective layers.
Several techniques have been applied to monitor the dissolution and precipitation kinetics of enamel and hydroxyapatite including techniques of scanning electron microscopy (SEM) [21] [22] [23] of enamel surfaces atomic force microscopy (AFM) [21] [22] [23] profilometry and more recently nano-indentation [21] . Typically, the majority of published methods involved subjecting slices of enamel to a collection of acid challenges [24] , ranging in pH from 2.3 to 6.3 or the effect of varying compositions of the solution and effectively changing the saturation level of the solution. To the author’s best knowledge, however, the hypothetical effect of natural polymers on reducing or restoring the acid erosion of human dental enamel evaluated by a stylus-based inductive gauge has not been reported so far.
1.2. Salvadora persica “Miswak”
Throughout the world, different species of plants have been used as oral treatments, the most significant of these being “Miswak” also known as “Arak tree” (as shown in Figure 1) [25] [26] . Miswak is made from “Salvadora persica” belonging to the family “Salvadoraceae S. persica” and is a small tree with a crooked trunk whose twigs have been approved by the World Health Organization (WHO) for oral hygiene [27] [28] and which is also consistent with Primary Health Care Approach (PHCA) principles [29] . The conventional meaning of “Miswak” is “stick” used on teeth and gums to clean
Figure 1. (a) Salvadora persica tree in Saudi Arabia with a maximum height of 3 m, (b) Miswak, and (c) Image of use Miswak.
them and also used for religious purposes in the Middle East [30] [31] . Aqueous extracts of Salvadora persica “Miswak” has been reported as possessing a number of medically beneficial properties including being anti-caries, anti-fungal, antibacterial and anti-periopathic. It has also been shown to contain several chemical constituents such as trimethylamine (C3H9N), salvadorine, silicon dioxide (SiO2), vitamin C (C6H8O6), fluoride (F−), chloride (Cl−), sulphur, resins, sterol, and flavonoides. It comprises the following compounds: lauric (C12H24O2), myristic (C14H28O2) and palmitic (C16H32O2) acids; polysaccharide and lignin derivatives of phenols and furans; sterols (also known as steroid alcohols) [31] .
A number of studies have been carried out on the anti-microbial effect of “Miswak” extract on cariogenic and periodontal pathogens. Also, Salvadora persica “Miswak” extracts showed a significant effect as regards reducing dental plaque and caries. But, so far, no study has been undertaken on the potential effects of “Miswak” on dental acid erosion. Hence, this investigation was carried out to examine the effects of “Miswak” on the acid eroded enamel surface at nano-mechanical scale. Studies involved two phases: i) extraction of aqueous solution extracts from Salvadora persica “Miswak” and fully characterizing these, and ii) the testing of the hypothesis that aqueous extracts of Salvadora persica (AESP) treatment forms a protective layer on the eroded enamel surface.
2. Materials and Methods
2.1. Sample Preparations
For this study, non-erupted human premolar teeth, as shown in Figure 2, were used. In brief, the teeth were extracted and disinfected from orthodontic clinics in Makkah, KSA, from 25-to-35-year-old subjects of either gender. Only macroscopically sound enamel surfaces were selected for this study. All the teeth that had been chosen for use would not have had any restoration or defect such as caries, enamel hypoplasia or other defects. Ideally, before storage stage, all pre-molar teeth were cleaned with a toothbrush under running tap water. Then, all samples were stored in artificial saliva at an air-
Figure 2. Schematic illustration of human premolar tooth.
conditioned temperature of 20˚C ± 1˚C and a relative humidity of 40% ± 5% RH until use. After that, all samples to be treated were placed in their respective solutions and rinsed with reagent water ca. 30 seconds.
2.2. Extraction of Salvadora persica “Miswak”
2.2.1. Plant Materials
Dried roots of “Miswak”, as shown in Figure 1(b), were purchased from a local market in Makkah city, Kingdom of Saudi Arabia, and these had been cut in the 2015/2016 season. All the authors, in consultation with an experienced “Arak” merchant, identified the plant known as Salvadora persica “Miswak”.
2.2.2. Preparation of Salvadora persica “Miswak” Extracts
Dried roots of “Miswak” were sliced using a sharp knife then the pieces were ground to a powder with a blending machine. Then, 100 mL of deionized distilled water (from Sigma-Aldrich Chemie GmbH, Switzerland) was added to 10 mg of powder in a sterile well-capped flask; the extract was allowed to soak for 48 hrs. in the refrigerator. After that, the combination was centrifuged at 2000 rpm for around 10 minutes. The supernatant was filtered using a 0.45 mm membrane and stored in sterile screw-capped vials in the refrigerator until used. This procedure is adequate and consistent with the following publications [32] [33] .
2.2.3. Polysaccharide Isolated from Salvadora persica “Miswak” Extracts
The supernatant from water soaked “Miswak” was precipitated with three volumes of acetone and stored overnight in a 4-degree refrigerator. After that, the precipitate was collected and washed three times again with acetone in order to obtain crude polysaccharide of around 2.0 g. The crude polysaccharide was dissolved in sterile deionized distilled water and then dialyzed against distilled water. The remaining quantity was lyophilized to provide total polysaccharide. This procedure is consistent with the following [34] [35] .
2.2.4. Polysaccharides Composition
Three qualitative analyses of carbohydrates were carried out to confirm the nature of the carbohydrate. Firstly, Molisch’s test in which 2 mL of carbohydrate solution and 2 drops of α-naphthol solution were added together in a test tube and using a dropper and carefully inclining the tube and pouring dropwise, concentrated H2SO4 was added. The violet color at the junction of the two liquids was observed, this is because of unstable condensation product formation of β-naphthol with furfural produced by the dehydration of the carbohydrate. Secondly, Fehling’s test in which equal volumes of the test carbohydrate, Fehling A and Fehling B solution was added together and placed in a boiling water bath for several minutes. The production of brownish-red or yellow precipitate of cuprous oxide (CuO) suggests the presence of reducing sugars in the given test solution. Lastly, the Iodine test in which 2 drops of iodine solution and 2 mL of the carbohydrate test solution were mixed and a blue-black color was spotted which indicates the presence of polysaccharides. Iodine forms colored adsorption complexes with polysaccharides.
2.2.5. Solubility Test
The polysaccharide obtained from Salvadora persica “Miswak” was assessed for solubility in water (H2O), acetone (C3H6O), chloroform (CHCl3), and ethanol (C2H6O) in accordance with the British Pharmacopoeia (BP) specification.
2.2.6. Fourier Transform Infrared (FTIR)
The FTIR spectrum of the polysaccharides was recorded using a PerkinElmer Spectrum 100 FTIR spectrometer (PerkinElmer, Norwalk, CT, USA) equipped with a MIR TGS detector. The FTIR spectra were recorded between the 4000 to 400 cm−1 region at a room temperature of 20˚C ± 1˚C and a relative humidity of greater than 40% ± 5% RH. All the data analysis was collected automatically by using appropriate software (Spectrum 100 software). Each enamel sample was scanned as three different replicates under the similar settings, all of which gave identical spectra. The mean spectra of these three replicates’ data were then used in detailed data and statistical analysis.
2.2.7. Nuclear Magnetic Resonance (NMR)
The principal component analysis of 600 MHz 1H-NMR spectra of the polysaccharides was recorded using Bruker Avance III WM 600 MHz spectrometers (Karlsruhe, Germany). The sample was dissolved in D2O at a room temperature of 20˚C ± 1˚C and a relative humidity of greater than 40% ± 5% RH. The combination of peak volumes in the 2D-HSQC spectra was completed via standard Bruker TOPSPIN 3.0 software.
2.3. Test Solution
To prepare the citric acid solutions 50% (w/w), the citric acid was added to alter the pH and controlled by using a pH meter (Model: Knick pH meter 765 Calimatic, Knick Elektronische Messgerate GmbH & Co. KG, Berlin, Germany).
2.4. Enamel Erosion Samples Treatment
The enamel samples were treated at a room temperature of 20˚C ± 1˚C and a relative humidity of greater than 40% ± 5% RH with CAS for 120 seconds, to replicate the common intake times of soft drinks. For the erosion studies, three samples (n = 3) were prepared for every citric acid (CAS). For each sample, 30 mL of CAS was used. After treatment, the samples were immediately rinsed with deionized water for 30 seconds and dried with compressed air. Then, samples were stored in the same environment that would be used for testing by the Taly-surf® test-rig. For the SEM study, immediately after treatment with the CAS, one-half of the surface of the enamel sample was exposed to AESP to obtain a treated area.
2.5. Testing Procedures
The surface roughness, Ra, is defined as an average roughness of the profile around the mean line (generated by a standard filter or least squares) and it is possibly the most frequently used parameter. If z = f(x) is the profile measured from the reference mean line and L are the profile lengths measured from the reference mean line, L is the profile length being evaluated, then the average surface roughness, Ra, value is defined in Equation (2) by [36] [37] [38] [39] [40] :
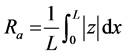 (2)
(2)
The 3D average surface roughness can be represented by a function of z(xi, yi) with xi = i∆x and yj = j∆y, where i = 1, 2, 3, ∙∙∙, M and j = 1, 2, 3, ∙∙∙, N. In addition, ∆x and ∆y are sampling intervals. Likewise, M and N symbolize the number of sampling data points in the x-direction and y-direction. The 3D average surface roughness parameters are passed on the residual surface η(x, y), which is the difference between the original surface z(xi, yi) and the reference datum f(xi, yi). Then, the average value of the surface roughness, Sa, is defined in Equation (3) as [36] [37] [38] [39] [40] :
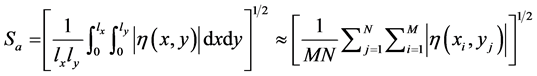 (3)
(3)
Bear in mind, Ra and/or Sa is an arithmetic mean parameter of the roughness profile. The sampling interval is insensitive to changes. Hence, the surface roughness profile of enamel teeth was analyzed quantitatively to determine the standard statistical parameter of average roughness, Ra, using a commercial precision test-rig Taly-surf® (from Taylor Hobson Precision, Inc.). Table 1 shows the specification of the device, and it conforms to British Standards (BS). Also, Figure 3 shows the image of Taly-surf® with conisphere tip r = 2 µm and pre-molar teeth with the surface profile. The errors of the surface roughness were calculated from the standard deviation (±SD) of the absolute values of height deviation and the data traces were auto-levelled to a linear least-squares straight line and then filtered with a typical cut-off.
During the course of the investigation, every single test was repeated at least three times at different and new locations on the enamel surface in order to ensure the repeatability and reproducibility of the results. The new location was at least ±300 µm from the previous test. This method should avoid any data variation of the counterbody
Table 1. Test-rig specifications.
Figure 3. Image of Taly-surf® with conisphere tip r = 2 µm (from Taylor Hobson) and pre-molar teeth with surface profile.
surface, e.g., due to micro-tribological behavior, which may appear during the test performance and indeed will affect the measurements in the following tests. All experiments were performed with a typical arrangement of sphere-on-flat applying a linear sliding contact at constant velocity over a specific distance. Tests were achieved by using a forward scan mode motion at scan length of 5 mm. For more details, see publication [41] [42] [43] [44] .
2.6. Calibration Method
A standard ball of 22.0161 mm diameter is used to calibrate the precision instrument (from Taylor Hobson Precision, Ltd.), as shown in Figure 4. A series of ten calibration trials have been performed. This is an acceptable series of experiments as these calibration trials are predominantly about design interpretation and relative behavior to other precision systems is always vulnerable to variations in materials and dimensions. Calibration results showed that the cantilever beam system at only one end was a linear mass-spring system (R2 > 0.99), under operational and environmental conditions, with an absolute uncertainties value of <1% and measurement resolution down to at worst 50 nm. For more details, see publication [45] .
2.7. Scanning Electron Microscopy (SEM)
The surface topography of all pre-molar samples was analyzed by SEM of at least ten scan areas (scan size: 10 µm × 10 µm) measured at an arbitrarily located area on the surface of the sample. The SEM was performed with an LEO 440i (LEO Elektronen- mikroskopie GmbH, Oberkochen, Germany) operated at 15 kV. To study the surface visualization and to improve the reflectivity for a higher fidelity in optical profiling, all samples were fully coated with gold (25 nm thickness in 60 seconds) using Bio-Red SEM Coating System.
3. Results and Discussions
Every test was repeated at least three times, and the results were presented as mean ±SD value. All citric acid solution of the current investigation was characterized by pH values. The pH value of the CAS was 3.3. As has been previously mentioned, the present work aims to study the effect of aqueous extracts of Salvadora persica on human dental enamel acid erosion. Surface roughness, Ra, has been extensively used to determine the total average roughness and the coverage net (before and after the treatment). Figure 5 illustrates an example of a human dental enamel surface roughness before and after the treatment of AESP.
3.1. Stylus-Base Data Analysis
The mean surface roughness, Ra, of the pre-molar teeth was measured using “ball-on- flat” arrangement and “single scan mode”. For group I: pre-molar protective, under normal conditions (where there is no inhibitor, and the tooth has been washed with deionized water for 30 seconds), the average surface roughness of the pre-molar tooth was 3.38 µm (±SD = 0.02 µm). Indeed, after the premolar was exposed to the AESP (for approximately 2 minutes), the performance of the human dental enamel was improved, and the average roughness of the premolar tooth was Ra ≈ 2.56 µm (±SD = 0.035 µm), which means that the surface net was Ra ≈ 0.82 µm. However, after the sample was immersed again in CAS, the surface was damaged by 0.63 µm as the surface roughness of the sample was Ra ≈ 3.19 µm (±SD = 0.027 µm). In addition, the remaining layer of the
Figure 4. Measurement calibration on a sphere of known radius.
Figure 5. A typical example of a human dental enamel surface roughness before and after treatment.
protective zone was around Ra ≈ 0.19 µm with a 95% level of confidence, as shown in Figure 6.
On the other hand, for group II: pre-molar restoring, under the normal conditions (where there is no inhibitor, and the tooth has been washed with deionized water for 30 seconds), the average surface roughness of the pre-molar tooth was 3.58 µm (±SD = 0.084 µm). After the pre-molar was exposed to the CAS (for around 2 minutes), the performance of the human dental enamel was degraded, and the average roughness of the pre-molar tooth was Ra ≈ 3.92 µm (±SD = 0.075 µm), which means that the surface net was Ra ≈ 0.34 µm. AESP was introduced to the human dental enamel after CAS (for around 2 minutes), the surface roughness was measured, and it gives us the Ra ≈ 2.97 µm (±SD = 0.058 µm), indicating that the surface net was Ra ≈ 0.95 µm (a few layers of the pre-molar after exposure to AESP was improved). Finally, the sample was exposed to CAS again for around 2 minutes, and the surface roughness value was Ra ≈ 3.30 µm (±SD = 0.17 µm), indicating that the surface net was Ra ≈ 0.33 µm. Surprisingly, the net value between two different mediums (normal-CAS-AESP) or (CAS-AESP-CAS) were Ra ≈ 0.61 µm and Ra ≈ 0.62 µm, respectively. Besides, the remaining layer of the restoration zone was around Ra ≈ 0.01 µm after treatment for two minutes with CAS, as shown in Figure 7, with a 95% level of confidence.
3.2. FTIR Data Analysis
The IR spectrum of polysaccharide isolated from Salvadora persica “Miswak” is presented in Figure 8. Peaks from 900 to 1400 are due to the fingerprint areas for carbohydrates, the absorption peaks at 1500 cm−1 and 1600 cm−1 represents the acetyl group.
Figure 6. Effect of different medium on human dental enamel at different test conditions (normal, AESP and CAS).
Figure 7. Effect of different medium on human dental enamel at different test conditions (normal, CAS, AESP and CAS).
Figure 8. IR of polysaccharide isolated from Salvadora persica “Miswak”.
The sharp band at 2900 cm−1 is characteristic of methyl C-H stretching associated with aromatic rings. The very obvious absorption at 3000 cm−1 is due to the stretching vibration and angular vibration of O-H linkage. The intensity of bands of about 3300 cm−1 in the IR spectrum was due to the hydroxyl stretching vibration of the polysaccharide, and as predicted these were broad, which make up the gross structure of carbohydrates. This is all in line with the polysaccharide structure that is neither starch nor cellulose, see [34] [46] [47] [48] [49] .
3.3. NMR Data Analysis
The 1H-NMR of polysaccharide isolated from Salvadora persica “Miswak” is illustrated in Figure 9. There is spectral evidence of the presence of polysaccharide. The peaks in the anomeric region and more peaks in the region of 3.3 ppm and 4.9 ppm, are indicative of the presence of polysaccharide in the extraction. This is an encouraging result and is indeed consistent with the results obtained in [49] [50] [51] .
3.4. UV Data Analysis
The UV-vis spectra showed that the polysaccharide had an absorption peak at 190 nm, which is the UV characteristic for the polysaccharide, which is in agreement with the data published in [34] . Figure 10 shows the UV-vis spectra of polysaccharide isolated from Salvadora persica “Miswak”.
Figure 9. The 1H-NMR of polysaccharide isolated from Salvadora persica “Miswak”.
Figure 10. The UV-vis spectra of polysaccharide isolated from Salvadora persica “Miswak”.
3.5. SEM Data Analysis
The surface topography of all pre-molar samples was analyzed by Scanning Electronic Microscopy (SEM) after all samples were fully coated with gold (25 nm thickness in 60 seconds). Figure 11(a) shows the SEM image of the effects of a single application of CAS (pH = 3.3 and t = 120 seconds) on the dental enamel surface. Whereas, Figure 11(b) shows the SEM image of the enamel surface treated with AESP (t = 120 second). Besides, Figure 11(c) and Figure 11(d) show a close-up view of the CAS and AESP images, respectively. For all treated samples illustrated in Figure 11, surface flaws and defects such as voids or rough surfaces are observable. SEM images of treated enamel surfaces with CAS show severe erosion with voids and loss of material over the whole surface compared to the images of AESP treatment where the surfaces seem to be much smoother with very flat areas. This can be seen only for the sections treated with CAS (see Figure 8(a)), the characteristic of eroded HAP prism structure of enamel and periphery is visible on the micrograph. Thus, it can be assumed that this interaction causes
Figure 11. SEM micrograph of the sound human enamel surfaces with different condition (a, b) and close-up features (c, d).
the loss of HAP crystals at the surface and results in the erosion process. To demonstrate this effect of acid erosion on dental enamel, the etched enamel prisms core surfaces after the acid erosive treatment are illustrated in SEM images.
The AESP treated enamel surfaces seen in Figure 11(b) are fairly smooth compared to the enamel surface treated with CAS. Therefore, it is hypothesized that the polymers perhaps adsorb on the eroded enamel surface and form protective layers covering the enamel surface following AESP treatment. Therefore, when the tooth was treated with the AESP the morphology of the surface changed and the etch pattern characteristics for CAS treatment on the surface of enamel are not apparent after this process. It can thus be suggested that the protective layer, which absorbs directly onto the human enamel surface may be due to the presence of polysaccharide. To confirm the presence of polysaccharide, the polysaccharide was isolated from extraction and some tests were carried out to characterize this. The polysaccharide is a combination of a number of varied macromolecular substances, and the composition and yield of the polymer can differ according to the methods of isolation. The polysaccharide indicated negative Fehling’s reagent and iodine-potassium iodide reactions, showing that it did not contain reducing sugar and starch type polysaccharide. It is considered as soluble in water and practically insoluble in ethanol, acetone, and chloroform.
The SEM images illustrated distinctive etch morphologies that support the surface roughness data and interpretations of the current investigation. There is a strong correlation between the Ra value and the SEM morphology image for both CAS and AESP. For those CAS treated, the surface profile was strongly eroded which corresponds to the measured value of Ra. On the other hand, for those AESP treated, the surface profile was less affected. Thus, the surface roughness corresponds to the value of Ra.
In addition, the SEM images show some changes in the surface morphology of enamel such as the presence of erosion and porosity. This finding is an encouraging result, and is in agreement with the following publications [52] [53] [54] [55] .
4. Conclusion
The scientific results obtained from this study show that the AESP derived can significantly reduce surface roughness value, Ra, few sub-nano meter level of human dental enamel affected by citric acid. It was also illustrated for the first time, measured with high precision test-rig Taly-surf® (from Taylor Hobson, Inc.), that AESP protects the human dental enamel against the effects of citric acid solutions. The protection is mainly due to the “shielding” effect of the polymers, which is self-formed directly on the surface of the human enamel during treatment with AESP. Perhaps, the modification of acidic soft drinks with such natural polymers (polysaccharide) may decrease the erosive effect considerably. Further investigation is essential to characterize the external polymer layer formed on the enamel surface. An important outcome of the studies herein is the ability to assess the effectiveness of inhibitors on enamel dissolution that could be expanded to include many types of substrate. In conclusion and in brief, the new methodology that has been used in this paper has enormous potential in the field of micro-mechanisms and nano-technology.
Ethical Statement
There is no ethical issue regarding this paper.
Conflicts of Interest
The authors have no conflicts of interest.
Funding Source
None.
Cite this paper
Bawazeer, T.M., Alsoufi, M.S., Katowah, D. and Alharbi, W.S. (2016) Effect of Aqueous Extracts of Salvadora persica “Miswak” on the Acid Eroded Enamel Surface at Nano-Mechanical Scale. Materials Sciences and Applications, 7, 754-771. http://dx.doi.org/10.4236/msa.2016.711059
References
- 1. West, N.X. and Joiner, A. (2014) Enamel Mineral Loss. Journal of Dentistry, 42, S2-S11.
https:/doi.org/10.1016/S0300-5712(14)50002-4 - 2. Robinson, C., Shore, R.C., Brookes, S.J., Strafford, S., Wood, S.R. and Kirkham, J. (2000) The Chemistry of Enamel Caries. Critical Reviews of Oral Biology and Medicine, 11, 481- 495.
https:/doi.org/10.1177/10454411000110040601 - 3. Featherstone, J.D.B. (1999) Prevention and Reversal of Dental Caries: Role of Low Level Fluoride. Community Dentistry and Oral Epidemiology, 27, 31-40.
https:/doi.org/10.1111/j.1600-0528.1999.tb01989.x - 4. Robinson, C. (2009) Fluoride and the Caries Lesion: Interactions and Mechanism of Action. European Archives of Paediatric Dentistry, 10, 136-140.
https:/doi.org/10.1007/BF03262674 - 5. Darby, E.T. (1892) Dental Erosion and the Gouty Diathesis: Are They Usually Associated? Dental Cosmos, 34, 629-640.
- 6. Miller, W.D. (1907) Experiments and Observations on the Wasting of Tooth Tissue Variously Designated as Erosion, Abrasion, Chemical Abrasion, Denudation, Etc. Dental Cosmos, 49, 1-23.
- 7. Margolis, H.C., Zhang, Y.P., Lee, C.Y., Kent, R.L. and Moreno, E.C. (1999) Kinetics of Enamel Demineralization in Vitro. Journal of Dental Research, 78, 1326-1335.
https:/doi.org/10.1177/00220345990780070701 - 8. Mahoney, E.K. and Kilpatrick, N.M. (2003) Dental Erosion: Part 1. Aetiology and Prevalence of Dental Erosion. New Zealand Dental Journal, 99, 33-41.
- 9. Davis, R.E., Marshall, T.A., Qian, F., Warren, J.J. and Wefel, J.S. (2007) In Vitro Protection against Dental Erosion Afforded by Commercially Available, Calcium-Fortified 100 Percent Juices. Journal of the American Dental Association, 138, 1593-1598.
https:/doi.org/10.14219/jada.archive.2007.0109 - 10. Wongkhantee, S., Patanapiradej, V., Maneenut, C. and Tantbirojn, D. (2006) Effect of Acidic Food and Drinks on Surface Hardness of Enamel, Dentine, and Tooth-Coloured Filling Materials. Journal of Dentistry, 34, 214-220.
https:/doi.org/10.1016/j.jdent.2005.06.003 - 11. Rubinstein, E., Hauge, C., Sommer, P. and Mortensen, T. (1993) Esophageal and Gastric Potential Difference and PH in Healthy-Volunteers Following Intake of Coca-Cola, Red Wine, and Alcohol. Pharmacology & Toxicology, 72, 61-65.
https:/doi.org/10.1111/j.1600-0773.1993.tb01340.x - 12. Williams, C.G., Macpherson, J.V., Unwin, P.R. and Parkinson, C. (2008) Laser Scanning Confocal Microscopy Coupled with Hydraulic Permeability Measurements for Elucidating Fluid Flow across Porous Materials: Application to Human Dentine. Analytical Sciences, 24, 437-442.
https:/doi.org/10.2116/analsci.24.437 - 13. Amjad, Z., Koutsoukos, P.G. and Nancollas, G.H. (1981) The Remineralization of Fluoride- Treated Bovine Enamel Surfaces. Journal of Dental Research, 60, 450-450.
- 14. LeGeros, R.Z. (1990) Chemical and Crystallographic Events in the Caries Process. Journal of Dental Research, 69, No. 567.
https:/doi.org/10.1177/00220345900690s113 - 15. Hannig, C., Hamkens, A., Becker, K., Attin, R. and Attin, T. (2005) Erosive Effects of Different Acids on Bovine Enamel: Release of Calcium and Phosphate in Vitro. Archives of Oral Biology, 50, 541-552.
https:/doi.org/10.1016/j.archoralbio.2004.11.002 - 16. Sonju Clasen, A.B., Ogaard, B., Duschner, H., Ruben, J., Arends, J. and Sonju, T. (1997) Caries Development in Fluoridated and Non-Fluoridated Deciduous and Permanent Enamel in Situ Examined by Microradiography and Confocal Laser Scanning Microscopy. Advances in Dental Research, 11, 442-447.
https:/doi.org/10.1177/08959374970110041001 - 17. Duschner, H., Gotz, H. and Ogaard, B. (1997) Fluoride-Induced Precipitates on Enamel Surface and Subsurface Areas Visualised by Electron Microscopy and Confocal Laser Scanning Microscopy. European Journal of Oral Sciences, 105, 466-472.
https:/doi.org/10.1111/j.1600-0722.1997.tb00232.x - 18. Ten Cate, J.M. (1999) Current Concepts on the Theories of the Mechanism of Action of Fluoride. Acta Odontologica Scandinavica, 57, 325-329.
https:/doi.org/10.1080/000163599428562 - 19. Tanaka, M., Moreno, E.C. and Margolis, H.C. (1993) Effect of Fluoride Incorporation into Human Dental Enamel on Its Demineralization in Vitro. Archives of Oral Biology, 38, 863- 869.
https:/doi.org/10.1016/0003-9969(93)90095-4 - 20. Beyer, M., Reichert, J., Heurich, E., Jandt, K.D. and Sigusch, B.W. (2010) Pectin, Alginate and Gum Arabic Polymers Reduce Citric Acid Erosion Effects on Human Enamel. Dental Materials, 26, 831-839.
https:/doi.org/10.1016/j.dental.2010.04.008 - 21. Cheng, Z.J., Wang, X.M., Cui, F.Z., Ge, J. and Yan, J.X. (2009) The Enamel Softening and Loss during Early Erosion Studied by AFM, SEM and Nanoindentation. Biomedical Materials, 4, No. 1.
https:/doi.org/10.1088/1748-6041/4/1/015020 - 22. Attin, T., Becker, K., Hannig, C., Buchalla, W. and Hilgers, R. (2005) Method to Detect Minimal Amounts of Calcium Dissolved in Acidic Solutions. Caries Research, 39, 432-436.
https:/doi.org/10.1159/000086852 - 23. Wang, L.J., Tang, R.K., Bonstein, T., Orme, C.A., Bush, P.J. and Nancollas, G.H. (2005) A New Model for Nanoscale Enamel Dissolution. Journal of Physical Chemistry B, 109, 999- 1005.
https:/doi.org/10.1021/jp046451d - 24. Barbour, M.E., Parker, D.M., Allen, G.C. and Jandt, K.D. (2003) Enamel Dissolution in Citric Acid as a Function of Calcium and Phosphate Concentrations and Degree of Saturation with Respect to Hydroxyapatite. European Journal of Oral Sciences, 111, 428-433.
https:/doi.org/10.1034/j.1600-0722.2003.00059.x - 25. Kouidhi, B., Al Qurashi, Y.M.A. and Chaieb, K. (2015) Drug Resistance of Bacterial Dental Biofilm and the Potential Use of Natural Compounds as Alternative for Prevention and Treatment. Microbial Pathogenesis, 80, 39-49.
https:/doi.org/10.1016/j.micpath.2015.02.007 - 26. Halawany, H.S. (2012) A Review on Miswak (Salvadora persica) and Its Effect on Various Aspects of Oral Health. The Saudi Dental Journal, 24, 63-69.
https:/doi.org/10.1016/j.sdentj.2011.12.004 - 27. Amir Alireza, R.G., Afsaneh, R., Seied Hosein, M.S., Siamak, Y., Afshin, K., Zeinab, K., Mahvash, M.J. and Amir Reza, R. (2014) Inhibitory Activity of Salvadora persica Extracts against Oral Bacterial Strains Associated with Periodontitis: An In-Vitro Study. Journal of Oral Biology and Craniofacial Research, 4, 19-23.
https:/doi.org/10.1016/j.jobcr.2014.01.001 - 28. Paster, B.J., Boches, S.K., Galvin, J.L., Ericson, R.E., Lau, C.N., Levanos, V.A., Sahasrabudhe, A. and Dewhirst, F.E. (2001) Bacterial Diversity in Human Subgingival Plaque. Journal of Bacteriology, 183, 3770-3783.
https:/doi.org/10.1128/JB.183.12.3770-3783.2001 - 29. Almas, K. and Al-Bagieh, N. (1999) The Antimicrobial Effects of Bark and Pulp Extracts of Miswak, Salvadora persica. Biomedical Letters, 60, 71-75.
- 30. Hattab, F.N. (1997) Meswak: The Natural Toothbrush. The Journal of Clinical Dentistry, 8, 125-129.
- 31. Ahmad, H. and Rajagopal, K. (2014) Salvadora persica L. (Meswak) in Dental Hygiene. The Saudi Journal for Dental Research, 5, 130-134.
https:/doi.org/10.1016/j.sjdr.2014.02.002 - 32. Lafi, T.A. and Ababneh, H. (1995) The Effect of the Extract of the Miswak (Chewing Sticks) Used in Jordan and the Middle East on Oral Bacteria. International Dental Journal, 45, 218- 222.
- 33. Mohamed, S.A. and Khan, J.A. (2013) Antioxidant Capacity of Chewing Stick Miswak Salvadora persica. BMC Complementary and Alternative Medicine, 13, 1-6.
https:/doi.org/10.1186/1472-6882-13-40 - 34. Das Biswajit, D.S., Chandra, C.R., Jashabir, C. and Saumendu, D.R. (2014) Optimization and Characterization of Purified Polysaccharide from Terminalia Belarica Gum as Pharmaceutical Excipient. International Journal of Pharmaceutical Research & Allied Sciences, 3, 21-29.
- 35. Xu, H.-S., Wu, Y.-W., Xu, S.-F., Sun, H.-X., Chen, F.-Y. and Yao, L. (2009) Antitumor and Immunomodulatory Activity of Polysaccharides from the Roots of Actinidia eriantha. Journal of Ethnopharmacology, 125, 310-317.
https:/doi.org/10.1016/j.jep.2009.06.015 - 36. Dong, W.P., Sullivan, P.J. and Stout, K.J. (1992) Comprehensive Study of Parameters for Characterizing Three-Dimensional Surface Topography I: Some Inherent Properties of Parameter Variation. Wear, 159, 161-171.
https:/doi.org/10.1016/0043-1648(92)90299-N - 37. Dong, W.P., Sullivan, P.J. and Stout, K.J. (1993) Comprehensive Study of Parameters for Characterizing Three-Dimensional Surface Topography II: Statistical Properties of Parameter Variation. Wear, 167, 9-21.
https:/doi.org/10.1016/0043-1648(93)90050-V - 38. Dong, W.P., Sullivan, P.J. and Stout, K.J. (1994) Comprehensive Study of Parameters for Characterising Three-Dimensional Surface Topography: III: Parameters for Characterising Amplitude and Some Functional Properties. Wear, 178, 29-43.
https:/doi.org/10.1016/0043-1648(94)90127-9 - 39. Dong, W.P., Sullivan, P.J. and Stout, K.J. (1994) Comprehensive Study of Parameters for Characterising Three-Dimensional Surface Topography: IV: Parameters for Characterising Spatial and Hybrid Properties. Wear, 178, 45-60.
https:/doi.org/10.1016/0043-1648(94)90128-7 - 40. Thomas, T.R. (1981) Characterization of Surface Roughness. Precision Engineering, 3, 97- 104.
https:/doi.org/10.1016/0141-6359(81)90043-X - 41. Alsoufi, M.S. (2016) Tactile Perception of Passenger Vehicle Interior Polymer Surfaces: An Investigation Using Fingertip Blind Observations and Friction Properties. International Journal of Science and Research (IJSR), 5, 1447-1454.
- 42. Alsoufi, M.S., Suker, D.K., Alsabban, A.S. and Azam, S. (2016) Experimental Study of Surface Roughness and Micro-Hardness Obtained by Cutting Carbon Steel with Abrasive Water Jet and Laser Beam Technologies. American Journal of Mechanical Engineering, 4, 173- 181.
- 43. Alsoufi, M.S. and Bawazeer, T.M. (2015) Quantifying Assessment of Touch-Feel Perception: An Investigation Using Stylus Base Equipment and Self-Touch (Human Fingertip). Umm Al-Qura University: Journal of Engineering & Architecture, 1, 1-16.
- 44. Suker, D.K., Alsoufi, M.S., Alhusaini, M.M. and Azam, S.A. (2016) Studying the Effect of Cutting Conditions in Turning Process on Surface Roughness for Different Materials. World Journal of Research and Review (WJRR), 2, 16-21.
- 45. Alsoufi, M.S. and Bawazeer, T.M. (2015) The Effect of Aggressive Biological Materials on a Painted Automotive Body Surface Roughness. American Journal of Nano Research and Applications, 3, 17-26.
- 46. Leopold, L.F., Leopold, N., Diehl, H.-A. and Socaciu, C. (2011) Quantification of Carbohydrates in Fruit Juices Using FTIR Spectroscopy and Multivariate Analysis. Spectroscopy, 26, 93-104.
https:/doi.org/10.1155/2011/285890 - 47. Nejatzadeh-Barandozi, F. and Enferadi, S.T. (2012) FT-IR Study of the Polysaccharides Isolated from the Skin Juice, Gel Juice, and Flower of Aloe vera Tissues Affected by Fertilizer Treatment. Organic and Medicinal Chemistry Letters, 2, 1-9.
https:/doi.org/10.1186/2191-2858-2-33 - 48. Martins Emejea, C.I., Byrnb, S., Fortunakc, J. and Olobayo Kunlea, S.O. (2011) Extraction and Physicochemical Characterization of a New Polysaccharide Obtained from the Fresh Fruits of Abelmoschus esculentus. Iranian Journal of Pharmaceutical Research, 10, 237-246.
- 49. Ding, X., Hou, Y.L., Zhu, Y.X., Wang, P.P., Fu, L., Zhu, H.Q., Zhang, N., Qin, H., Qu, W., Wang, F. and Hou, W.R. (2015) Structure Elucidation, Anticancer and Antioxidant Activities of a Novel Polysaccharide from Gomphus clavatus Gray. Oncology Reports, 33, 3162- 3170.
- 50. León de Pinto, G., Martínez, M. and Sanabria, L. (2001) Structural Features of the Polysaccharide Gum from Acacia glomerosa. Food Hydrocolloids, 15, 461-467.
https:/doi.org/10.1016/S0268-005X(01)00063-7 - 51. Kang, J., Cui, S.W., Phillips, G.O., Chen, J., Guo, Q. and Wang, Q. (2011) New Studies on Gum Ghatti (Anogeissus latifolia) Part III: Structure Characterization of a Globular Polysaccharide Fraction by 1D, 2D NMR Spectroscopy and Methylation Analysis. Food Hydrocolloids, 25, 1999-2007.
https:/doi.org/10.1016/j.foodhyd.2010.11.020 - 52. Bitter, N.C. (1998) A Scanning Electron Microscope Study of the Long-Term Effect of Bleaching Agents on the Enamel Surface in Vivo. General Dentistry, 46, 84-88.
- 53. Fu, B., Hoth-Hannig, W. and Hannig, M. (2007) Effects of Dental Bleaching on Micro- and Nano-Morphological Alterations of the Enamel Surface. American Journal of Dentistry, 20, 35-40.
- 54. Pinto, C.F., Oliveira, R.D., Cavalli, V. and Giannini, M. (2004) Peroxide Bleaching Agent Effects on Enamel Surface Microhardness, Roughness and Morphology. Brazilian Oral Research, 18, 306-311.
https:/doi.org/10.1590/S1806-83242004000400006 - 55. Lia Mondelli, R.F., Garrido Gabriel, T.R.C., Piola Rizzante, F.A., Magalhães, A.C., Soares Bombonatti, J.F. and Ishikiriama, S.K. (2015) Do Different Bleaching Protocols Affect the Enamel Microhardness? European Journal of Dentistry, 9, 25-30.
https:/doi.org/10.4103/1305-7456.149634













