Materials Sciences and Applications
Vol. 3 No. 12 (2012) , Article ID: 25408 , 5 pages DOI:10.4236/msa.2012.312125
Surface Modification of Hollow Glass Microspheres

College of Material Science and Engineering, Donghua University, Shanghai, China.
Email: nziokafredrick@yahoo.com
Received September 25th, 2012; revised October 18th, 2012; accepted November 24th, 2012
Keywords: Hollow Glass Microspheres; 3-Aminopropyltriethoxysilane; Hydroxylate
ABSTRACT
Hollow Glass Microspheres are high-strength, low-density additives made from water resistant and chemically-stable soda-lime-borosilicate glass. These hollow glass microspheres offer a variety of advantages over conventional irregularly-shaped mineral fillers or glass fiber. Their spherical shape helps reduce resin content in a variety of applications. They also create a ball bearing effect that can result in higher filler loading and improved flow. In this research, amine terminated hollow glass microspheres were prepared by adopting three different routes. The results were investigated using FT-IR and SEM to establish the formation of amine groups and observe the morphological structure of the modified HGMs. The results obtained were used to select a suitable less toxic and environmental friendly modification method based on the chemicals used.
1. Introduction
This research paper reports the results of a research on the modification of hollow glass microspheres carried out using three different modification methods to attach amine terminal groups to the surface of hollow glass microspheres. Among other uses, this modified hollow glass microspheres can be employed as a light filler material for polymeric composite to improve mechanical properties and weight reduction.
After a brief overview on general definitions and properties of these particulate filler materials, the paper focuses on the different modification techniques under study. The salient features of the modified hollow glass microspheres, observed under FT-IR and SEM studies, are summarized under results and discussions.
With the development of modern science and technology, there has been increasing need for the modification of existing polymeric materials to improve their properties in different application fields. Thermoplastic polyurethanes (TPU) are a part of the polyurethane group and are the first thermoplastic elastomers. They are a class of materials that combine the process ability of thermoplastics with rubberlike elastic properties. The main advantages of TPU are their excellent tensile strength, resistance against abrasion and wear, ozone and oils, and flexibility at low temperatures [1].
Hollow glass microspheres (HGMs) have a potential as inorganic filler for TPU composites of low density. HGMs consist of an outer stiff glass and an inner inert gas, which results in some unique properties, such as light weight and low thermal conductivity. Based on these properties, HGMs have been used in preparing composites with various polymers [2-5]. HGM composites, for example, syntactic foams, exhibit multifunctional properties, including high specific compressive strength [6-8], high thermal stability, low density, and low moisture absorption [2,9,10]. These properties make them more suitable for application in areas such as aeronautical and marine structures as compared to solid particle-filled composites and open-cell structured foams.
M. M. Ashton-Patton et al. [11] studied development of a low density polyethylene (LDPE)/hollow glass microsphere (HGM) composite. He found that, loading the LDPE with microspheres reduce its density and allows use of microspheres made from various compositions of glass. Since the bulk density of the hollow glass microspheres is much less than that of LDPE, the use of a composite reduces the weight of the material.
S. N. Patankar et al. [12] studied the performance of low density high strength sodium borosilicate HGM in high density polyethylene (HDPE) matrix. They found that it was crucial to ensure a strong interfacial bonding between the reinforcement and the matrix to enhance the properties of the resultant composites. They achieved this by use of compatibilizer, polyethylene-graft-maleic anhydride (PE-g-MAH), which showed possibility of enhancing the compatibility and interfacial adhesion between sodium borosilicate HGM and HDPE. They found that, in absence of compatibilizer the HGM /HDPE interface was very discrete with no sign of bonding between HGM and the matrix.
The reason for surface modification is to promote excellent interfacial adhesion between the matrix and HGMs. This can be achieved by treating the surface of the HGMs with proper organic materials. One of the best techniques is fabricating amine terminated HGMS (HGMNH2). For applications in thermoplastic polyurethane, these amine groups in HGM-NH2 lead to hydrogen bonding with urethane linkages in the TPU matrix resulting in better interfacial adhesion than TPU/HGM where the HGM are not modified [13].
Z.-G. An et al. [14] fabricated Glass/Ni-Fe-P ternary alloy core/shell composite hollow microspheres, in which process, a coupling procedure to make the surface of the HGMS catalytic for electroless plating was employed with 3-aminopropyltriethoxy silane as the coupling agent. He found out that, the coupling treatment could improve the uniformity of Ni-Fe-P deposits remarkably. The effects of the coupling agent were examined by SEM analysis. The SEM image of the product obtained without coupling treatment showed that, the alloy films were coarse and uneven and there existed large bare areas without any deposits. In contrast, products obtained via coupling-activating-electroless plating process had relatively smooth and uniform coatings. Furthermore, the coupling-activating pretreatment gave a much more steady and faster plating reaction.
G. Arslan et al. [15] studied Surface modification of glass beads with a self-assembled monolayer of 3-Aminopropyltriethoxysilane (APTES). The characterization of the self assembled monolayers was done by reaction between the amine terminal groups and thionylchloride of Rhodamine-B dye using fluorescence microscopy. They further analyzed the reaction steps using infrared spectroscopy (FT-IR).
Other than surface modification, good interface formation results from clean surface, and generation of a rough surface for interfacial interlocking. This is also assisted by good wetting of the substratum by the adhesive/cohesive materials, adequate flow and adaptation for intimate interaction, and acceptable curing when phase changes are required for final joint formation [16].
2. Experimental Details
2.1. Materials
HGM composed of sodium borosilicate (grade HGM im 30 k, particle size = 10 - 25 µm, density = 0.66 g/cm3 was obtained from 3M corporation China). 3-Aminopropyltriethoxysilane (APTES, Sinopharm Chemical Reagent co., Ltd, China) was used as a silane coupling agent to form an amine-terminated organic compound on the HGM. To hydroxylate (attach hydroxyl groups) the hollow glass microspheres, sodium hydroxide, piranha solution (Mixture of sulphuric acid and hydrogen peroxide), and hydrochloric acid were used in three different experiments as explained in the sections below. N-propylamine (Sinopharm Chemical Reagent co., Ltd, China) and ethanol were used as catalyst and solvent respectively.
2.2. Procedures
Amine terminated HGMs were fabricated for this work using three different techniques under the same conditions. The initial step in each of the three experiments was for attaching hydroxyl groups on the surface of the HGMs for successful subsequent reaction.
For hydrochloric acid (HCl) to attach hydroxyl groups on the surface of HGMs, 10 grams of the hollow glass microspheres were dispersed in a mixture of 20 ml concentrated HCl and 180 mL of deionized water for 1 hour at 90˚C, separated from this solution and washed twice with deionized water. The microspheres were dried at 70˚C for 8 hours and re-dispersed in 300 ml 3-aminopropyltriethoxy silane (KH550, Silane-coupling agent), n-propylamine and ethanol solution, stirred at 60˚C for 1 hour. The microspheres were filtered off, cleaned with ethanol and then distilled water. The final product was dried in a vacuum drying oven at 80˚C for 24 hours.
In a different study, the glass beads were treated for 1 hour in boiling piranha solution (3:1 concentrated H2SO4:H2O2. Note: Piranha is an extremely dangerous strong oxidant which cleans hydroxylate the surface of HGMs), the glass beads were removed from the cleaning solution, rinsed using distilled water and dried at 70˚C for 8 hours prior to self assembly of monolayers using silane coupling agent. The freshly dried glass beads were reacted with APTES in ethanol and n-propylamine as a catalyst for 1 hour at 60˚C with stirring in a reflux as previously described above. The HGMs were removed from the solution, followed by a sequence of 2 washes with ethanol and water to remove any physisorbed APTES. The samples obtained were dried for 24 hours at 80˚C in vacuum.
To use less toxic chemicals in fabricating amine terminated HGMs, 10 g of HGMS were added to NaOH (0.5 mol/l) aqeous solution (400 ml). This solution was stirred for 1 hour at 90˚C to create hydroxyl groups on the surface of HGMs and the solution was filtered off. The filtered HGMs were washed 3 times using distilled water and dried at 70˚C for 8 hours. The resulting HGMs were reacted with APTES in a solution of ethanol (200 ml) and n-propylamine (0.2 g) catalyst. This reaction was done in a reflux at 60˚C for 1 hour. The resulting sample was filtered off and washed three times with ethanol then distilled water and dried under vacuum at 80˚C for 24 hours.
As shown in previous studies by T. Paunikallio et al. [17] and J. W. Kim et al. [18] using solid state 29Si NMR spectroscopy, two ethoxy groups of the silane coupling agent can react with hydroxyl groups on the surface of HGMs or with another silane. This reaction can result in a structure as shown Figure 1.
The molecular structure of the HGM-NH2 was confirmed by Fourier transform infrared (FT-IR, Magna 750, Nicolet, Madison, WI) analyses. FT-IR measurements were carried out using KBr (Aldrich Chemicals, Milwaukee, WI) as a standard sample over a range of 600 - 4000 cm–1. Scanning electron microscopy (SEM, S- 37004, Hitachi, Tokyo, Japan) was employed to observe the morphologies of the HGMs before and after surface modification in order to establish which method gave a better surface structure.
3. Results and Discussions
3.1. Fourier Transform Infrared Spectroscopy
Figure 2 shows FT-IR spectrums of HGMs modified using the methods explained above. The FT-IR curve of HGMs shows a characteristic stretching peak between 1060 - 1002 cm–1 corresponding to Si-O-Si bond(around 1100 cm–1). The FT-IR spectrum of HGMs-NH2 (2(a) and 2(c)), shows stretching peaks at 3399 cm–1, 1640 cm–1 for Figure 2(a) and 3381 cm–1, 1638 cm–1 for Figure 2(c) which correspond to the primary amine in HGMs-NH2. However Figure 2(b) doesn’t exhibit this characteristic peaks and thus piranha solution is not favorable to hydroxylate the surface of HGMs for anchoring amine groups.
3.2. Scanning Electron Microscopy
The morphologies of the hollow glass microspheres were observed using SEM before and after surface modification. The SEM Microphotographs obtained are presented and discussed in the following section.
Figure 3 shows the SEM microphotographs at different magnification for unmodified HGMS. The results suggest a smooth clean surface, which would not promote interfacial adhesion between the filler material and polymer matrix. Comparing this with Figure 4, where hydrochloric acid in distillated water solution was used to hydroxylate the surface of the HGMs, it can be observed that, there was the formation of a dense layer due to the reaction between the OH and APTES thus formation of amine terminated HGMs.
In using piranha solution (Figure 5), the results were more or less the same as the ones for the original HGMs, this suggests a very low level of hydroxylation also confirmed by the FT-IR results discussed earlier. Piranha solution is extremely dangerous due to its high level of oxidation and should be avoided as the results obtained are equally very poor. Figure 6 shows the results obtained in application of sodium hydroxide to hydroxylate the surface of HGMs. The results are similar to those in Figure 4, thus the efficiency of NaOH in attaching hydroxyl groups on the surface of the HGMs is equally high although less than that resulting from HCL as shown by the density of the crosslink on the surface of the HGMS.
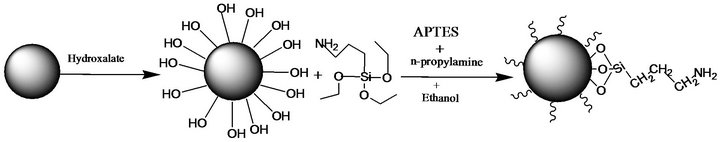
Figure 1. Reaction route for fabrication Amine terminated HGMs.
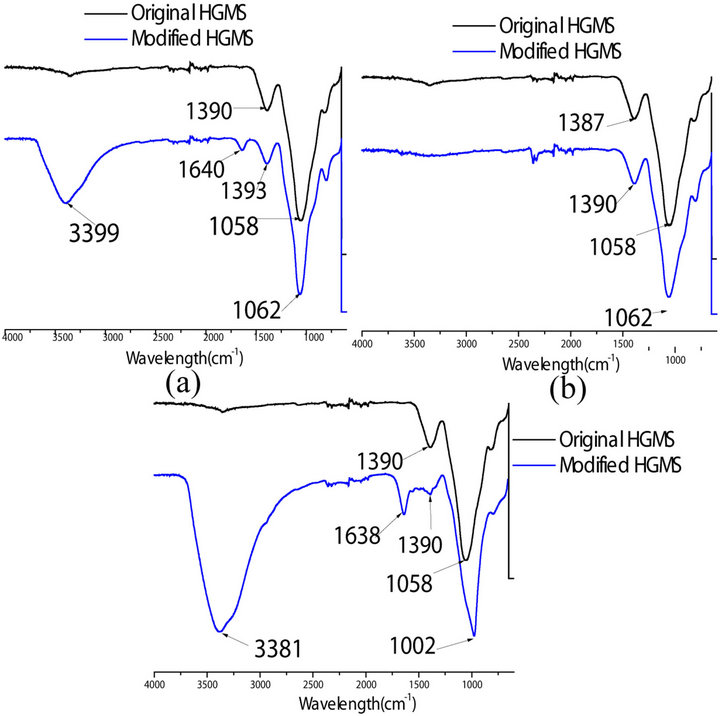
Figure 2. FTIR spectrum of obtained from modification of hollow glass microspheres: (a) Using HCl to attach OH groups on the surface of the HGMs; (b) Using Piranha solution to clean and hydroxylate the surface of HGMs and (c) Using NaOH to attach OH groups on the surface of HGMS.
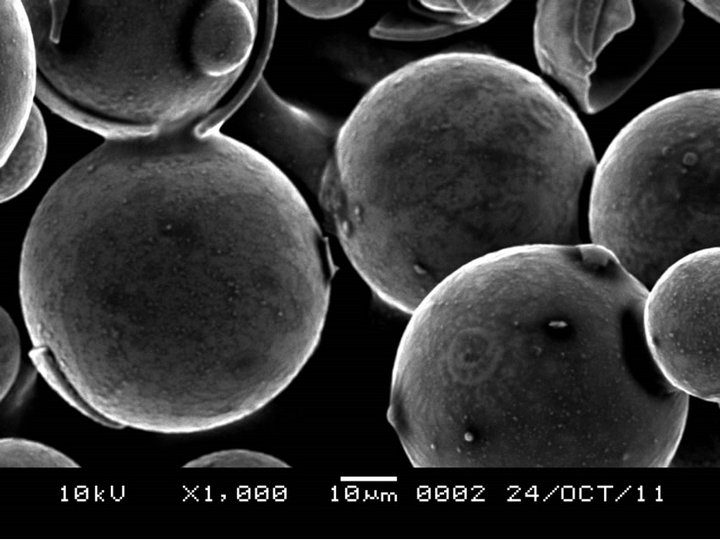
Figure 3. SEM image of HGMS before surface modification.

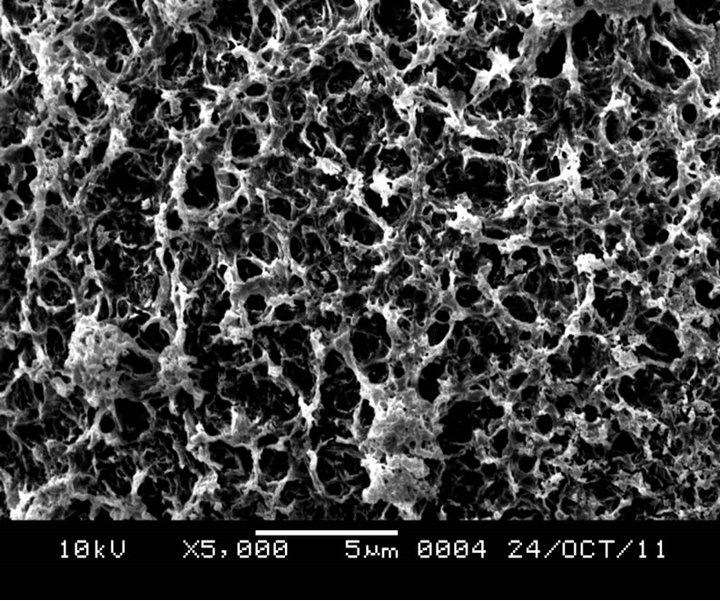
Figure 4. SEM image of HGMs after surface modification using HCl to hydroxylate its surface surface.

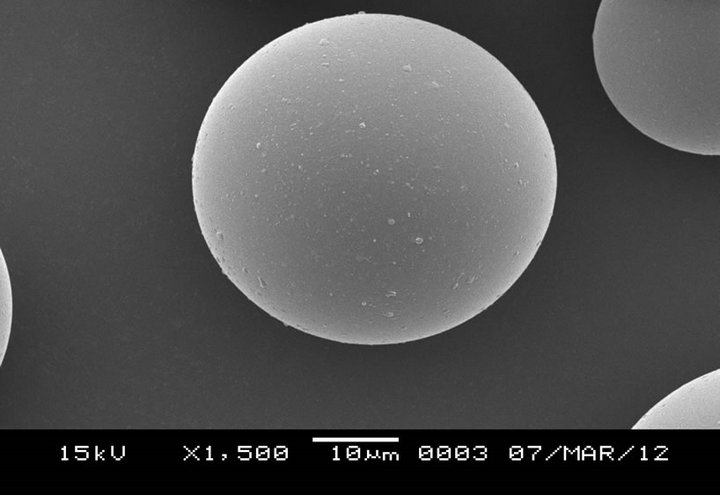

Figure 5. SEM image of HGMs after surface modification using Piranha to hydroxylate its surface.



Figure 6. SEM image of modified HGMs using NaOH to attach hydroxyl groups on its surface.
4. Conclusions
Surface modification is crucial in ensuring excellent interfacial adhesion between the polymeric matrix and HGMs. A strong interfacial bonding between the reinforcement and the matrix enhances the properties of the resultant composites by facilitating good load transfer between the matrix and the reinforcing filler. These are key requirements for the application of HGMs as fillers in polymers.
From the results obtained with sodium hydroxide which is much less toxic than hydrochloric and sulphuric acids. It can be concluded that, NaOH is a favorable agent in attaching hydroxyl groups on the surface of HGMs for the subsequent reaction with silane coupling agent in fabrication of amine terminated hollow glass microspheres. Optimal chemical ratios and percentages used in the experiments were established after several trials under different conditions.
REFERENCES
- A. Voda, et al., “Investigation of Soft Segments of Thermoplastic Polyurethane by NMR, Differential Scanning Calorimetry and Rebound Resilience,” Polymer Testing, Vol. 25, No. 2, 2006, pp. 203-213. doi:10.1016/j.polymertesting.2005.10.007
- E. M. Wouterson, et al., “Specific Properties and Fracture Toughness of Syntactic Foam: Effect of Foam Microstructures,” Composites Science and Technology, Vol. 65, No. 11-12, 2005, pp. 1840-1850. doi:10.1016/j.compscitech.2005.03.012
- G. Tagliavia, M. Porfiri and N. Gupta, “Vinyl Ester— Glass Hollow Particle Composites: Dynamic Mechanical Properties at High Inclusion Volume Fraction,” Journal of Composite Materials, Vol. 43, No. 5, 2009, pp. 561- 582. doi:10.1177/0021998308097683
- J. S. Huang and L. J. Gibson, “Elastic Moduli of a Composite of Hollow Spheres in a Matrix,” Journal of the Mechanics and Physics of Solids, Vol. 41, No. 1, 1993, pp. 55-75. doi:10.1016/0022-5096(93)90063-L
- G. Gladysz, et al., “Three-Phase Syntactic Foams: Structure-Property Relationships,” Journal of Materials Science, Vol. 41, No. 13, 2006, pp. 4085-4092. doi:10.1007/s10853-006-7646-9
- H. S. Kim and M. A. Khamis, “Fracture and Impact Behaviours of Hollow Micro-Sphere/Epoxy Resin Composites,” Composites Part A: Applied Science and Manufacturing, Vol. 32, No. 9, 2001, pp. 1311-1317. doi:10.1016/S1359-835X(01)00098-7
- H. S. Kim and P. Plubrai, “Manufacturing and Failure Mechanisms of Syntactic Foam under Compression,” Composites Part A: Applied Science and Manufacturing, Vol. 35, No. 9, 2004, pp. 1009-1015. doi:10.1016/j.compositesa.2004.03.013
- N. Gupta and R. Nagorny, “Tensile Properties of Glass Microballoon-Epoxy Resin Syntactic Foams,” Journal of Applied Polymer Science, Vol. 102, No. 2, 2006, pp. 1254- 1261. doi:10.1002/app.23548
- A. Calahorra, O. Gara and S. Kenig, “Thin Film Parylene Coating of Three-Phase Syntactic Foams,” Journal of Cellular Plastics, Vol. 23, No. 4, 1987, pp. 383-398. doi:10.1177/0021955X8702300402
- N. Bilow and P. M. Sawko, “Coating Processes for Increasing the Moisture Resistance of Polyurethane Baffle Material,” Journal of Cellular Plastics, Vol. 11, No. 4, 1975, pp. 207-212. doi:10.1177/0021955X7501100404
- M. M. Ashton-Patton, M. M. Hall and J. E. Shelby, “Formation of Low Density Polyethylene/Hollow Glass Microspheres Composites,” Journal of Non-Crystalline Solids, Vol. 352, No. 6-7, 2006, pp. 615-619. doi:10.1016/j.jnoncrysol.2005.11.058
- S. N. Patankar and Y. A. Kranov, “Hollow Glass Microsphere HDPE Composites for Low Energy Sustainability,” Materials Science and Engineering: A, Vol. 527, No. 6, 2010, pp. 1361-1366. doi:10.1016/j.msea.2009.10.019
- H. Im, S. C. Roh and K. K. Chang, “Fabrication of Novel Polyurethane Elastomer Composites Containing Hollow Glass Microspheres and Their Underwater Applications,” Industrial & Engineering Chemistry Research, Vol. 50, No. 12, 2011, pp. 7305-7312. doi:10.1021/ie102600q
- Z.-G. An, J.-J. Zhang and S.-L. Pan, “Fabrication of Glass/Ni-Fe-P Ternary Alloy Core/Shell Composite Hollow Microspheres through a Modified Electroless Plating Process,” Applied Surface Science, Vol. 255, No. 5, 2008, pp. 2219-2224. doi:10.1016/j.apsusc.2008.07.067
- G. Arslan, M. O. B. Gunduz, X. L. Zhang and M. Ersoz, “Surface Modifcation of Glass Beads with Aminosilane Monolayer,” Turkish Journal of Chemistry, Vol. 30, 2006, pp. 203-210.
- S. J. Marshall, et al., “A Review of Adhesion Science,” Dental Materials, Vol. 26, No. 2, 2010, pp. e11-e16. doi:10.1016/j.dental.2009.11.157
- T. Paunikallio, M. Suvanto and T. T. Pakkanen, “Viscose Fiber/Polyamide 12 Composites: Novel Gas-Phase Method for the Modification of Cellulose Fibers with an Aminosilane Coupling Agent,” Journal of Applied Polymer Science, Vol. 102, No. 5, 2006, pp. 4478-4483. doi:10.1002/app.24789
- J. W. Kim, L. U. Kim and C. K. Kim, “Size Control of Silica Nanoparticles and Their Surface Treatment for Fabrication of Dental Nanocomposites,” Biomacromolecules, Vol. 8, No. 1, 2006, pp. 215-222. doi:10.1021/bm060560b

