Journal of Modern Physics
Vol.05 No.18(2014), Article ID:52202,10 pages
10.4236/jmp.2014.518199
Electron Spin and Proton Spin in the Hydrogen and Hydrogen-Like Atomic Systems
Stanisław Olszewski
Institute of Physical Chemistry, Polish Academy of Sciences, Warsaw, Poland
Email: olsz@ichf.edu.pl
Copyright © 2014 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 16 October 2014; revised 12 November 2014; accepted 5 December 2014
ABSTRACT
The mechanical angular momentum and magnetic moment of the electron and proton spin have been calculated semiclassically with the aid of the uncertainty principle for energy and time. The spin effects of both kinds of the elementary particles can be expressed in terms of similar formulae. The quantization of the spin motion has been done on the basis of the old quantum theory. It gives a quantum number n = 1/2 as the index of the spin state acceptable for both the electron and proton particle. In effect of the spin existence the electron motion in the hydrogen atom can be represented as a drift motion accomplished in a combined electric and magnetic field. More than 18,000 spin oscillations accompany one drift circulation performed along the lowest orbit of the Bohr atom. The semiclassical theory developed in the paper has been applied to calculate the doublet separation of the experimentally well-examined D line entering the spectrum of the sodium atom. This separation is found to be much similar to that obtained according to the relativistic old quantum theory.
Keywords:
Spin Effect and Its Semiclassical Quantization, Electron and Proton Elementary Particles, Electron Drift in the Hydrogen Atom, Separation of the Doublet Spectral Lines

1. Introduction
In physics we look usually for general rules which govern the properties of a physical object, or a set of such objects. For example the Bohr atomic model gives a rather perfect description of several quantum parameters characterizing the hydrogen atom, but not the spin effects. The main items obtained from the Bohr description have been confirmed both on the experimental way, as well as on the quantum-mechanical footing which is considered to be a more flexible formalism than the old quantum theory. Simultaneously, however, quantum mechanics seemed to be enough complicated to give no transparent idea on the spin effects of the charged particles entering the atom. In consequence a treatment of the spin effects of the electron and proton was evi- dently absent in such simple model as the semiclassical Bohr approach to the hydrogen atom; see e.g. [1] . The aim of the present paper is to bridge this gap.
A general warning on the treatment of spin is that it should not be seeked as a result of the circulation effect of a particle about its own axis (see e.g. [2] ), and this view is shared also in the present approach. But instead of the motion about an axis which crosses the particle body, a charged particle may perform its spontaneous circulation in the magnetic field about an axis located outside the particle mass. The sense of such behaviour is―as we shall see―that in effect of the particle interaction with the magnetic field created by the particle motion, the particle energy becomes much lowered below the zero value of energy which can be assumed to be associated with the particle at rest.
In defining the position of the axis of the particle circulation in the magnetic field, the uncertainty relation for energy and time can be of use [3] - [5] . Beyond of time 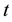 and energy
and energy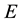 , the principle contains also a reference to the particle mass
, the principle contains also a reference to the particle mass 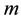 and the speed of light
and the speed of light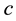 :
:
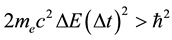 . (1)
. (1)
Evidently the rule (1) derived for electrons in [3] - [6] does apply to the particles which obey the Fermi statistics. But, for example, instead of electrons of the mass 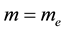 considered in [3] - [6] , we can have also the gas of the proton particles of the mass
considered in [3] - [6] , we can have also the gas of the proton particles of the mass 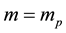 distributed in the field of a negative background which makes the gas electrically neutral. A reasoning of [3] - [6] repeated in the case of an ensemble of the proton particles gives the result
distributed in the field of a negative background which makes the gas electrically neutral. A reasoning of [3] - [6] repeated in the case of an ensemble of the proton particles gives the result
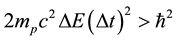 . (2)
. (2)
This makes (2) different from (1) solely by a replacement of 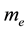 in (1) by
in (1) by 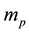 in (2). Certainly
in (2). Certainly 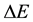 and
and 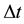 in (2) refer to the proton particle.
in (2) refer to the proton particle.
A consequence of the principle in (1) and (2) is a rule that two Fermi particles of the same kind cannot approach together to an arbitrarily small distance but they should be separated at least by the interval which―in view of (1)―is equal to [6]
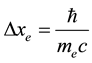 (3)
(3)
for electrons, but becomes equal to
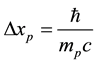 (4)
(4)
for the protons case; see e.g. [7] for the proton mass, spin angular momentum and spin magnetic moment.
The minimal distances (3) and (4) between particles represent respectively the Compton length of the electron and proton particle, on condition that the rationalized Planck constant 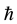 is replaced by the original Planck constant
is replaced by the original Planck constant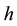 . The kind of the formulae given in (3) and (4) has been derived before in [8] - [10] ; see also [11] .
. The kind of the formulae given in (3) and (4) has been derived before in [8] - [10] ; see also [11] .
In Section 2 we apply (3) and (4) to define the positions of the axes of a spontaneous particle circulation giving, respectively, the electron and the proton spin. Before these motions take place we assume that the particle energy of the electron 


2. Spinning Process of the Electron and Proton
A general law of physics is that any particle tends to assume a possibly lowest level of energy. In case of a charged particle this can be attained in effect of the particle circulation about some axis along which the particle motion induces the presence of the magnetic field. This situation implies that the kinetic energy of the orbital motion is associated with a particle. The axis of the motion can be located outside the extension area of the particle mass. As a distance of the axis from the particle location (

The magnetic field 



where 






in the case of the electron particle, and

in the proton case.
The mechanical angular momenta of electron and proton become respectively

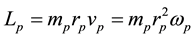
For the sake of simplicity the same size of charge 
The 



The 






Evidently the acceleration (13) vanishes when the particle velocity becomes a constant


and

With the aid of (3) and (4) we obtain from (14), (15) and (16):

and

on condition the absolute values of 



for the electron and

for the proton particle. In effect we have

Evidently the formulae obtained in (19)-(21) do not depend on the particle mass. But a mass dependent parameter becomes the magnetic moment 

(which is the Bohr magneton) and for proton

called also the theoretical nuclear magneton applied in considering the nuclear particles [7] . The ratio between (23) and (22) is defined by

which is not very far from the ratio obtained from the experimental data for the magnetic moment of electron and proton [7] . In many cases the experiments performed on the nuclear magnetic momenta 



The energy of a spinning particle in the magnetic field is respectively represented by

for an electron, and by

for a proton. Therefore the gain of energy in the magnetic field due to formation of the particle spin is large. This gain of energy is expensed to provide the kinetic energy to a spinning particle having its velocity close to
3. Magnetic Flux of a Spinning Particle, Conservation of Energy and Quantization of the Spin Motion
A parameter concerning spin which has its established experimental counterpart is the magnetic flux. Let us choose for an elementary planar area of that flux the circle

for electrons, and the circle

for protons. From (27), (28), as well as for the magnetic field strength taken respectively from (17) and (19), we obtain

and

respectively in the electron and proton case. An evident result is that

Therefore the flux extended over the elementary areas in (27) and (28) does not depend on the particle kind represented by the particle mass. Moreover, the flux calculated in (29) and (30) is equal to a constant quantum term observed experimentally since a long time in superconductors [14] .
The time derivative of the flux term is zero, so we have the fundamental relation of electrodynamics

Physically this means that a linear integral over 

Having the magnetic flux

the spin motion can be quantized according to a rule of the old quantum theory [12] [15] . It originates from a general rule given by Sommerfeld that momentum 



here 

By taking into account the first equation in (33) we obtain for (35) the relation

from which the spin quantum number becomes:

This is a well-known result confirmed experimentally by the measurements on the gyromagnetic ratio in ferromagnets [16] performed a time before the spin discovery [17] .
4. Drift Velocity of a Spinning Electron in the Electric Field of the Proton Nucleus
Till the present time no other field than 

where 





But it is easy to check from (17) and (38) that

is the fine-atomic-structure constant [2] [19] , so

The result in (41) is precisely the electron velocity on the lowest orbit of the Bohr atom [1] . Therefore a combined action of the spin magnetic force of the electron and electrostatic force acting between electron and the proton nucleus, gives the speed of electron equal to that possessed on the lowest quantum state in the hy- drogen atom. The spin action of the proton on the electron spin moment present on the orbit has been neglected.
In effect the velocity along the lowest orbit of the Bohr’s hydrogen atom can be considered as a consequence of a drift motion being a result of superposition of many spinning rotations along very small orbits having their radii equal to (7) and travelled with a speed equal to

is

whereas the travel time along the spin orbit calculated from (7) and (3) is equal to

In consequence the number of spinning circular motions which take place in course of the electron drift along the first Bohr orbit is equal to

This is a number independent of the mass

The mass 

5. Semiclassical Approach to the Doublet Separation in the Sodium Atom
Experimentally the doublet separations in the spectra of atoms ascribed to the presence of the electron spin are
Figure 1. A scheme representing the motion of a spinning electron along the shortest (lowest) circular Bohr orbit of the hydrogen atom. The orbit circle is represented by a dashed line, the separation distance between two circles enclosing the motion is twice the radius re given by the Formulae (3) and (7). For the number of the spin oscillations along the orbit see Formula (45).
well known since a long time; see e.g. [20] . The problem is with a theoretical approach to these values. In the author’s opinion no satisfactory agreement between experiment and theory has been reported in this domain. Our aim is to calculate a doublet separation for the sodium atom in the case of the electron transition between two levels being on the same atomic shell 

[20] . The level energies are approached by the quantum-defect method. We follow first the idea developed by the old quantum theory, next the formalism of the present paper is applied.
The considered electron of the sodium atom is the valence electron moving outside the atomic core. The electron energy is given by the formula

Here 




We apply

for term 

for term
A difference of energy (48)

calculated respectively from (50) and (51) gives the length of the spectroscopic line equal to

which is not far from the experimental length
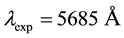
measured for the examined doublet [20] .
A proposal of calculating the doublet separation based on the relativistic old quantum theory applies the following formula for the change of energy connected with that separation [21] [22]

Here


For the effective nuclear charge equal to that applied before in (52), i.e.

moreover


we obtain

A semiclassical approach of the present paper is based on the interaction energy of two magnetic dipoles. One of them is provided by the angular momentum of the electron circulating about the atomic core, another dipole is due to the electron spin. For the sake of simplicity we assume that the magnetic momenta of the orbital motion and the spin motion are either parallel, or antiparallel, in their mutual arrangement. For both cases the absolute value of the coupling energy is the same. On the level of type 






and

We assume that momenta (60) and (61) are located at the nucleus. The absolute value of the spin magnetic moment (located at the electron position) is the same in both cases being equal to the Bohr magneton 
The electron in course of an excitation does not change its spin, but a separation distance between the magnetic momenta of the orbital motion and the spin momentum is changed. For state 

and for state

Therefore in case of a parallel arrangement of the orbital momentum and spin momentum the energy change of the momenta interaction due to the electron excitation becomes:

(the dot products of the vector joining the spin and orbital momenta with these momenta can be neglected be- cause the vector is assumed to be normal to the momenta).
The results obtained in (59) and (64) differ solely by a factor

A substitution of the values



in 

than the experimental doublet separation equal to 6 angstroms [20] . A substitution of 
6. A Look on the Dirac Theory and the Present Theory of the Electron Spin
A difference of both theoretical treatments of spin is evident. Dirac’s theory is essentially a relativistic quantum- mechanical approach to the electron motion; see e.g. [2] [19] [23] [24] . After the Hamiltonian of the problem is linearized, the four-dimensional matrices are applied as substitutions of the Hamiltonian operator. In the presence of an external electromagnetic field a simplification of the problem can be obtained by separating large and small components of the Dirac equation. In this way the spin-dependent interaction energy with the field can be calculated. The spin magnetic moment is coupled with the spin angular momentum by a constant term which is twice as large as in the classical electrodynamics. This implies that the spin quantum number should have the size of 1/2. Dirac’s electron particle considered in the field of the Coulomb potential gives rather complicated formulae for the electron wave functions which have no counterpart in the present semiclassical theory.
An advantage of the Dirac theory is that it gives an insight into antiparticles like positron, and presents an interval in the energy spectrum of particles and antiparticles of the size equal to
The theory of the present paper is much different than the Dirac approach. First the method is essentially of a semiclassical nature since no wave functions are considered. A basic reference to the quantum theory is the uncertainty principle applied to the changes of energy and time; see (1) and (2). The term

included in the formalism is obtained in effect of the derivation procedure of the principle; see [3] - [5] . A further analysis of the change 
Another advantage of the present theory is that both Fermi particles―electron and proton―can be considered on an equal footing because of the fermion character of these particles; see (1) and (2) which differ solely in their mass symbol. This allowed us to obtain an insight into the spin and magnetic moment of protons together with similar electron properties. The theoretical results obtained for both kinds of the particles are confirmed by the experimental data to a large degree.
Moreover, the Dirac theory assumes that certain magnetic field should be present in order to obtain a spinning electron particle, but the size of such field is not defined. In the present approach the size and source of the magnetic field acting on the particles are the results of the theory.
7. Summary
A semiclassical model of two spinning charged particles (electron and proton) has been proposed on the basis of a quantum uncertainty principle for energy and time and the classical electromagnetic theory. The main reason of a spontaneous formation of a spinning particle is a strong lowering of the particle energy in the magnetic field associated with the existence of the spin circulation.
The mechanical angular momentum connected with the spin is found to be the same for electron and proton, and the mass difference between the particles becomes sound only for the magnetic spin moment. This very fact is confirmed by experiment (see e.g. [7] ) which provides us with the ratio of the magnetic moments similar to that obtained by the present theory.
It could be noted that the mechanical moment of a proton equal to that of a spinning electron seemed to surprise many physicists since a long time; see e.g. [25] . This kind of feeling is stimulated by the fact that the magnetic moment of proton is about 
When a spinning electron meets the electrostatic field of a proton, it can be demonstrated that the resulted drift velocity of the electron becomes equal to the velocity of that particle on the lowest quantum level of the Bohr model of the hydrogen atom.
The effect of the spectral doublet separation has been also examined for the atomic sodium taken as an example. A semiclassical calculation of the present paper gives almost the same result as it is provided by the relativistic old quantum theory.
References
- Bohr, N. (1922) The Theory of Spectra and the Atomic Constitution. Cambridge University Press, Cambridge.
- Landau, L.D. and Lifshitz, E.M. (1972) Quantum Mechanics (in Russian). Izd. Nauka, Moscow.
- Olszewski, S. (2011) Journal of Modern Physics, 2, 1305. http://dx.doi.org/10.4236/jmp.2011.211161
- Olszewski, S. (2012) Journal of Modern Physics, 3, 217. http://dx.doi.org/10.4236/jmp.2012.33030
- Olszewski, S. (2012) Quantum Matter, 1, 127. http://dx.doi.org/10.1166/qm.2012.1010
- Olszewski, S. (2014) Journal of Modern Physics, 5, 1264. http://dx.doi.org/10.4236/jmp.2014.514127
- Tolansky, S. (1948) Hyperfine Structure in Line Spectra and Nuclear Spin. 2nd Edition, Methuen, London.
- Ruark, A.E. (1928) Proceedings of the National Academy of Sciences of the United States of America, 14, 322. http://dx.doi.org/10.1073/pnas.14.4.322
- Flint, H.E. (1928) Proceedings of the Royal Society A, London, 117, 630. http://dx.doi.org/10.1098/rspa.1928.0025
- Flint, H.E. and Richardson, O.W. (1928) Proceedings of the Royal Society A, London, 117, 637. http://dx.doi.org/10.1098/rspa.1928.0026
- Jammer, M. (1966) The Conceptual Development of Quantum Mechanics. McGraw-Hill, New York.
- Slater, J.C. (1967) Quantum Theory of Molecules and Solids. Vol. 3, McGraw-Hill, New York.
- Landau, L.D. and Lifshitz, E.M. (1969) Mechanics. Electrodynamics (in Russian). Izd. Nauka, Moscow.
- Kittel, C. (1987) Quantum Theory of Solids. 2nd Edition, Wiley, New York.
- Onsager, L. (1952) The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 43, 1006- 1008. http://dx.doi.org/10.1080/14786440908521019
- Beck, E. (1919) Annalen der Physik, 305, 109-148. http://dx.doi.org/10.1002/andp.19193651802
- Uhlenbeck, G.E. and Goudsmit, S.A. (1925) Die Naturwissenschaften, 13, 953-954. http://dx.doi.org/10.1007/BF01558878
- Matveev, A.N. (1964) Electrodynamics and the Theory of Relativity (in Russian). Izd. Wyzszaja Szkola, Moscow.
- Schiff, L.I. (1968) Quantum Mechanics. 3rd Edition, McGraw-Hill, New York.
- White, H.E. (1934) Introduction to Atomic Spectra. McGraw-Hill, New York.
- Millikan, R.A. and Bowen, I. (1924) Physical Review, 23, 1.
- Rubinowicz, A. (1933) Handbuch der Physik. In: Geiger, H. and Scheel, K., Eds., Vol. 24, Part 1, Springer, Berlin.
- Rose, M.E. (1961) Relativistic Electron Theory. Wiley, New York.
- Avery, J. (1976) Creation and Annihilation Operators. McGraw-Hill, New York.
- Kobos, A.M. (2013) Postĕpy Fizyki, 64, 86.





