Advances in Bioscience and Biotechnology
Vol. 4 No. 12 (2013) , Article ID: 41065 , 8 pages DOI:10.4236/abb.2013.412140
Hypothemycin production and its derivatives diversifying of Aigialus parvus BCC 5311 influenced by cultural condition
![]()
National Center for Genetic Engineering and Biotechnology, National Science and Technology Development Agency, Pathum Thani, Thailand
Email: *wai.pra@biotec.or.th
Copyright © 2013 Kanokarn Kocharin et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 16 September 2013; revised 31 October 2013; accepted 16 November 2013
Keywords: Hypothemycin; Aigialus parvus; Aigialomycin; Aigialospirol; Fractional Factorial Design; Derivative Diversifying
ABSTRACT
Many metabolites produced by various microorganisms have proven their usefulness in the area concerning human health. However, most of their diverse natural compound biosyntheses are hardly discovered. These metabolites might have specific or novel functions and these diverse active compounds can be achieved by biosynthesis, semi-biosynthesis, or chemical synthesis. A strategy to exploit the biosynthesis potential of a fungal strain is to use various culture conditions and to evaluate the chemical profiles of the culture extracts. The value of this approach was demonstrated with the fungal strain Aigialus parvus BCC 5311, producer of hypothemycin, aigialospirol, and aigialomycin A-D. The optimization of hypothemycin production and its derivative diversity by Aigialus parvus BCC 5311 was carried out using qualitative (general factorial design) and quantitative analysis (two-level fractional factorial design). Qualitative analysis revealed that soluble starch and yeast extract were shown to be the best carbon and nitrogen source respectively for the production of hypothemycin, aigialospirol and aigialomycin A-D. Quantitative analysis showed that the initial pH of culture medium is the most important factor that affects the production of hypothemycin and its derivatives (aigialospirol and aigialomycin A-D) production. Optimal medium composition used in a 5 L bioreactor generated a specific growth rate of A. parvus BCC 5311 of 0.0295 h−1, biomass yield of 1.6 g(gstarch−1, hypothemycin yield of 13.6 mg(gbiomass−1, and hypothemycin production rate of 0.6 mg(L−1(day−1. The maximum concentration of 58.0 mg(L−1 of hypothemycin was obtained at 120 h of culturing. Furthermore, the Aigialomycin A-D and Aigialospirol obtained were diversified towards various cultural conditions used. The high amount of hypothemycin produced and the diversity of derivatives obtained from this study should be useful for future mass production.
1. INTRODUCTION
Diversifying bioactive metabolites for specific or novel functions and for their usefulness in the area of human health are proved to be attractive by means of biosynthesis, semi-biosynthesis, or chemical synthesis [1,2]. Recently, the biosyntheses of such bioactive metabolites are the most appealing, using various microorganisms. Unfortunately, the discoveries of their novel functions are hardly successful and only a small fraction has been produced [3]. The isolated microorganisms from natural resources might have lost their ability to produce diverse compounds in synthetic medium [3,4], especially for those fungi isolated from insects. This problem concerning the loss of compounds may cause their absence in novel compound discovery or mislead to other compounds of higher bioactivity. Nevertheless, generating compound analogues may require strategies of biosynthesis incorporated with semi-biosynthesis [1]. Hypothemycin (Figure 1) is a 14-membered resorcylic acid lactone that exhibits anti-malarial and anti-fungal properties, in conjunction with having cytotoxicity against various murine and human cell lines. Hypothemycin derivatives structures, aigialomycin A-E, were described by Isaka et al. [5]. Hypothemycin and aigialomycin D exhibit in vitro antimalarial activity with IC50 value of
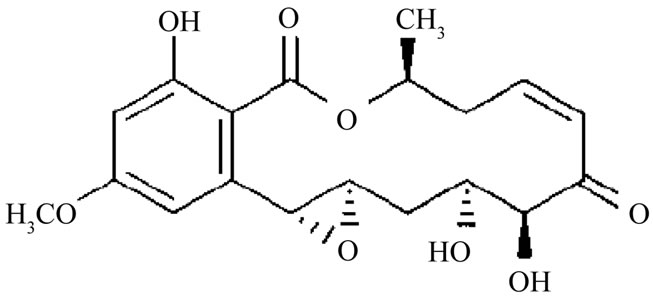
Figure 1. Chemical structure of hypothemycin.
2.2 and 6.6 µg×mL−1 respectively [5]. The former exhibits moderate antibiotic activity against the protozoan, Tetrahymena furgasoni, as well as the plant pathogenic fungi, Ustilago maydis and Botrytis allii [5,6]. This compound is also used as a kinase inhibitor, especially against kinases that contain a conserved cysteine residue in their ATP-binding site [7]. Such kinases play an important role in signal transduction pathways regulating cell proliferation, cell differentiation and apoptosis [8-12]. Hypothemycin is isolated from Hypomyces trichothecoides [6,7], H. subiculosus [13], Coriolus versicolor [14], and Aigialus parvus BCC 5311, a marine mangrove fungus [5]. This fungus produces hypothemycin as the major secondary metabolite, along with aigialone, aigialospirol, and five resorcylicmacrolides [5]. In view of the potential application of hypothemycin in the medicinal and agricultural areas, there is a need to enhance the yield of hypothemycin from A. parvus BCC 5311, likewise with diversifying other derivatives (Aigialomycin A-D, and Aigialospirol). The present study described the identification of the optimal carbon and nitrogen sources influencing hypothemycin and derivatives of Aigiaromycin A-D, Aigialosprirol, and Aigialone production in A. parvus BCC 5311 using a general factorial design and a two-level fractional factorial design. Significant levels of other nutritional factors were determined in order to reduce the cultivation time of this fastidious fungus that produces useful metabolites. These experimental designs were also applied as a tool that aids in achieving high diversity of hypothemycin and derivatives produced by the fungus. The nutritional factors were determined individually towards diversified compounds obtained.
2. MATERIALA AND METHODS
2.1. Fungal Strain and Cultivation
Aigialusparvus BCC 5311 was kindly provided by Prof. E. B. G. Jones, National Center for Genetic Engineering and Biotechnology (BIOTEC), National Science and Technology Development Agency, Thailand and has been deposited in BIOTEC Culture Collection.
2.2. Inoculum
The fungus was initially grown on potato dextrose agar (PDA) with sea water at 25˚C for 30 - 35 days. An agar block (1 cm3) containing mycelia was cut into small pieces, transferred to 25 mL of potato dextrose broth (PDB) and incubated for 5 - 7 days at 25˚C on a rotary shaker (200 rpm) (New Brunswick, NJ, USA).
2.3. Fermentation Condition
About 10% (v/v) of the seed culture were transferred into 50 mL of liquid medium (20 g×L−1 sugar, 1.5 g×L−1 nitrogen source, 3 g×L−1 KH2PO4, 2 g×L−1 MgSO4×7H2O, 0.2 g×L−1 CaCl2 and 1 mL×L−1 of trace element solution) and incubated for 14 days at 25˚C in a rotary shaker (200 rpm). The trace element solution contained (per L) 14.3 g of ZnSO4×H2O, 2.5 g of CuSO4×5H2O, 0.5 g of NiCl2×6H2O and 13.8 g of FeSO4×H2O. For scale up studies, fungus was then incubated with agitation (300 rpm) and aeration (0.5 vvm) for 18 days at 25˚C in a 5 L fermenter (Marubishi Co., Ltd., Nonthaburi, Thailand). The medium used (initial pH of 5.0) was a working 4 L volume containing (per L) 5 g of soluble starch, 2 g of yeast extract, 3 g of KH2PO4, 2 g of MgSO4×7H2O, 0.2 g of CaCl2, 3 mL of trace element solution and 3 mL of vitamin solution (Blackmores, NSW, Australia). Vitamin complex consisted of 75 mg of vitamin B1 (thiamine hydrochloride), 10 mg of Vitamin B2 (riboflavin), 50 mg of nicotinamide, 25 mg of calcium pantothenate, 10 mg of vitamin B6 (pyridoxine hydrochloride), 25 mcg of vitamin B12 (cyanocobalamin), 15 mcg of biotin, 500 mg of vitamin C (derived from ascorbic acid 260 mg and calcium ascorbate 290.5 mg), 10 mg of choline bitartrate, 10 mg of Inositol, 10 mg of zinc amino acid chelate (zinc 2 mg), 175 mg of calcium phosphate, and 75 mg of magnesium phosphate.
2.4. Experimental Design
A general factorial design was used to determine the optimal carbon and nitrogen sources. About 20 g×L−1 of 4 different carbon sources (glucose, lactose, soluble starch, and carboxy methyl-cellulose (CMC)) and 1.5 g×L−1 of 4 nitrogen sources ((NH4)2SO4, yeast extract, fish meal and casamino acid (CASA)) were used. When the optimal carbon and nitrogen sources had been obtained, a twolevel fractional factorial design of 2n−1 with resolution IV was applied to 6 selected factors influencing hypothemycin production, namely, soluble starch concentration, yeast extract concentration, amount of trace solution, amount of vitamin solution, sea salt amount and initial pH of medium. Experiments were conducted in duplicate and 5 center points were added in order to increase the power of the test. In the two-level fractional factorial design of 2n−1, factors influencing hypothemycin production were analyzed using analysis of variance (ANOVA) and Design Expert software (Version 7.0.b1.1Stat-Ease Inc., Minneapolis, USA) for experimental design, data analysis and linear model building. Optimal fermentation conditions for enhanced yield of hypothemycin were obtained by solving the regression equation and also by analyzing the interactions using Design Expert software.
2.5. Biomass Determination
Biomass content was determined by filtering whole medium through Whatman No. 1 filter paper. The filter cake was washed with distilled water and dried at 105˚C - 110˚C for 24 - 48 hours until a stable weight was achieved. The culture filtrate was subjected to metabolite and sugar analyses.
2.6. Hypothemycin, Aigiallomtcin A-D, and Aigialospirol Quantification
Fermentation medium was centrifuged at 10,000 g for 10 min and filtered through 0.22 μm filter paper. Hypothemycin and derivatives were detected using HPLC employing reverse phase NovaPak C18 column (Waters, MA, USA) and 30% acetonitrile as mobile phase at a flow rate of 1 mL×min−1, monitoring spectrophotometrically at 220 nm (Waters 996 Photodiode Array Detector). The concentration of hypothemycin was determined from a standard curve (250 to 440 mg×L−1). Standard samples of hypothemycin and aigialomycins were isolated from Aigialus parvus BCC 5311 as previously described [5,15].
2.7. Sugar Determination
Medium was centrifuged at 10,000 g for 10 min and supernatant filtered through 0.22 μm filter paper. Filtrate was subjected to HPLC analysis using Sugar-Pak column (Waters, MA, USA) at 90˚C, with water as mobile phase at a flow rate of 0.6 mL×min−1. Sugars were detected refractometrically (Waters 410 Differential Refractometer Detector, Millipore Corp., Milford, MA, USA). Residual soluble starch in culture broth was detected by the addition of iodine solution. A standard curve for starch determination was prepared by using soluble starch solution in the concentration range of 0.2 - 2.0 mg×L−1. The amount of starch-iodine complex was measured at 620 nm (Thermo, WI, USA).
3. RESULTS
3.1. Optimization of Hypothemycin Production
3.1.1. General Factorial Design (Qualitative Optimization)
A. parvus BCC 5311 was cultivated on different carbon and nitrogen sources. The highest biomass yield was obtained from soluble starch and CASA (9.7 ± 0.8 g×L−1) as carbon and nitrogen sources, respectively (Table 1). The highest hypothemycin and aigialomycin B and D productions were obtained on soluble starch and yeast extract (17.1 ± 0.9 and 4.6 ± 0.3 mg×L−1, respectively). The highest aigialomycin A and aigialospirol productions were obtained on glucose and ammonium sulfate (8.0 ± 0.1 and 4.4 ± 0.4 mg×L−1, respectively). When interactions between the carbon and nitrogen sources were considered, the highest hypothemycin production was obtained when soluble starch and yeast extract were used, and thus these two were selected as the sole carbon and nitrogen sources in he further experiment, two-level fractional factorial design (quantitative analysis), to obtain the optimal conditions of other nutritional requirements. Results obtained from general factorial design revealed that all derivatives were diversified towards their carbon and nitrogen sources used in the cultural medium.
3.1.2. Two-Level Fractional Factorial Design (Quantitative Optimization)
Six factors (trace element solution, vitamin, soluble starch, yeast extract, sea salt and initial pH) were selected and studied for their effects on the production of biomass, hypothemycin, aigialospirol, and aigialomycin A-D. Levels of each factor used in the two-level fractional factorial design are listed in Table 2, which were assigned as high (+1), low (−1), or center point level (0). The amount of factors used in this study was based on minimal growth requirement of the fungus. The highest biomass concentration of 14.6 ± 12.1 g×L−1 was obtained in the medium supplemented with 3 mL×L−1 vitamin, 20 g×L−1 soluble starch, 1 mL×L−1 trace element solution, 1 g×L−1 yeast extract, 2 g×L−1 sea salt, and initial pH of 5.0; the highest hypothemycin concentration of 60.6 ± 15.7 mg×L−1 was obtained in the medium supplemented with 3 mL×L−1 trace element solution, 20 g×L−1 soluble starch, 1 g×L−1 yeast extract, 1 mL×L−1 vitamin, 2 g×L−1 sea salt, and initial pH of 5.0; the highest biomass yield of 1.2 ± 0.1 g×gstarch−1 was obtained in the medium supplemented with 3 mL×L−1 vitamin, 8 g×L−1 sea salt, 1 mL×L−1 trace element solution, 5 g×L−1 soluble starch, 1 g×L−1 yeast extract, and initial pH of 7.0; and the highest yield of hypothemycin of 9.7 ± 2.9 mg×gstarch−1 was obtained in the medium supplemented with 3 mL×L−1 trace element solution, 3 mL×L−1 vitamin, 2 g×L−1 yeast extract, 5 g×L−1 soluble starch, 2 g×L−1 sea salt, and initial pH of 5.0 (Table 3). Table 4 shows all the derivatives produced by the fungus when two-level fractional factorial design was applied. Aigialomycin A-D and Aigialospirol were produced at different levels in different culture conditions. The highest production of Aigialomycin B and D and Aigialospirol were obtained on soluble starch (5 g×L−1), 14.9 ± 5.0 and 2.6 ± 0.3 mg×L−1, respectively. However, the highest amount of Aigialomycin A and C were pro-
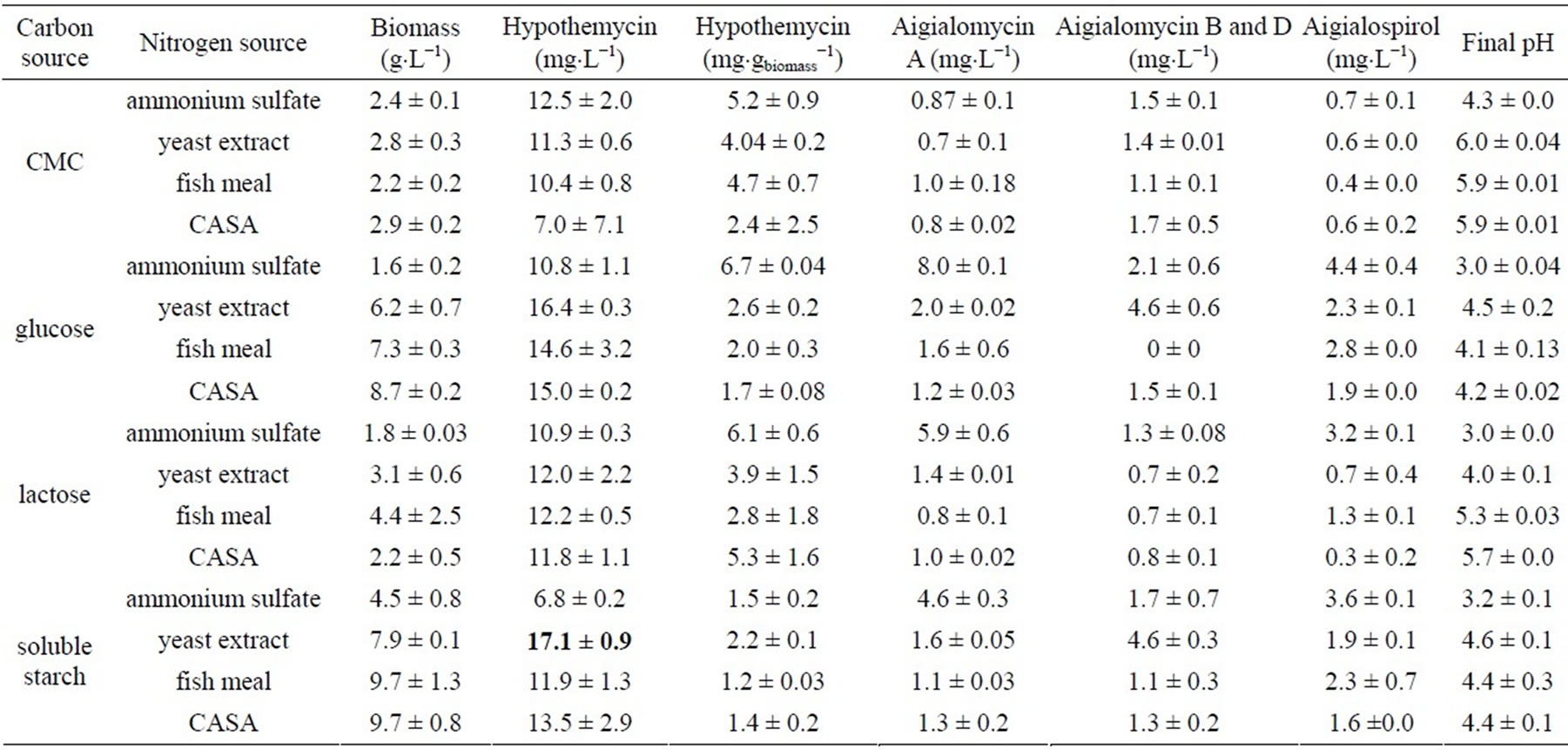
Table 1. General factorial design for biomass and hypothemycin production by A. parvus BCC 5311.
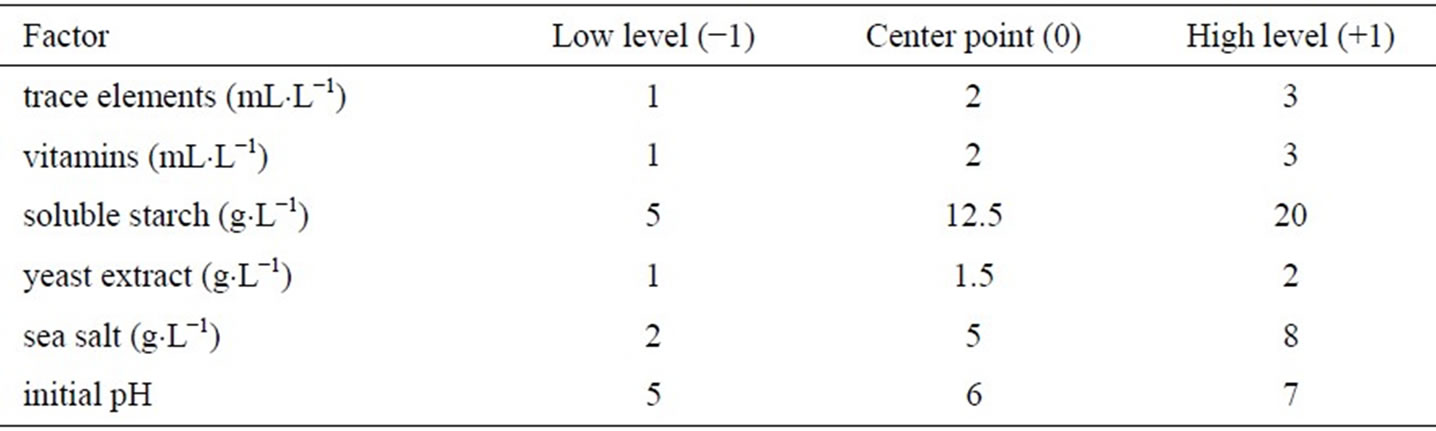
Table 2. Factors and levels used in a two-level fractional factorial design.
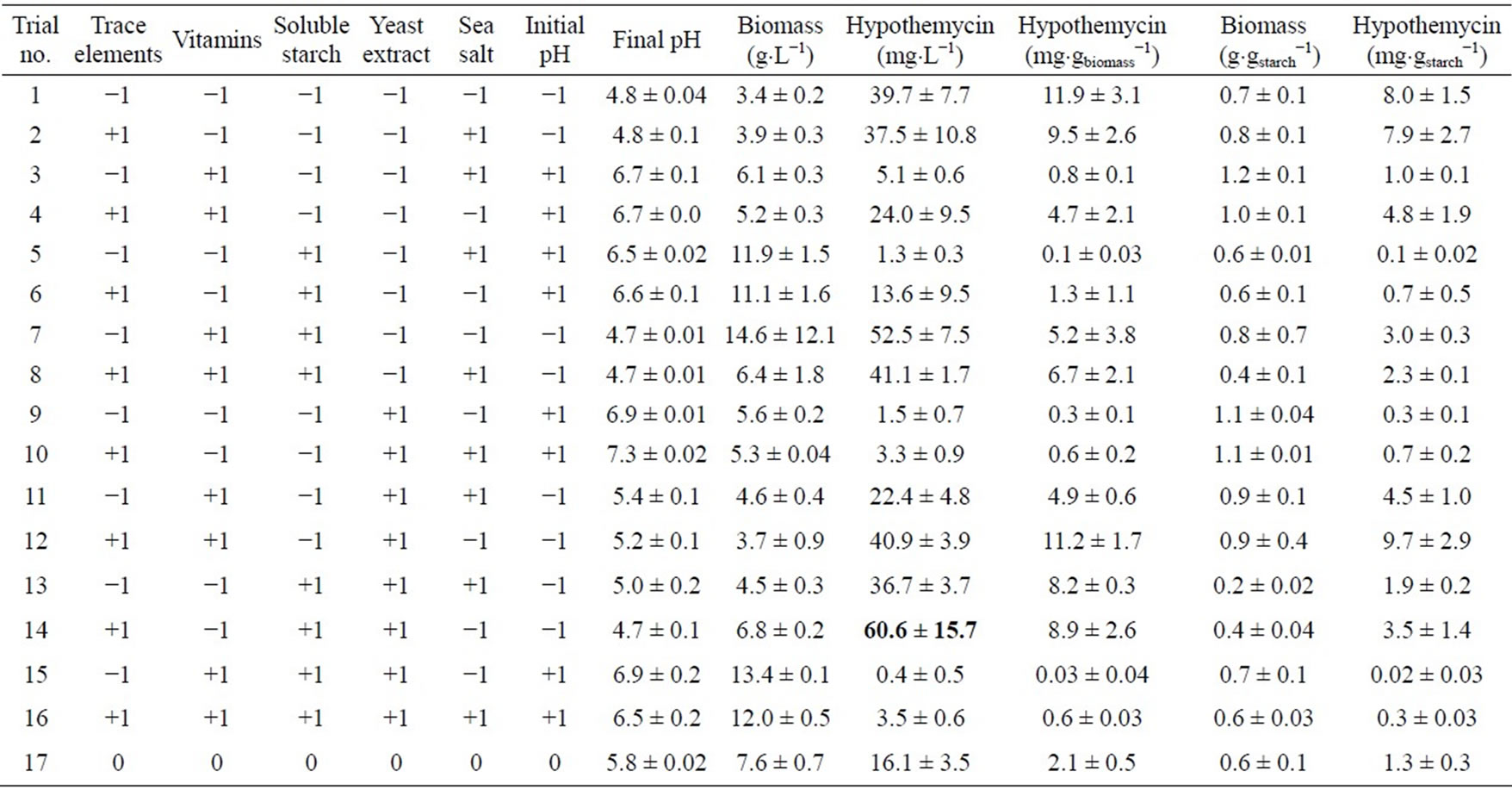
Table 3. Two-level fractional factorial design of biomass and hypothemycin production by A. parvus BCC 5311.
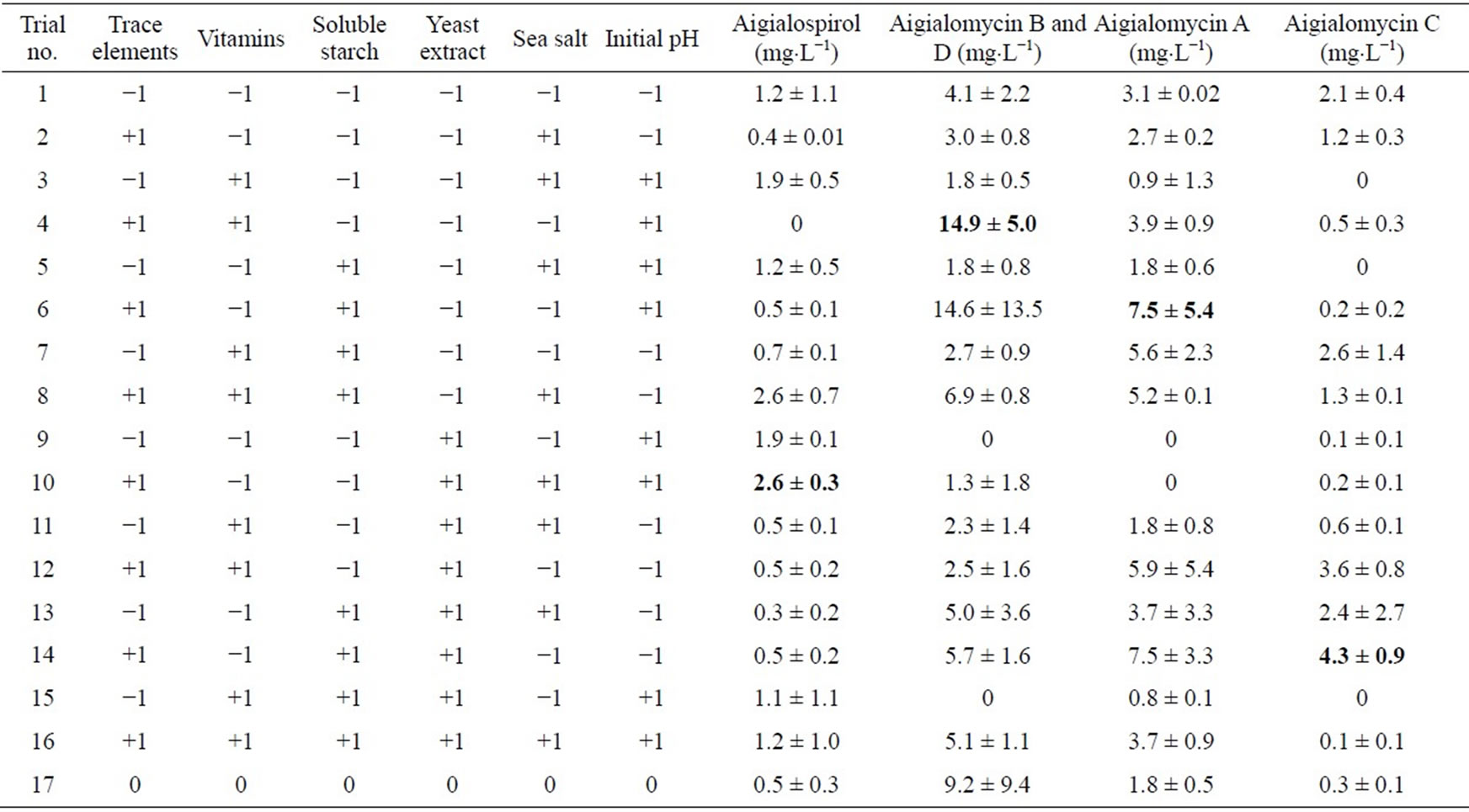
Table 4. Two-level fractional factorial design of hypothemycin derivative production by A. parvus BCC 5311.
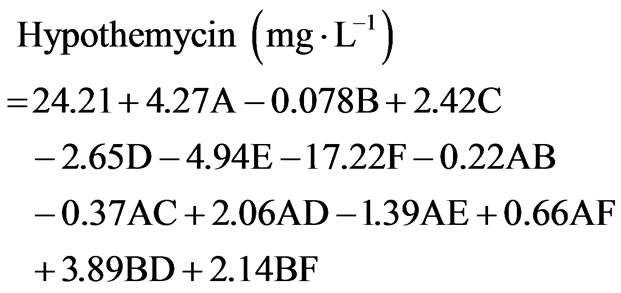
duced on soluble starch (20 g×L−1), 7.5 ± 5.4 and 4.3 ± 0.9 mg×L−1, respectively. This high concentration and diversity of derivatives were obtained by using two-level fractional factorial design. The variation of cultural conditions is very important to achieve a high production of hypothemycin, aigialomycin A-D, and aigiarospirol. By applying multiple regression analysis on the experimental data, the following equations were formulated to describe the biomass and hypothemycin production:
 (1)
(1)
 (2)
(2)
where A = trace element, B = vitamin, C = soluble starch, D = yeast extract, E = sea salt, F = initial pH.
Equation (1) indicates that soluble starch had constructive effects on biomass production; bearing the highest positive coefficient (2.68). Nevertheless, trace element supported high hypothemycin production, which can be seen from the positive coefficient of 4.27 (Equation (2)). The highest estimated coefficient value was used as a gauge for initial pH, indicating that this factor was most effective in the hypothemycin production, resulting in a larger amount of hypothemycin at initial pH of 5.0 compared to initial pH of 7.0 (Figure 2(b)). On the other hand, a high initial pH of 7.0 had positive effects on biomass production (Figure 2(a)). An interaction between yeast extracted concentration and vitamin level on hypothemycin production brought about a greater hypothemycin yield at low concentration of yeast extracted (1 g×L−1) and high level of vitamin (3 mL×L−1) (Figure 3). A culture medium supplemented with vitamins giving plentiful hypothemycin production, especially at low levels of nitrogen concentration, might be due to vitamins that promotes the growth of this fastidious fungus. Based on the highest hypothemycin production, high concentration, and diversity of aigialomycin A-D and aigialospirol obtained, the medium contained 5 g×L−1 soluble starch, 2 g×L−1 yeast extract, 3 mL×L−1 trace element solution, 3 mL×L−1 vitamin solution, and the initial pH of 5.0 was selected for scaling up in a 5 L bioreactor.
3.2. Hypothemycin and Derivative Production in Bioreactor
Media conditions obtained from the two-level fractional factorial design were used in the production of hypothemycin by A. parvus BCC 5311 in the 5 L bioreactor. Figure 4 shows the time profile of growth and hypothe-
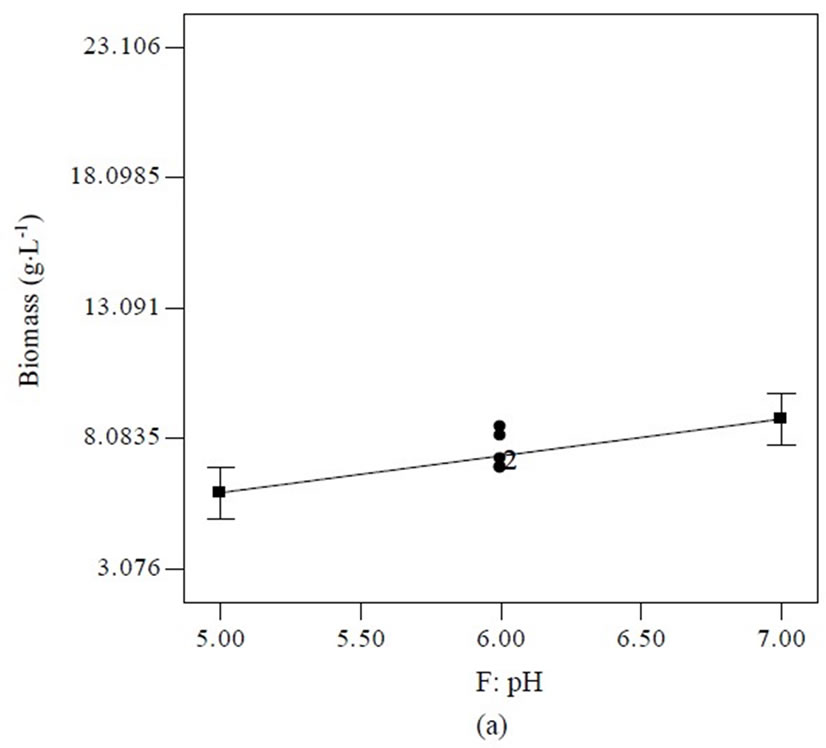
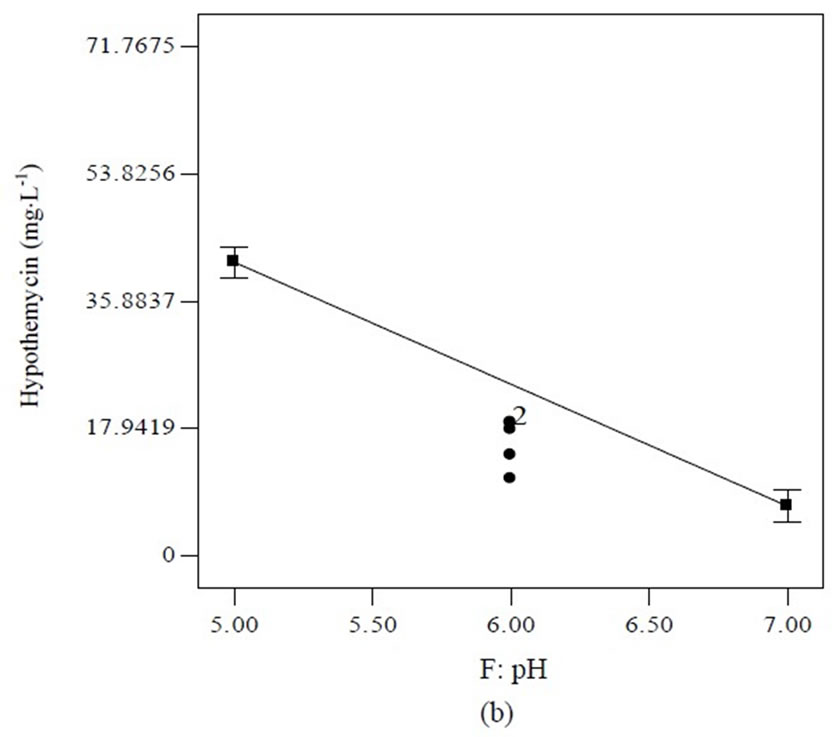
Figure 2. Effect of initial pH on biomass (a) and hypothemycin (b) production of A. parvus BCC 5311.
mycin production on media consisted of 5 g×L−1 soluble starch, 2 g×L−1 yeast extract, 3 mL×L−1 trace element solution, 3 ml×L−1 vitamin solution, with the initial pH of 5.0. The specific growth rate (m) of A. parvus BCC 5311 was 0.0295 h−1, biomass yield (YXS) of 1.6 g×gstarch−1, hypothemycin yield (YXP) of 13.6 mg×gbiomass−1, and hypothemycin production rate (qp) of 0.6 mg×L−1×day−1. The maximum concentration of hypothemycin (58.0 mg×L−1) was obtained at 120 h, and after which hypothemycin production decreased. On the other hand, hypothemycin derivatives increased, especially aigialomycin A, B and D increased (Figure 5). The optimal conditions allowed cultivation time and hypothemycin production to be reduced from 18 to 5 days. Thus, cultivation in an optimized medium gave rise to increased diversity of hypothemycin derivatives produced by A. parvus BCC 5311, which should be useful in the mass production of
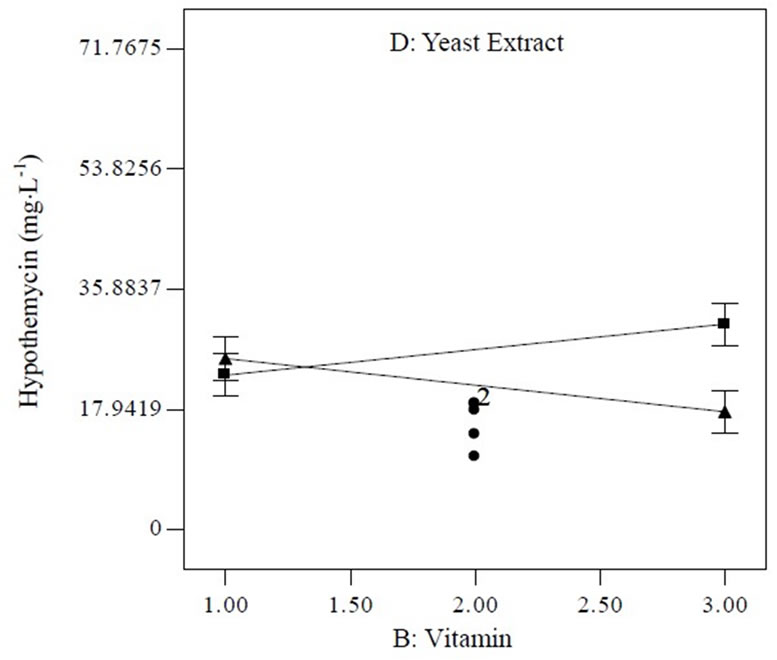
Figure 3. Interaction plot between vitamin and yeast extract concentration (dash line [D+], high level; solid line [D−], low level of yeast extract concentration) influencing hypothemycin production of A. parvus BCC 5311.
hypothemycin and its derivatives for future application in the medicinal and agricultural fields.
4. DISCUSSION
This study gave the strategies of nutritional factors triggering a variety of metabolites in the group of hypothemycin. The study shows the diversifying of compounds using different carbon and nitrogen sources. All metabolites increased significantly with optimized conditions attained and high levels of production were achieved. This proves that diversifying of metabolites can be obtained using nutritional requirements of the fungi. In addition, it is very important to vary nutritional factors that may minimize the loss of any useful compounds. Although the mechanism of hypothemycin synthesis and transition of its derivatives (aigialomycin A-D, Aigialone, and aigialospirol) are unknown, the regulation of polyketide synthase, which is responsible for the synthesis of hypothemycin and its derivatives, might be affected from cultural conditions such as pH, carbon and nitrogen sources, and temperature etc. [16]. This diversifying of hypothemycinderivatives is useful for the production of specific purposes of each derivative as bioactivecompound, this strategy is similar to the report of [2]. In order to have diversified metabolites, one should consider cultivation conditions, which influencesmicroroganisms growth followed by the efficiency in metabolite production. Some metabolite productions might increase significantly; some might be lost due to unknown nutritional factors [3]. This might risk missing some important bioactive compounds. Some metabolite productions may need special substrates to trigger their synthesis; especially microorganisms isolated from extreme
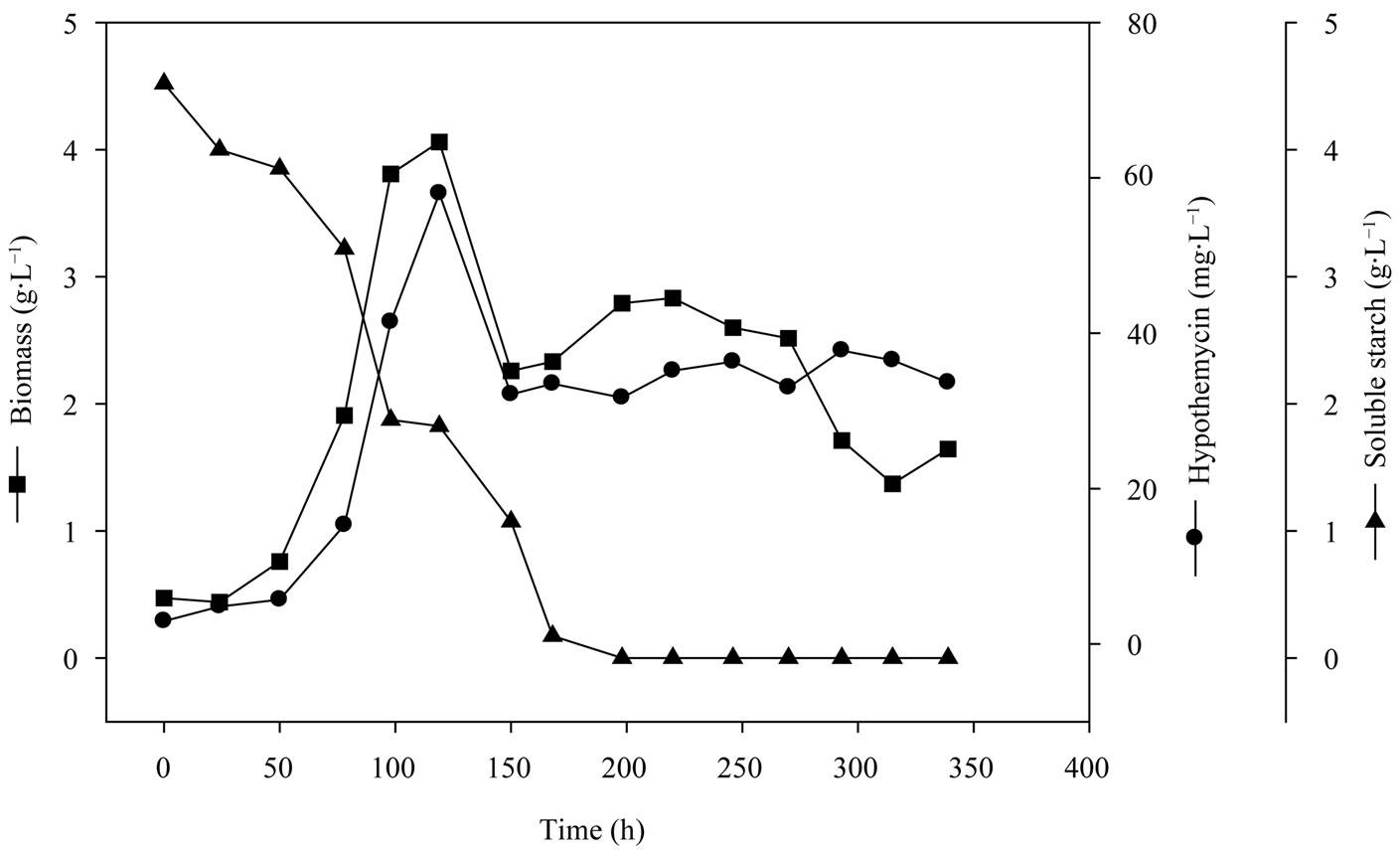
Figure 4. Time profile of growth and hypothemycin production of A. parvus BCC 5311 in 5 L bioreactor.
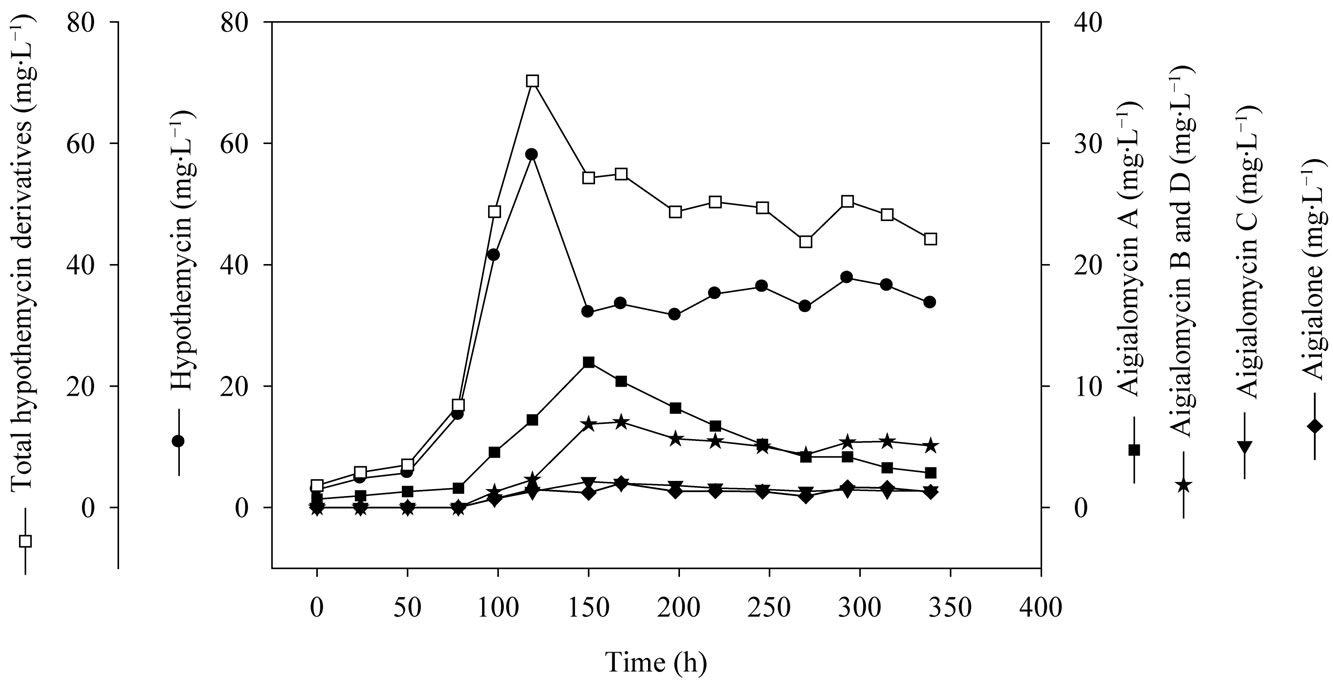
Figure 5. Time profile of hypothemycin and derivatives production of A. parvus BCC 5311 in a 5-L bioreactor.
environments f.ex. enthomopathogenic fungi, marine fungi, hot spring microorganisms [4]. There are many attempts to screen for bioactive compounds from natural resources and plenty known and new compounds were reported [2,3,17]. Some attempts provided metabolite profiling for drug discovery and some constructed large compound libraries to obtained new compound search from nature. All these are information provided for later molecular genetics studies [17].
5. CONCLUSION
By the use of experimental design, this helps to shorten the cultivation time of the fastidious A. parvus BCC 5311 along with maximized production of major derivatives of hypothemycin. Furthermore, other useful derivatives (Aigialomycin A-D, Aigialone, and Aigialospirol) produced by this fungus were diverse with other nutritional factors variation and their production was increased significantly. This high diversity of hypothemycin derivatives is important and factors, which triggerred these derivatives were analyzed to be initial pH of the culture and soluble starch was a good carbon source for production of biomass and hypothemycin derivatives by A. parvus BCC 5311.
REFERENCES
- Fu, X., Albermann, C., Zhang, C. and Thorson, J.S. (2005) Diversifying vancomycin via chemoenzymatic strategies. Organic Letters, 7, 1513-1515. http://dx.doi.org/10.1021/ol0501626
- Knight, V., Sanglier, J.J., Di, T.D., Braccili, S., Bonner, P., Waters, J., Hughes, D. and Zhang, L. (2003) Diversifying microbial natural products for drug discovery. Applied Microbiology and Biotechnology, 62, 446-458. http://dx.doi.org/10.1007/s00253-003-1381-9
- Umeno, D., Tobias, A.V. and Arnold, F.H. (2005) Diversifying carotenoid biosynthetic pathways by directed evolution. Microbiology and Molecular Biology Reviews, 69, 51-78. http://dx.doi.org/10.1128/MMBR.69.1.51-78.2005
- Petit, R.K. (2009) Mixed fermentation for natural product drug discovery. Applied Microbiology and Biotechnology, 9, 1916-1919.
- Isaka, M., Suyarnsestakorn, C. and Tanticharoen, M. (2002) Aigialomycin A-E, new resoculicmacrolides from the marine mangrove fungus Aigialus parvus. Journal of Organic Chemistry, 67, 1561-1566. http://dx.doi.org/10.1021/jo010930g
- Nair, M.S.R. and Carey, S.T. (1980) Metabolites of pyrenomycetes, XIV Structure and partial stereochemistry of the antibiotic macrolideshypothemycin and dihydrohypothemycin. Tetrahedron Letters, 21, 2011-2012. http://dx.doi.org/10.1016/S0040-4039(00)71472-9
- Nair, M.S.R., Carey, S.T. and James, J.C. (1981) Metabolites of pyrenomycetes, XIV: Structure and partial stereochemistry of the antibiotic macrolideshypothemycin and dihydrohypothemycin. Tetrahedron, 37, 2445- 2449. http://dx.doi.org/10.1016/S0040-4020(01)88900-6
- Schaeffer, H.J. and Weber, M.J. (1999) Mitogen-activated protein kinases: Specific messages from ubiquitous messengers. Molecular and Cellular Biology, 19, 2435- 2444.
- Robinson, M.J. and Cobb, M.H. (1997) Mitogen-activated protein kinase pathways. Current Opinion in Cell Biology, l9, 180-186. http://dx.doi.org/10.1016/S0955-0674(97)80061-0
- Camacho, R., Staruch, M.J., DaSilva, C., Koprak, S., Sewell, T., Salituro, G. and Dumont, F.J. (2000) Hypothemycin inhibits the proliferative response and modulates the production of cytokines during T Cell activation. Immunopharmacology, 44, 255-265. http://dx.doi.org/10.1016/S0162-3109(99)00085-5
- Kolch, W. (2000) Meaningful relationships: The regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochemical Journal, 351, 289-305. http://dx.doi.org/10.1042/0264-6021:3510289
- Schirmer, A., Kennedy, J., Murli, S., Reid, R. and Santi, D.V. (2006) Targeted covalent inactivation of protein kinases by resorcylic acid lactone polyketides. Proceedings of the National Academy of Sciences USA, 103, 4234-4239. http://dx.doi.org/10.1073/pnas.0600445103
- Wee, J.L., Sundermann, K., Licari, P. and Galazzo, J. (2006) Cytotoxic hypothemycin analogues from Hypomyces subiculosus. Journal of Natural Products, 69, 1456- 1459. http://dx.doi.org/10.1021/np060258o
- Agatsuma, T., Takahashi, A., Kabuto, C. and Nozoe, S. (1993) Revised structure and stereochemistry of hypothemycin. Chemical & Pharmaceutical Bulletin, 41, 373- 375. http://dx.doi.org/10.1248/cpb.41.373
- Vongvilai, P., Isaka, M., Kittakoop, P., Srikitikulchai, P., Kongsaeree, P. and Thebtaranonth, Y. (2004) Ketene acetal and spiroacetal constituents of the marine fungus Aigialus parvus BCC 5311. Journal of Natural Products, 67, 457-460. http://dx.doi.org/10.1021/np030344d
- Prathumpai, W., Kocharin, K., Phimmakong, K. and Wongsa, P. (2007) Effects of different carbon and nitrogen sources on naphthoquinone production of Cordyceps unilateralis BCC1869. Applied Biochemistry and Biotechnology, 136, 223-232. http://dx.doi.org/10.1007/BF03259856
- Larsen, T.O., Smedsgaard, J., Nielsen, K.F., Hansen, M.E. and Frisvad, J.C. (2005) Phenotypic taxonomy and metabolite profiling in microbial drug discovery. Natural Product Reports, 22, 672-695. http://dx.doi.org/10.1039/b404943h
NOTES
*Corresponding author.

