Advances in Bioscience and Biotechnology
Vol. 4 No. 2A (2013) , Article ID: 28444 , 9 pages DOI:10.4236/abb.2013.42A040
Occurrence of tetracycline residues and antimicrobial resistance in gram negative bacteria isolates from cattle farms in Spain
![]()
1Research Centre in Animal Health, CISA-INIA, Madrid, Spain
2National Centre for Environmental Health, Group of Water Pollution, Instituto de Salud Carlos III, Madrid, Spain
Email: torre@inia.es
Received 18 December 2012; revised 20 January 2013; accepted 28 January 2013
Keywords: Antimicrobial Resistance; Gram Negative Bacteria; Cattle Manure; Tetracycline
ABSTRACT
The usage of antibiotics in animal husbandry has dramatically increased the concentration of antibiotic residues and has promoted the development and abundance of antibiotic resistance in manure. When it is spread onto agricultural land, both residues of antibiotics and bacteria carrying antibiotic resistance genes may be introduced into the environment. In this research, we isolated resistant gram negative bacteria from manure produced in two dairy and two beef cattle farms, located in Madrid (Spain), to determine their resistance to seventeen representative antibiotics commonly used in veterinary therapy. A total of 63 isolates were used to assess the overall bacterial antimicrobial resistance on cattle manure samples. Predominant species were Escherichia coli and Comamonas testosteroni accounting for 25% and 19.6% of the total, respectively. The most found antimicrobial resistances in gram-negative bacteria were to tetracycline (66.7%), sulphamethoxazole (55.6%), ampicillin (52.4%), cephalothin (46.0%), chloramphenicol (44.4%), nalidixic acid (39.7%) and trimethroprimsulphamethoxazole (33.3%). The mean of resistance and the percentage of multi-resistant bacteria in beef farms were higher and statistically significant when compared to dairy farms which is opposite from the findings of the previous studies. The presence of three tetracyclines in all manure samples was also examined with stable recoveries (76% - 82%) and high sensitivity (limit of quantification 0.015 - 0.03 μg/kg). The concentrations of tetracyclines detected (<0.015 - 10 mg/kg) were consistent to the theoretical tetracycline levels in manure in Spain according to the excretion rate of these antibiotics and the values reported in scientific literature in other European countries.
1. INTRODUCTION
Many studies have highlighted the spread of veterinary antibiotic residues around the world. After their application to prevent and to treat animal diseases, most of the veterinary antibiotics, almost in 90% unchanged form, reach the environment through direct urination or defecation on the fields or through dispersion on agricultural lands as fertilizers [1]. Thus, it is not surprising that antibiotics are commonly found in animal manure [2], soils and waters [3] since more than two decades ago.
Despite the fact that the individual amounts of antibiotics and their metabolites introduced into the environment are likely low, continuous introduction can lead to cumulative high long-term concentrations. Among the adverse consequences, their environmental impact [4,5] and the potential for development of antimicrobial resistance through continuous exposure [6-8] are of particular concern.
The current information regarding the implications of veterinary antibiotics on the terrestrial environment and impacts on human health is still limited. Antibiotics have only received attention as environmental contaminants. Different countries have conducted a priorization of veterinary antibiotics [9-11] and have initiated monitoring programs for the characterization of antibiotic distribution in the environment. This resulted in an European legislation on veterinary antibiotics which requires ecotoxicological assessment of their environmental risks before they can be marketed [12]. On the contrary, the potential for development of antibiotic-resistant bacteria in the environment has only recently raised social concerns. Current European surveillance plans on antimicrobial resistance only include monitoring on volumes of sales of antibiotics and on levels of resistance in zoonotic and indicator bacteria from humans, animals and food. But research on antimicrobial resistance on swine manure [13-15], poultry litter [16], dairy manure [17] and farm surroundings [17-22] is only advancing during the past years. But the problem cannot be reliably quantified as there is insufficient surveillance data. Specifically in Spain, one of the major farm animals’ producers and veterinary antibiotics consumer in the EU (1102 Tons in 2009 [23]) there is only few available data about antibiotic residues and antimicrobial resistance in animal manure and farm environments. During recent years, the manure production of Spain has been estimated on 111 tonnes per year [24], from which cattle manure represents a 41% of the total (49.87 tonnes per year). Manure is usually employed as a fertilizer in peninsular Spain where most soils have low organic matter content.
This paper compares the percentage of antimicrobial resistance found in gram negative bacteria and their resistance patterns in cattle manures from two dairy and two beef farms located in Madrid, Spain. Tetracyclines were also quantified and compared to the theoretical mean values in animal manure according to the recent publish values of veterinary antibiotics sold in Spain. The gram negative bacteria were selected because it is an abundant and representative fecal flora in cattle manure, whereas tetracyclines were selected because they are commonly used in dairy and beef cattle and show a high persistence in the environment.
2. MATERIAL AND METHODS
2.1. Collection of Manure Samples
Manure samples were collected from two dairy cattle farms and two beef cattle farms located in the center of Spain, Community of Madrid, which housed more than 300 cattle per enclosed building. Samples were collected from each farm in the summer (July 2010) and winter (January 2011). Overall, eight manure samples were taken for analysis in this study.
Each manure sample was prepared by mixing an equal amount of 5 - 10 discrete subsample collected from manure heaps using an Auger sampling at a depth of 10 cm below the surface. All samples were placed into plastic containers (three containers per sample) and immediately stored on ice and transported to the laboratory. One of the containers was stored at 4˚C and processed within 24 hours for the microbiological assay. The second was employed in the physic-chemical characterization of the manure and the last one was stored at –20˚C for a maximum of one month for later chemical analysis.
2.2. Antibiotic Resistance of Gram-Negative Bacteria
The microbiological approach is summarized in Figure 1. Two main steps were realized: firstly antibiotic resistant gram-negative bacteria were isolated from cow manure samples, and secondly, bacterial identification and a complete antibiotic resistant profile of selected strains were attempted. The methodological scheme is detailed in subsections 2.2.1 and 2.2.2.
2.2.1. Isolation of Resistant Gram-Negative Bacteria
The cow manure samples were diluted in peptone water solution on a 1:10 (w/v) ratio and macerated with an automatic homogenizer. Resistant gram-negative bacteria were isolated following the standard CLSI protocol (National Committee for Clinical Laboratory Standards, CLSI, 2001). Aliquots (100 µl) were growth by triplicate on MacConkey agar (MAC, difco/becton Dickinson, Sparks, MD) amended with tetracycline (16 ug/ml) (MAC+TET), nalidixic acid (32 ug/ml) (MAC+NAL) and cloramphenicol (32 ug/ml) (MAC+C), representing members of tetracyclines, quinolones and phenicols respectively.
2.2.2. Identification of Resistant Gram-Negative Bacteria and Antimicrobial Susceptibility Testing
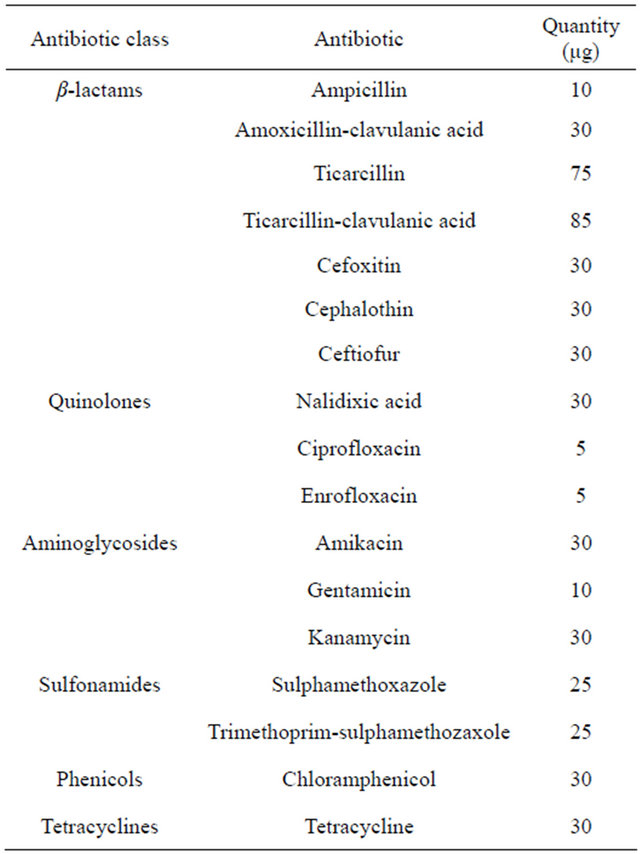
Five of the resistant bacterial isolated in the antibiotic plates were selected for bacterial identification and antimicrobial susceptibility testing. For their identification, bacteria were regrown in no-selective medium (Lab Lemco, Oxoid) and identified by bioquimic methodologies using the Rapid method (RapID ONE and RapID NF Plus, Oxoid). Antimicrobial susceptibility of strains was tested against 17 antimicrobials using the diffusion method recommended by the Clinical and Laboratory Standards Institute (CLSI). Selected antibiotics are detailed in Table 1. The test was performed using Mueller Hinton (MH) agar (Oxoid) plates containing the appropriate concentrations of antibiotics. Disk contents and
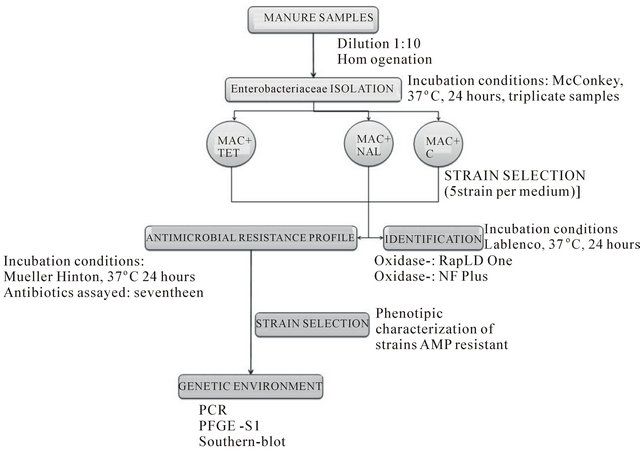
Figure 1. Flowchart outlining the microbiological approach for determining the antibiotic resistance in gram-negative bacteria.
growth inhibition zones were in accordance with criteria set by the standard protocol for bacteria isolated from animals.
2.3. Chemical Analysis of Tetracyclines
2.3.1. Samples Extraction and Clean-Up
The treatment of manure samples is shown in the flowchart in Figure 2. Before analyzing, samples were dried for 24 h at 100˚C and then crushed and passed through 2 mm sieve. Desiccated solid cow manure (1 g) was mixed to 30 ml methanol, 3 ml EDTA (0.5 mg/ml) and 7 ml buffer phosphate (0.14 M). The pH of the solution was adjusted below 3 using chlorhidric acid (1 M). The mixture was vortexed in automatic agitation for 1 hour (rpm) and then placed into ultrasonic bath for 10 min and centrifuged at approximately 3500 rpm for 10 min. The resultant supernatant was decanted into a glass bottle and the extraction was repeated for one more time. The two supernatants were combined, filtered through 0.45 μm membrane filter (PVDF Acrodis® LC, Pall, USA) and diluted with water (30 ml methanol: 200 ml water). The methanol/water solution was passed through a Water Oasis HLB cartridge (Waters, Milford MA, USA), previously conditioned with 20 ml methanol: 10 ml Milli-Q water, at a flow rate of 10 mL/min to concentrate and purify the diluted manure extract. In the last step, the cartridges were washed (water and methano l5%), dried under nitrogen flows, eluted (´2) from the cartridge with 3 ml methanol and concentrated to dryness under nitrogen flows. The residue was dissolved in 2 ml methanol for LC-MS analysis.
2.3.2. Analytical Quantification
The analytical method applied has been previously described by Jacobsen et al. [25] and Babić et al. [27]. The concentrations of selected antibiotics (Oxitetraciclina (OTC) (Aldrich 05875-10 g), Doxicilina (DX) (Sigma D9892) and Clortetraciclina (CTC) (Fluka 46133); 99% purity) were determined with an LC-MS system consisting of Varian Liquid Chromatography con bomb ProStar and automatic autosampler (Varian, Autosample 410, USA). A Zorbax SB C18 column (4.6 ´ 150 mm, 5 μm pore size, Agillent) thermostated at 25˚C - 30˚C was used for separation of antibiotics. Gradient elution was carried out with 0.1% formic acid in 99.9% acetonitrile (v/v) (mobile phase A) and 0.1% formic acid in 99.9% Milli-Q water (v/v) (mobile phased B). The injection volume was 10 μl and the flow rate was 0.2 ml/min. The separation of antibiotics was achieve with a gradient program described as follows: A:B was 1:99 at 0 min and maintained for 2 min, 8:92 at 2.1 min, 10:90 at 6 min, 60:40 at 15 min, 95:5 at 18 min and maintained 10 min, 1:99 at 28.1 min and maintained 12 min for column equilibration. All the four antibiotics could be eluted within 25 min.

Figure 2. Flowchart outlining the sample preparation and analytical detection procedures.
The triple-cuadruple mass spectrometer (MS) (Varian 1200 L) was equipped with an electrospray ionisation (ESI) source and operated in the positive ion mode. The ESI source values were: capillary voltage 4.5 kV, cone voltage 30 V, source temperature 120˚C, desolvation temperature 200˚C and pressure of the desolvation and cone gas 24 and 50 psi respectively. The precursor ion parent [M+H]+ (oxitetracycline: 461 m/z, doxitetracycline: 323 m/z, chlortetracycline: 479 m/z) was used for quantification in the selected ion recording (SIR) mode for each antibiotic, while the product ion daughter
Table 1. Antibiotic classes, compounds and quantities used for the antibiotic resistance study.
(oxitetracycline: 446 m/z, doxitetracycline: 157 m/z, chlortetracycline: 479 m/z) was used for confirmation purpose.
2.3.3. Method Validation
Analytical procedures were checked for linearity, accuracy, precision, limit of detection (LOD) and limit of quantification (LOQ). Concentrations in the samples were calculated by external standard method based on the peak area of the monitored product ion daughter. The repeatability of the LC/MS method was evaluated by injection of standard solutions (0.5, 5, 10 µg/l) into the system. The standard solutions were prepared from the corresponding stock solutions (2 mg/ml MeOH) by subsequent dilutions. Results indicate that good linearity was achieved (r2 > 0.8) for the calibration curves of all selected antibiotics in the studied concentration range.
Recoveries for the entire procedure were determined using samples taken from the farms. To determine the influence of different matrixes on LC/MS analyses, manure samples were fortified with chlortetracycline, oxytetracycline and doxicycline at three concentration levels (approximately 0.5, 5 and 10 mg/kg). Since these fortified samples contained target compounds, blanks (nonfortified manure samples) were also analysed as well as procedural and instrumental blanks to avoid laboratory contamination and analytical interferences. The fortified and non-fortified samples were extracted and analyzed using the entire procedure. For each matrix and concentration, recoveries were determined by triplicate samples comparing the obtained concentrations with initial fortified levels. Table 2 shows that the recoveries of the test antibiotics ranged from 76% to 82% which fell within the analytical recommended range [28]. The limit of detection (LOD) was estimated at a signal-to-noise ratio (S/N) of 3, while the limit of quantification (LOQ) value was estimated by using S/N of 5 (Table 2). These results confirmed that the LOD and LOQ achieved with the developed method are sufficient to determine the antibiotic concentrations in field manure samples.
2.4. Statistical Analysis.
The variations in the average number of resistance between different kinds of production (beef, dairy) were compared using an ANOVA test. A Pearson’s X2 test was used to measure the bivariate probability of association between kind of production (beef, dairy) and categories of resistance. Resistance was categorized into resistance (to 1 or 2 antibiotic classes) and multi-resistance (i.e. resistant to ≥3 antibiotic classes (Karczmarczyk et al., 2011). All statistical studies were conducted using SPSS v.15.0 software.
3. RESULTS AND DISCUSSION
A total of 63 isolates were used to assess the overall antimicrobial resistance on cattle manure samples. The predominant species found were Escherichia coli and Comamonas testosteroni accounting for 25% and 19.6% of the total, respectively. Other identified species were Proteus vulgaris (12.5%), Pseudomonas aeruginosa (10.7%), Serratia marcenscens (8.9%), Burkholderia cepacia (5.4%), Enterobacter cloacae (5.4%), Moraxella osloensis (3.6%), Providencia rettgeri (3.6%), Alcaligenes fecalis (3.6%) and Myroides odoratum (1.8%). The most found antimicrobial resistances in gram negative bacteria were to tetracycline (66.7%), sulphamethoxazole (55.6%), ampicillin (52.4%), cephalothin (46.0%), chloramphenicol (44.4%), nalidixic acid (39.7%) and trimethroprim-sulphamethoxazole (33.3%) (Table 3). This profile is similar to previous findings in Spanish cattle
Table 2. Recoveries, LOD (limit of detection) and LOQ (limit of quantification) of selected antibiotics.

Table 3. Comparison between dairy and beef cattle manures: Occurrence of resistance (in percentage) in Gram-negative bacteria.
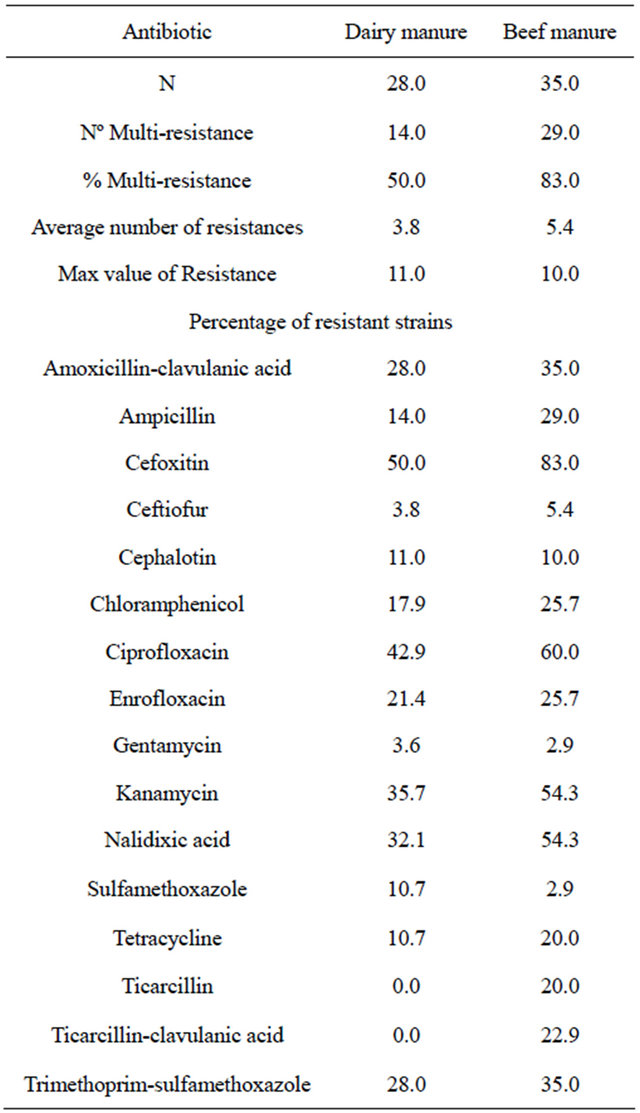
where the most common antimicrobial resistances were to sulfonamides, followed by tetracycline, aminoglycosides and ampicillin. It is also similar to the studies performed in European livestock, where the antibiotic resistance phenotype most commonly found was tetracycline, ampicillin, aminoglycosides and sulfonamides [29,30]. According to surveillance data, bacteria are more resistant to antibiotics that have been used for a long time in human and veterinary medicine, such as tetracyclines [31].
In order to establish differences between the different cattle production systems (dairy or beef), both mean of antimicrobial resistance and multi-resistance profiles were compared. The mean of resistance in beef farms (5.37) was higher and statistically significant (p = 0.018) compared to dairy farms (3.79). The percentage of multiresistant bacteria in beef farms (83%) was also higher and statistically significant (p = 0.04) compared to dairy farms (50%). This result is unexpected, given that in previous studies carried out in the United States, the authors found that the multi-resistance profile was higher in Escherichia coli isolates from dairy cattle than in those isolated from beef cattle [32]. The scarce observational studies available in scientific literature, usually find that cattle from conventional dairies harbor a higher prevalence of antimicrobial resistant enteric bacteria compared to beef cattle farms, given that dairies usually use more antibiotics than beef in the United States [33,34]. Due to the lack of studies in other geographical areas it is particularly interesting to obtain relevant data to compare both production systems in those respective areas.
The elevated resistance levels of tetracycline found in animal manure could be associated with its higher consumption compared with other veterinary antibiotics, since the use of antibiotics has been identified as an important risk factor for the development of antimicrobial resistance [1,35]. The sale pattern of veterinary antimicrobials in Europe has been recently analyzed by the European Medicines Agency (EMA). As per day, data have been provided by nine European countries. The contribution of tetracyclines to the total amounts sold was high, representing more than 40% of the total tonnes sold in 2009 [35]. The total sales by country during 2009, in tonnes of active ingredient, were 487.28 tonnes in France, 267.26 tonnes in the Netherlands, 176.89 tonnes in the United Kingdom, 38.35 tonnes in Denmark, 36.17 tonnes in the Czech Republic, 16.4 tonnes in Switzerland, 2.28 tonnes in Finland, 1.17 tonnes in Sweden and 0.22 tonnes in Norway [36]. In Spain, monitoring data from the use of antibiotics in animals indicated that tetracyclines were also the highest selling antibiotic family, accounting for 344.36 tonnes in 2009 (31% of the total) [23]. Therefore, Spain is the second European country in consumption of tetracycline after France. Taking into account that the estimated manure output from farm animals in Spain is nearly 110,840,522 tonnes of dry matter per year [24] and considering the percentage of excretion of each antibiotic group, data suggest that tetracyclines should be expected to represent the antibiotics with the highest theoretical concentration in animal manure in Spain (Table 4). This theoretical value (2.24 mg/kg manure) (Table 4) is very close located to the average concentration of tetracyclines (3.2 mg/kg manure) found in cattle manure in this study (Table 5).
Our results showed concentrations of 0.7 - 10 mg/kg for oxytetracycline and <0.02 - 0.5 mg/kg for chlortetracycline. In contrast, doxycycline was not identified in any of the samples analysed. These values are slightly higher than those reported in other European countries for cattle manure: 0.82 mg/kg for oxytetracycline and
Table 4. Theoretical tetracycline concentrations in animal manure (mg/kg) in Spain.

Table 5. Tetracycline concentrations in cattle manure (mg/kg).
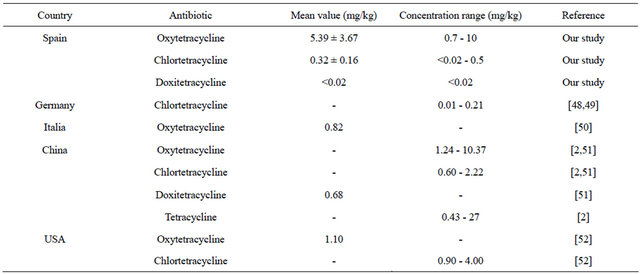
0.01 - 0.21 mg/kg for chlortetracycline (Table 5). Values up to 10.37 mg/kg and 4 mg/kg (Table 5) have been found in the United States and China for oxytetracycline and chlortetracycline respectively.
The long-term risk of antibiotic residues and antimicrobial resistances has yet to be well elucidated. Very few data exist on the environmental effects of antibiotics under field conditions because their ecotoxicity to microorganisms, plants and soil fauna has been mainly investigated under laboratory conditions. Field data on the emergence of antibiotic resistance after manure fertilization on soils are also scarce and are currently under discussion. Some authors suggest that continuous introduction of antibiotics into the environment even at low concentrations, can lead to cumulative high long-term concentrations because they often have longer environmental half lives and can also contribute to the development of antibiotic-resistant microbial populations [6]. On the contrary, other researchers hypothesize that resistance among soil bacteria can returned to preapplication levels within 6 months of manure application [37]. Ghosg and LaPara [38] recommend more stringent control of animal manure as a viable approach to slow the proliferation of antibiotic-resistant bacteria.
The need to investigate potential environmental problems caused by veterinary antibiotics has been highlighted by the veterinary pharmacovigilance programme of the European Medicines Agency (EMA). The most important action to achieve it is leaded by the European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) project to obtain detailed data at package level of sales, but this information should always be complemented with field data on antibiotic residues and antimicrobial resistances on manure, soils and waters. Recent studies suggest that a screening of antibiotics on the en-vironment is required to ensure agro-ecosystem safety and reduce the adverse effects of antibiotic residuals [39]. It should be supported by predictive models that help to identify the high risk areas of antibiotic contamination to focus the surveillance programmes [40]. Some efforts are also being made to improve the knowledge on the effectiveness of livestock management, e.g. size of the operation or general hygienic measures, to decrease the release of antibiotics to the environment. Also the efficiency of different manure treatments is recently beginning to be explored focusing on reduction or elimination of the problem at source [41]. All together, these data could help in setting management priorities.
4. CONCLUSSION
The mean of resistance and the percentage of multi-resistant bacteria in beef farms were higher and statistically significant compared to dairy farms which is opposite from the findings of the previous studies. The average concentration of tetracyclines in catlle manures were similar to the theoretical levels expected in animal manure in Spain and showed slightly higher values than those reported in other European countries.
5. ACKNOWLEDGEMENTS
This work was financially supported by RTA2010-00066-C02-01 and S3009-AGR-1489. The authors thank to the technicians Elena Neves and Verónica Nogal for their assistance.
REFERENCES
- Heuer, H., Schimitt, H. and Small, K. (2011) Antibiotic resistance gene spread due to manure application on agricultural fields. Current Opinion in Microbiology, 14, 236-243. doi:10.1016/j.mib.2011.04.009
- Li, Y., Zhang, X., Li, W., et al. (2012) The residues and environmental risks of multiple veterinary antibiotics in animal faeces. Environmental Monitoring and Assessment, 179, 137-153.
- Kim, K.R., Owens, G., Kwon, S.I., et al. (2011) Occurrence and environmental fate of veterinary antibiotics in the terrestrial environment. Water, Air, and Soil Pollution, 214, 163-174. doi:10.1007/s11270-010-0412-2
- Sarmah, A.K., Meyer, M.T. and Boxall, A.B.A. (2006) A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere, 65, 725-759. doi:10.1016/j.chemosphere.2006.03.026
- Wei, R., Feng, G., Huang, et al. (2011) Occurrence of veterinary antibiotics in animal wastewater around farms in Jiangsu Province, China. Chemosphere, 82, 1408-1414. doi:10.1016/j.chemosphere.2010.11.067
- Witte, W. (1998) Medical consequences of antibiotic use in agriculture. Science, 279, 996-997. doi:10.1126/science.279.5353.996
- Khachatourians, G.G. (1998) Agricultural use of antibiotics and the evolution and transfer of antibiotic-resistant bacteria. Canadian Medical Association Journal, 159, 1129-1136.
- Chee-Sanford, J.C., Mackie, R.I., Koike, S., et al. (2009) Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. Journal Environment Quality, 38, 1086-1108. doi:10.2134/jeq2008.0128
- Boxall, A.B.A., Fogg, L.A., Kay, P., et al. (2003). Priorisation of veterinary medicines in the UK environment. Toxicology Letters, 142, 207-218. doi:10.1016/S0378-4274(03)00067-5
- Capelton, A., Courage, C., Rumsby, P., et al. (2006) Prioritising veterinary medicines according to their potential indirect human exposure and toxicity profiles. Toxicology Letters, 163, 213-223. doi:10.1016/j.toxlet.2005.10.023
- Kima Y., Junga, J., Kimb, M., Parkc J., Boxall, A.B.A. and Choi, K. (2008) Prioritizing veterinary pharmaceuticals for aquatic environment in Korea. Environmental Toxicology and Pharmacology, 26, 167-176. doi:10.1016/j.etap.2008.03.006
- (1992) DIRECTIVE 92/18/EEC of 20 March 1992 modifying the annex to council directive 81/852/EEC on the approximation of theilaws of member states relating to analytical, pharmacotoxicological and clinical standards and protocols in respect of the testing of veterinary medicinal products. 1-23.
- Binh, C.T.T., Heuer, H., Kaupenjohann, M. and Smalla, K. (2008) Piggery manure used for soil fertilization is a reservoir for transferable antibiotic resistance plasmids. FEMS Microbiology Ecology, 66, 25-37. doi:10.1111/j.1574-6941.2008.00526.x
- Hölzel, C.S., Harms, K.S., Küchenhoff, H., et al. (2010) Phenotypic and genotypic bacterial antimicrobial resistance in liquid pig manure is variously associated with contents of tetracyclines and sulfonamides. Journal Applied Microbiology, 108, 1642-1656. doi:10.1111/j.1365-2672.2009.04570.x
- Pan, X., Qiang, Z., Ben, W. and Chen, M. (2011) Residual veterinary antibiotics in swine manure from concentrated animal feeding operations in Shandong Province, China. Chemosphere, 84, 695-700. doi:10.1016/j.chemosphere.2011.03.022
- Brooks, J.P., McLaughlin, M.R., Scheffler, B. and Miles, D.M. (2010) Microbial and antibiotic resistant constituents associated with biological aerosols and poultry litter within a commercial poultry house. Science of the Total Environment, 408, 4770-4777. doi:10.1016/j.scitotenv.2010.06.038
- Edrington, T.S., Fox, W.E., Callaway, T.R., et al. (2009) Pathogen prevalence and influence of composted dairy manure application on antimicrobial resistance profiles of commensally soil bacteria. Foodborne Pathogenic Diseases, 6, 217-224. doi:10.1089/fpd.2008.0184
- Peak, N., Knapp, C.W., Yang, R.K., et al. (2007) Abundance of six tetracycline resistance genes in wastewater lagoons at cattle feedlots with different antibiotic use strategies. Environmental Microbiology, 9, 143-151. doi:10.1111/j.1462-2920.2006.01123.x
- Graham, J.P., Price, L.B., Evans, S.L., Graczyk, T.K. and Silbergeld, E.K. (2009) Antibiotic resistant enterococci and staphylococci isolated from flies collected near confined poultry feeding operations. Science of the Total Environmen, 407, 2701-2710. doi:10.1016/j.scitotenv.2008.11.056
- Graves, A.K., Liwimbi, L., Israel, D.W., et al. (2011) Distribution of ten antibiotic resistance genes in E. coli isolates from swine manure, lagoon effluent and soil collected from lagoon waste application field. Folia Microbiology, 56, 131-137.
- Walczak, J.J. and Xu, S. (2011) Manure as a source of antibiotic-resistant Escherichia coli and enterococci: A case study of a Wisconsin, USA family dairy farm. Water, Air and Soil Pollution, 219, 579-589. doi:10.1007/s11270-010-0729-x
- Barkovskii, A.L. and Bridges, C. (2012) Persistence and profiles of tetracycline resistance genes in swine farms and impact of operational practices on their occurrence in farms vicinities. Water, Air and Soil Pollution, 223, 49- 62.
- Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (2011) Datos de ventas de antimicrobianos en España en el año 2009. Ministerio de Sanidad, Politica Social e Igualdad. http://www.aemps.gob.es/informa/notasInformativas/medicamentosVeterinarios/2011/docs/ventas-antimicrobianos_ Espana-2009.pdf
- Ministerio Agricultura, Pesca y Alimentación (MAPA) (2007) Libro blanco de subproductos de origen animal no destinados a consume humano (SANDACH). Capitulo VI.-Valorización de los SANCHA, Madrid, ISBN 978- 84-491-0774-0, 155.
- Karczmarczyk, M., Abbott, Y., Walsh, C., Leonard, N. and Fanning, S. (2011) Characterization of multidrug-resistant Escherichia coli isolates from animals presenting at a university veterinary hospital. Applied and Environmental Microbiology, 77, 7104-7112. doi:10.1128/AEM.00599-11
- Jacobsen, A.M. and Halling-Sørensen, B. (2006) Multicomponent analysis of tetracyclines, sulfonamides and tylosin in swine manure by liquid chromatography-tandem mass spectrometry. Analytical and Bioanalytical Chemistry, 384, 1164-1174. doi:10.1007/s00216-005-0261-9
- Babić, S., Pavlović, D.M., Ašperger, D., et al. (2010) Determination of multi-class pharmaceuticals in wastewater by liquid chromatography-tandem mass spectrometry (LCMS-MS). Analytical and Bioanalytical Chemisty, 398, 1185-1194. doi:10.1007/s00216-010-4004-1
- U.S. Environmental Protection Agency (2007) US.EPA method 1694: Pharmaceuticals and personal care products in water, soil, sediment, and biosolids by HPLC/ MS/MS. EPA-821-R-08-002, Washington.
- Bonnedahl, J., Drobni, M., Gauthier-Clerc, M., Hernandez, J., Granholm, S., et al. (2009) Dissemination of Escherichia coli with CTX-M type ESBL between humans and yellow-legged gulls in the south of France. PLoS ONE, 4, e5958. doi:10.1371/journal.pone.0005958
- Enne, V.I., Cassar, C., Sprigings, K., Woodward, M.J. and Bennett, P.M. (2008) A high prevalence of antimicrobial resistant Escherichia coli isolated from pigs and a low prevalence of antimicrobial resistant E. coli from cattle and sheep in Great Britain at slaughter. FEMS Microbiology Letters, 278, 193-199. doi:10.1111/j.1574-6968.2007.00991.x
- Trisuwan, K., Rukachaisirikul, V., Phongpaichit, S., Preedanon, S. and Sakayaroj, J. (2011) Modiolide and pyrone derivatives from the sea fan-derived fungus Curvularia sp. PSU-F22. Archives of Pharmacal Research, 34, 709-714.
- Berge, A.C., Hancock, D.D., Sischo, W.M. and Besser, T.E. (2010) Geographic, farm, and animal factors associated with multiple antimicrobial resistance in fecal Escherichia coli isolates from cattle in the western United States. Journal of the American Veterinary Medical Association, 236, 1338-1344. doi:10.2460/javma.236.12.1338
- Davis, M.A., Hancock, D.D., Besser, T.E., Daniels, J.B., Baker, K.N. and Call, D.R. (2007) Antimicrobial resistance in Salmonella enterica serovar Dublin isolates from beef and dairy sources. Veterinary Microbiology, 119, 221-230. doi:10.1016/j.vetmic.2006.08.028
- Call, D.R., Davis, M.A. and Sawant, A.A. (2008) Antimicrobial resistance in beef and dairy cattle production. Animal Health Research Reviews, 9, 159-167. doi:10.1017/S1466252308001515
- Grave, K., Greko, C., Kvaale, M.K., et al. (2012) Sales of veterinary antibacterial agents in nine European countries during 2005-09: Trends and patterns. Journal of Antimicrobial Chemotherapy, 67, 3001-3008. doi:10.1093/jac/dks298
- European Medicines Agency (E.M.A.) (2011) Trends in the sales of veterinary antimicrobial agents in nine European countries (2005-2009). EMA/238630/2011.
- Sengeløv, G., Halling-Sørensen, B. and Aarestrup, F.M. (2003) Susceptibility of Escherichia coli and Enterococcus faecium isolated from pigs and broiler chickens to tetracycline degradation products and distribution of tetracycline resistance determinants in E. coli from food animals. Veterinary Microbiology, 95, 91-101. doi:10.1016/S0378-1135(03)00123-8
- Ghosh, S. and LaPara, T.M. (2007) The effects of subtherapeutic antibiotic use in farm animals on the proliferation and persistence of antibiotic resistance among soil bacteria. The Multidiscilinary Journal of Microbial Ecology, 1, 191-203. doi:10.1038/ismej.2007.31
- OK, Y.S., Kim S.C., Kim, K.R., et al. (2011) Monitoring of selected veterinary antibiotics in environmental compartments near a composting facility in Gangwon Province, Korea. Environmental Monitoring Assessment, 174, 693-701.
- De la Torre, A., Iglesias, I., Carballo, C., Ramírez, P. and Muñoz, M.J. (2012) An approach for mapping the vulnerability of European Union soils to antibiotic contamination. Science of the Total Environment, 414, 672-679. doi:10.1016/j.scitotenv.2011.10.032
- Du, L. and Liu, W. (2012) Occurrence, fate, and ecotoxicity of antibiotics in agro-ecoystems: A review. Agronomy for Sustainable Development, 32, 309-327. doi:10.1007/s13593-011-0062-9
- Winckler, C. and Grafe, A. (2001) Use of veterinary drugs in intensive animal production. Journal of Soils and Sediments, 1, 66-70. doi:10.1007/BF02987711
- Aust, M.O., Godlinski, F., Travis, G.R., Hao, X., et al. (2008) Distribution of sulfamethazine, chlortetracycline and tylosin in manure and soil of Canadian feedlots after subtherapeutic use in cattle. Environmental Pollution, 156, 1243-1251. doi:10.1016/j.envpol.2008.03.011
- Al-Ahmad, A., Daschner F.D. and Kummerer, K. (1999) Biodegradability of cefotiam, ciprofloxacin, meropenem, penicillin G and sulfamethoxazole and inhibition of waster bacteria. Archives of Environmental Contamination and Toxicology, 37, 158-163. doi:10.1007/s002449900501
- Boxall, A.B.A., Kay, P., Blackwell, P.A. and Fogg, L.A. (2004). Fate of veterinary medicines applied to soils. In: Kummerer, K., Ed., Pharmaceuticals in the Environment, Springer-Verlag, Berlin, 165-180.
- Kummerer, K. and Henninger, A. (2003) Promoting resistance by the emission of antibiotics from hospitals and households into effluent. Clinical Microbiology and Infection, 9, 1203-1214. doi:10.1111/j.1469-0691.2003.00739.x
- Jjemba, P.K. (2006) Excretion and ecotoxicity of pharmaceutical and personal care products in the environment. Ecotoxicology and Environmental Safety, 63, 113-130. doi:10.1016/j.ecoenv.2004.11.011
- Hamscher, G., Sczesny, S., Höper, H. and Nau, H. (2002) Determination of persistent tetracycline residues in soil fertilized with liquid manure by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Analytical Chemistry, 74, 1509-1518. doi:10.1021/ac015588m
- Arikan, O.A., Mulbry, W., and Rice, C. (2009) Management of antibiotic residues from agricultural sources: Use of composting to reduce chlortetracycline residues in beef manure from treated animals. Journal of Hazardous Materials, 164, 483-489. doi:10.1016/j.jhazmat.2008.08.019
- De Liguoro, M. Cibin, V., Capolongo, F., Halling-Sorensen, H. and Montesina, C. (2003) Use of oxytetracycline and tylosin in intensive calf farming: Evaluation of transfer to manure and soil. Chemosphere, 52, 203-212. doi:10.1016/S0045-6535(03)00284-4
- Zhao, L., Dong, H.Y. and Wang, H. (2010) Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Science of the Total Environment, 408, 1069-1075. doi:10.1016/j.scitotenv.2009.11.014
- Huang, C.H., Renew, J.E., Smeby, K.L., Pinkston, K. and Sedlak, D.L. (2003) Assessment of potential antibiotic contaminants in water and preliminary occurrence analysis. Water Resources Update, 120, 30-40.

