Journal of Environmental Protection
Vol. 3 No. 6 (2012) , Article ID: 20060 , 6 pages DOI:10.4236/jep.2012.36060
Evaluation of Nitrogen and Phosphorus Wastes Produced by Nile Tilapia (Oreochromis niloticus L.) Fed Azolla-Diets in Earthen Ponds*
![]()
1Research Unit on Wetlands, Department of Zoology and Genetic, Faculty of Sciences and Technics, University of Abomey-Calavi, Abomey-Calavi, Republic of Benin; 2Laboratory of Soil Sciences, Department of Crop Production, Faculty of Agricultural Sciences, University of Abomey-Calavi, Abomey-Calavi, Republic of Benin; 3Laboratory of Applied Hydrology, Faculty of Sciences and Technics, University of Abomey-Calavi, Abomey-Calavi, Republic of Benin; 4Research Unit in Environmental and Evolutionary Biology, FUNDP-University of Namur, Namur, Belgium.
Email: #y_abou@yahoo.com
Received February 18th, 2012; revised March 12th, 2012; accepted April 10th, 2012
Keywords: Azolla; Aquaculture; Environment; Fish Meal Replacement; Oreochromis niloticus; Nitrogen; Phosphorus; Nutrient Balance
ABSTRACT
Nitrogen (N) and phosphorus (P) wastes produced by Nile tilapia Oreochromis niloticus L. fed Azolla, an aquatic atmospheric nitrogen fixing fern, was evaluated for 90 days in pond experiment. Six isonitrogenous (29.2% crude protein) and isoenergetic (16.9 Kj·g–1) diets A0, A10, A20, A30, A40 and A50, containing 0%, 10%, 20%, 30%, 40% and 50% of Azolla meal (AM) respectively, as partial fishmeal (FM) substitutes, was provided to experimental fish. The Azolla-free diet A0 served as a control. Fish specific growth rate (SGR) was higher with the control diet, the lower values being obtained in A50-fed fish (P < 0.05). Crude protein and P content in experimental fish showed similar values. Evaluation of the nutrient wasted show identical values (84.8% - 87.8% of supplied) for total P (TP); while total N (TN) discharged into ponds by fish increased significantly when AM level greater than 30% in diets (P < 0.05), amounting 63.9% - 74.2% of that supplied. From these findings, the fern Azolla could be used in diet to sustain Nile tilapia growth and as “environmentally-friendly” ingredient to limit P loss, while providing N to the field, beneficially in tropical marshland pond where this nutrient is already limiting.
1. Introduction
One of the major problems currently facing aquaculture industry is the projected increase in production in order to meet the worldwide demand for fish. Beside from this challenge, aquaculture productions are unfortunately faced with the need to resolve another problem that constrains its sustainability. Indeed, the intensification of production leads to the release of organic wastes and inorganic nutrients, such as phosphorus (P) and nitrogen (N), which are known to enrich and promote eutrophication in aquatic ecosystems [1]. Thereby, environmental pollution associated with aquaculture becomes another critical issue for sustainability and future expansion of this activity [2]. In aquaculture, both P and N originate mainly from fish feeds [3] due to their high amounts in fishmeal (FM) that is rich in P [4,5]. Several studies have reported that the high P of fish meal-based diets is not well utilized by many fish species, such as common carp Cyprinus carpio L., gibel carp Carassius auratus gibelio or rainbow trout Onchorhynchus mykiss Walbaum [6-8]. As a consequence, P loading into the water by fish is generally high, and thus fishmeal-based-diets are considered as primary pollutants of aquatic ecosystems. In Benin, this situation causes generalized eutrophication that has compromised the use of many stagnant earthen ponds in small-scale farms. Therefore, one should appropriately balance this nutrient in feeds for farmed species. One of the numerous ways to reduce P waste produced by aquaculture is the use of FM substitutes that contain lower P with high availability [9]. Among the most popular alternative ingredients usable as FM substitutes, plants proteins appear to be suitable in practice to achieve this goal [10]. Azolla, a small floating freshwater fern that has been successfully used recently in fish farming [11-15] could be a good candidate. P content in that species is less than 0.77% dry matter [16], which is by far lower than the levels of 2% - 4% generally found in FM. This may encourage testing Azolla in low-polluting feeds. In particular, Azolla species develop a symbiotic relationship with a cyanobacteria named Anabaena azolla Strasburger, an atmospheric nitrogen fixing organism. Due to its presence in Azolla fronds cavity, Anabaena azollae make the fern naturally rich in nitrogen. This property is exploited in rice-field culture where Azolla is known as biofertilizer [17].
The main purpose of this study was to investigate P and N wastes discharged into water by Nile tilapia O. niloticus fed with diets containing gradual levels of AM in earthen ponds.
2. Material and Methods
2.1. Experimental Design and Set-Up
Eighteen small earthen ponds of 30 m2 (10 m × 3 m × 1 m) filled naturally from the water table were newly constructed for a 90-days experiment at Louho village, in Porto-Novo suburb, Benin (West Africa).
Male Nile tilapia O. niloticus (initial mean weight = 16.3 ± 0.1 g) from a same cohort was stocked at a density of 2 m–2 (60 fish·pond–1).
These ponds were randomly assigned to 6 triplicate (6 × 3) groups, each set attributed one of the experimental diets. Diets are isonitrogenous (29.2% crude protein) and isoenergetic (16.9 kJ·g–1), formulated using locally available ingredients and the freshwater fern Azolla filiculoides Lamarck. Diets were formulated to contain 0% (A0), 10% (A10), 20% (A20), 30% (A30), 40% (A40) and 50% (A50) of Azolla meal (AM). The Azolla-diets were compared with a control (A0) without AM.
Formulation and proximate composition of experimental diets are given in Table 1. They were prepared according to the procedure described in Abou et al. [14] and preserved in the refrigerator (+4˚C) until used for feeding fish.
Fish were hand-fed daily according to Melard [19]. Daily rations were divided into two parts, each distributed at 8:00 h and 16:00 h, respectively. They were adjusted every two weeks according to the fish biomass in each tank.
Once every fortnight, at least 40% of the fish in stock were sampled with a dip net, without entering the pond, and weighed. The daily ration was adjusted according to the actual body weight, and used for the next fortnight.
2.2. Analysis
The diets used were reduced in meal and preserved at –20˚C for biochemical analysis. 30 fishes were randomly sampled at the beginning from the initial batch, and at the
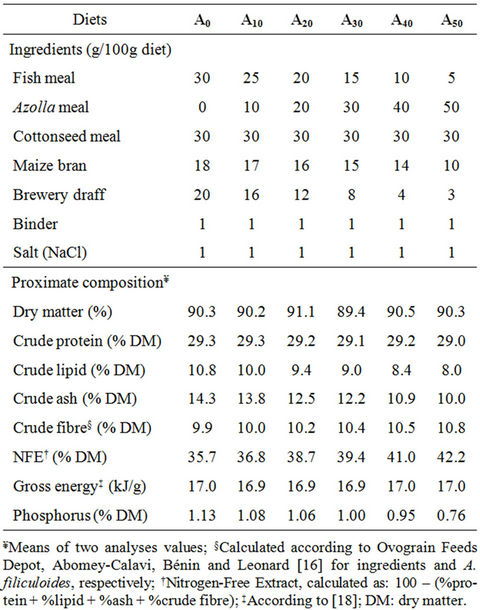
Table 1. Formulation and proximate composition of experimental diets.
end of the experiment 6 fish·pond–1, and stored at –20˚C for analysis of whole-body composition. Whole fish were mashed, using a Robot coupe food processor. Diets and whole fish were analyzed for dry matter (drying samples in an oven at 105˚C, [20]) and crude protein (N × 6.25, Kjeldahl method). Body P concentrations in fish were determined by persulphate digestion [21] with boric acid and sodium hydroxide.
2.3. Calculations
Growth and feed performances parameters were calculated as follow:


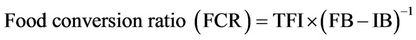

where Wi and Wf are initial and final mean wet weight in g; TFI the total feed intake; FB and IB are final and initial fish biomass in g; DPI the total dietary protein intake; TPG the total body protein gain.
Apparent retention rate (%) of P and N, and the amount of P and N retained and that is discharged into water as wastes (as faecal + urinary + gill) by fish were evaluated according to Cho et al. [22] as follow:
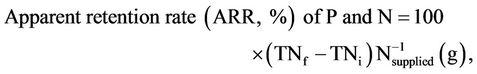

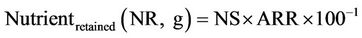
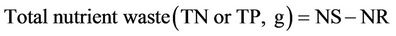
where: TNf and TNi are the final and initial nutrient (P or N) body content in (g); Nsupplied the quantity in (g) of nutrient (P or N) content in the total food supplied; Nfeed is the quantity of feed supplied.
During the experiment, very limited feed loss was noticed. As this amount was unknown, we assume for calculations that the amount lost is 0.
2.4. Statistical Analysis
Means for growth and feed performances, body composition, and nutrient balance were analyzed using one-way ANOVA, after verifying the homogeneity of their variance [23]. Values for percentage data and ratios were log-transformed prior to analyses. When the effect was significant, comparisons between treatment means was run using Duncan’s multiple range test [24] at P = 0.05.
3. Results
3.1. Growth Rate and Feed Utilization
As shown in Table 2, survival rate did not show any significant differences and values were higher in each treatments (P > 0.05). Fish specific growth rate decreased from fish fed the control diet to those fed A50 (P < 0.05)values ranging from 2.35 ± 0.04 %day–1 to 1.87 ± 0.06 %day–1. Significant differences appear at AM levels higher than 20% in diets. FCR increased from 1.26 for A0-fish to 1.68 for A50-fish (P < 0.05). Apparent net protein utilization decreased significantly at AM levels higher than 30% in diets (P < 0.05).
3.2. Body Composition and Nutrient Retention
No significant differences were found in body crude protein (range: 12.1% - 12.9% fresh matter) among all the experimental diets (P > 0.05). Body P (0.73% - 0.85% dry matter) content in fish showed unchanged values in all experimental fish (P > 0.05).
Total P or N supplied to fish and nutrient balance at the end of the experiment are presented in Table 3. Total P or N supplied decreased as AM increased in the diets, from 86.1 ± 0.7 to 47.9 ± 1.2 for TP and from 357.4 ± 3.0 to 292.3 ± 7.0 for TN (P < 0.05). No differences were found in P apparent retention rate (P > 0.05) whereas a decrease in N retention rate was only significant in fish fed A40 and A50. P waste discharged into the water showed significant decreasing values, from fish fed the control diets to those fed diets containing 50% AM (P < 0.05). Conversely, a similar quantity of N was wasted by all experimental fish (P > 0.05). The evaluation as percentage of the amount supplied show no difference for TP (84.8% - 87.8%), whereas TN increased from 63.9% to 74.2% when dietary AM increased from 0% to 50%, difference being significant when AM was more than 30% in diet (P < 0.05).
4. Discussion
In this study, all the diets used contain sufficient amounts of phosphorus to meet this need. Although a low level of methionine has been reported in Azolla filiculoides [25], the required aminoacids and minerals needed to balance the dietary deficiency at high content in AM could be

Table 2. Growth, feed performance and body composition (% fresh matter basis) of Nile tilapia fed in ponds with diets containing increasing level of Azolla filiculoides for 90 days. (A0 to A50: diet with 0% to 50% Azolla).
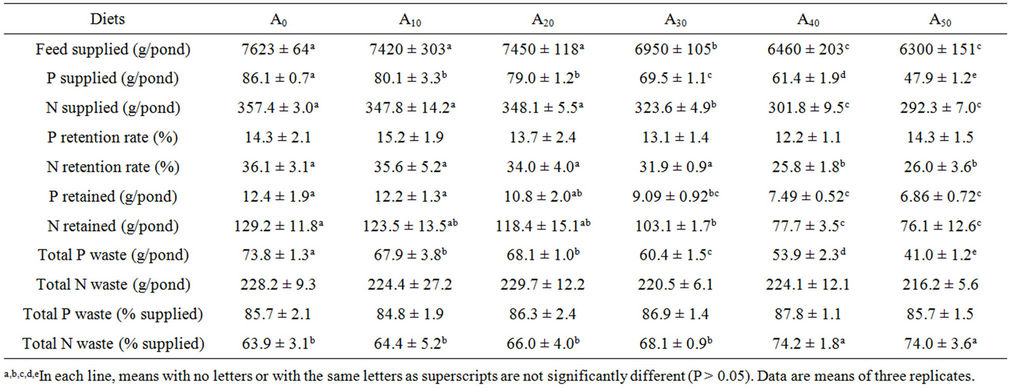
Table 3. Phosphorus and nitrogen budget from Nile tilapia fed Azolla meal diets in earthen ponds for 90 days. (A0 to A50: diet with 0% to 50% Azolla).
likely met from pond ecosystem through natural productivity and water [26]. Thus, since A. filiculoides showed relatively low fibre content, and no antinutrient factors have been detected in that fern, the reduction observed in growth could probably be due to the lower protein digestibility of this fern, as mentioned by Leonard et al. [11] and Micha and Leonard [27] in Oreochromis aureus Steindachner and O. niloticus, respectively. The improved protein efficiency and protein utilization in group of fish fed with diets A0, A10, A20 and A30 could implies that the potentiality of natural food failed to balance Azolla deficiency in A40 and A50. As a consequence, nitrogen retention in fish fed the last two diets was lower than that in fish fed the other diets. Although P content in diet decreased obviously with increasing AM level, a stable P retention was obtained in all experimental fish. According to Hernández et al. [28], P provided by FM-based diets generally surpasses the minimal requirements needed for optimal fish growth and in fact are less utilized by some cultivated species [5]. Therefore, it was suggested to provide fish with just the quantity required in order to keep the diets environmentally friendly [5]. Since tilapia P requirements vary from 0.90% [29] to 0.46% [30] depending on the species, fish size and diet composition, only P content in diet A50 comply with this suggestion. However, because the quantity of P or N supplied was adjusted fortnightly based on fish biomass, the amount provided in ponds receiving diets containing high AM was lower, and so the amount retained in fish decreased with increasing AM in diets.
Conversely, N waste discharged into ponds was similar in all groups of fish whereas lower P loaded decreased from ponds A0 to ponds A50. These findings confirm the lower utilization of nitrogen from Azolla at high levels of the fern in diets and its efficiency in reducing P output, and in turn to limit eutrophication. This result agreed with Jahan et al. [3], and Kaushik et al. [31] who found a reduction in P discharge when feeding fish with diets containing lower P, in which FM was replaced by corn gluten meal. These results could be compared to studies relating dietary P (or N) to P (or N) discharged. In rainbow trout fed 2.2%, 1.4% and 1.0% dietary P, 1.24%, 0.64% and 0.54% P was excreted [32], respectively. Investigating salmonid cage culture, Phillips et al. [33] found 79% loss of N and 85% loss of P supplied with feed, which amounted to 104 kg·N·ton–1 fish production. Values in our study were similar for N and slightly higher for P, ranging from 69.5% to 80.7% and from 89.6% to 91.2%, respectively. In contrary, values obtained in this study are lower to those of Abou et al. [34] from the same experiment conducted in concrete tanks, who found that 90% and 80% of total phosphorus and total nitrogen supplied was wasted by the experimental fish; thus confirming the beneficial role of natural food in improving the nutrient utilization, and then in reduction in the amount wasted. When compared to that in control diet, the values of nutrient wasted by fish fed Azolla-diets were reduced, and the percentage of reduction increased with increasing AM level, reaching 8.7% to 46% for P and 2.9% to 8.8% for N at 50% Azolla-diet. The corresponding nutrient biomass ranged from 4.90 g to 25.9 g for P and 5.27 g to 15.8 g for N, which could not be negligible in preventing eutrophication in the field.
5. Conclusions
These results further contribute toward the comprehension of the role of Azolla in economical and environmental management of semi-intensive tilapia cultures in the tropics, where feed costs are a major factor limiting fish production and where, unfortunately, it is imperative to protect the ponds used, as many of them are stagnant earthen ponds. From the present study, the following conclusions could be drawn in low-polluting feed field:
1) Because of its low P content, Azolla meal could be used in diets for Nile tilapia to develop low-polluting feeds. However, more investigations are needed in that area;
2) High levels of Azolla meal in diets reduce growth, but positively, its non well-utilized nitrogen could enrich the fish ponds; which will be quite good in tropical marshland ponds where nitrogen is already limiting;
3) Hence, Azolla could be considered as “environmentally-friendly” ingredient that could limit the eutrophication, as well as preventing oligotrophication.
6. Acknowledgements
We are grateful to the BTC-CTB (Coopération Technique Belge), the FUNDP-University of Namur (Belgium) and the CUD (Commission Universitaire pour le Développement) of Belgium Kingdom, which allocated, respectively, a PhD grant and a postdoctorate fellowship (“ELAN” fellowship) to M. Abou.
REFERENCES
- GESAMP IMO/FAO/UNESCO-IOC/WMO/WHO/IAEA/ UN/NEP (Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection), “Monitoring the Ecological Effects of Coastal Aquaculture Wastes,” GESAMP, Rome, 1996.
- S. H. Sugiura, J. K. Babbitt, F. M. Dong and R. W. Hardy, “Utilization of Fish and Animal By-Product Meals in Low-Pollution Feeds for Rainbow Trout Onchorhnchus mykiss (Walbaum),” Aquaculture Research, Vol. 31, No. 7, 2000, pp. 585-593. doi:10.1046/j.1365-2109.2000.00476.x
- C. Y. Cho and D. P. Bureau, “Reduction of Waste Output from Salmonid Aquaculture Trough Feed and Feeding,” Progressive Fish-Culturist, Vol. 59, No. 2, 1997, pp. 155- 160. doi:10.1577/1548-8640(1997)059<0155:ROWOFS>2.3.CO;2
- Y. Buyukates, S. D. Rawles and D. M. Gatlin, “III Phosphorus Fractions of Various Feedstuffs and Apparent Phosphorus Availability to Channel Catfish,” North American Journal of Aquaculture, Vol. 62, No. 3, 2000, pp. 184-188. doi:10.1577/1548-8454(2000)062<0184:PFOVFA>2.3.CO;2
- S. H. Sugiura and R. W. Hardy, “Environmentally Friendly Feeds,” In: R. R. Stickney, Ed., Encyclopedia of Aquaculure, Wiley-Interscience, 2000, pp. 299-310.
- C. Ogino, L. Takeuchi, H. Takeda and T. Watanabe, “Availability of Dietary Phosphorus in Carp and Rainbow Trout,” Nippon Suisan Gakkaishi, Vol. 45, 1979, pp. 1527-1532. doi:10.2331/suisan.45.1527
- B. S. Nakashima and W. C. Leggett, “Natural Sources and Requirements of Phosphorus for Fishes,” Canadian Journal of Fisheries and Aquatic Sciences, Vol. 37, No. 4, 1980, pp. 679-686. doi:10.1139/f80-085
- S. Zhang, S. Xie, X. Zhu, W. Lei, Y. Yang and M. Zhao, “Meat and Bone Meal Replacement in Diets for Juvenile Gibel Carp (Carasius auratus gibelio): Effects on Growth Performance, Phosphorus and Nitrogen Loading,” Aquaculture Nutrition, Vol. 12, No. 5, 2006, pp. 353-362. doi:10.1111/j.1365-2095.2006.00431.x
- C. Y. Cho and D. P. Bureau, “A Review of Diet Formulation Strategies and Feeding Systems to Reduce Excretory and Feed Wastes in Aquaculture,” Aquaculture Research, Vol. 32, No. S1, 2001, pp. 349-360. doi:10.1046/j.1355-557x.2001.00027.x
- P. Jahan, T. Watanabe, S. Satoh and V. Kiron, “Reduction in Elemental Waste Loading from Commercial Carp Feeds by Manipulating the Dietary Phosphorus Levels,” Fisheries Sciences, Vol. 69, No. 1, 2003, pp. 58-65. doi:10.1046/j.1444-2906.2003.00588.x
- V. Leonard, C. Breyne, J.-C. Micha and Y. Larondelle, “Digestibility and Transit Time of Azolla filiculoides Lamarck in Oreochromis aureus (Steindachner),” Aquaculture Research, Vol. 29, 1998, pp. 159-165. doi:10.1111/j.1365-2109.1998.tb01120.x
- E. A. Fasakin, A. M. Balogun and O. A. Fagbenro, “Evaluation of Sun-Dried Water Fern, Azolla africana, and Duckweed, Spirodela polyrrhiza, in Practical Diets for Nile Tilapia, Oreochromis niloticus, Fingerlings,” Journal of Applied Aquaculture, Vol. 11, No. 4, 2001, pp. 83- 92. doi:10.1300/J028v11n04_09
- E. D. Fiogbé, J.-C. Micha and C. Van Hove, “Use of a Natural Aquatic Fern, Azolla microphylla, as a Main Component in Food for Omnivorous-Phytoplanktonophagous Tilapia, Oreochromis niloticus L.,” Journal of Applied Ichthyology, Vol. 20, No. 6, 2004, pp. 517-520. doi:10.1111/j.1439-0426.2004.00562.x
- Y. Abou, E. D. Fiogbé and J.-C. Micha, “A Preliminary Assessment of Growth and Production of Nile Tilapia, Oreochromis niloticus L., Fed Azolla-Based-Diets in Earthen Ponds,” Journal of Applied Aquaculture, Vol. 19, No. 4, 2007, pp. 55-69. doi:10.1300/J028v19n04_03
- Y. Abou, E. D. Fiogbé and J.-C. Micha, “Effects of Stocking Density on Growth, Yield and Profitability of Farming Nile Tilapia, Oreochromis niloticus L., Fed Azolla Diet, in Earthen Ponds,” Aquaculture Research, Vol. 38, No. 6, 2007, pp. 595-604. doi:10.1111/j.1365-2109.2007.01700.x
- V. Leonard, “Use of an Aquatic Fern (Azolla filiculoides) in Two Species of Tropical Fish (Oreochromis niloticus and Tilapia rendalli),” Doctoral Dissertation, Catholic University of Louvain, Louvain-la-Neuve, 1997.
- F. Carrapiço, G. Teixeira and M. Adélia Diniz, “Azolla as a Biofertilizer in Africa. A Challenge for the Future,” Revista de Ciêcias Agrárias, Vol. 23, 2000, pp. 120-138.
- A. G. J. Tacon, “Standard Methods for the Nutrition and Feeding of Farmed Fish and shrimp,” Argent Laboratories Press, Washington DC, 1990.
- Ch. Mélard, “Bases Biologiques de l’Élevage Intensif du Tilapia du Nil O. niloticus,” Cahiers d’Ethologie Appliquée, Vol. 5, No. 3, 1986, pp. 1-224.
- AOAC, “Official Methods of Analysis,” 15th Edition, Association of Official Analytical Chemists, Arlington, 1990.
- A. Gross and C. E. Boyd, “A Digestion Procedure for the Simultaneous Determination of Total Nitrogen and Total Phosphorus in Pond Water,” Journal of the World Aquaculture Society, Vol. 29, No. 3, 1998, pp. 300-303. doi:10.1111/j.1749-7345.1998.tb00650.x
- C. Y. Cho, J. D. Hynes, K. R. Wood and H. K. Yoshida, “Development of High-Nutrient-Dense, Low-Pollution Diets and Prediction of Aquaculture Waste Using Biological Approaches,” Aquaculture, Vol. 124, No. 1-4, 1994, pp. 293-305. doi:10.1016/0044-8486(94)90403-0
- H. O. Hartley, “Smallest Composite Designs for Quadratic Response Surface,” Biometrics, Vol. 15, No. 4, 1959, pp. 611-624. doi:10.2307/2527658
- D. B. Duncan, “Multiple Range and Multiple F-Tests,” Biometrics, Vol. 11, No. 1, 1955, pp. 1-42. doi:10.2307/3001478
- N. C. Sanginga and C. Van Hove, “Amino Acids of Azolla as Affected by Strains and Population Density,” Plant and Soil, Vol. 117, No. 2, 1989, pp. 263-267. doi:10.1007/BF02220720
- E. W. Becker, “Nutritional Properties of Microalgae: Potential and Constrains,” In: A. Richmond, Ed., Handbook of Microalgae Mass Culture, CRC Press, Boca Raton, 1986.
- J.-C. Micha and V. Leonard, “Digestibility of the Aquatic Fern Azolla filiculoides Lamarck in Two Species of Tilapia: The Phytoplanktonophagous Oreochromis niloticus (L.) and the Macrophytophagous Tilapia rendalli (Boulenger),” Bulletin des Séances de l’Académie Royale des Sciences d’Outre-Mer, Vol. 47, 2001, pp. 147-157.
- A. Hernández, S. Shuichi, V. Kiron and T. Watanabe, “Phosphorus Retention in Rainbow Trout Fed Diets with Low Fish Meal and Alternative Protein Ingredients,” Fisheries Sciences, Vol. 70, No. 4, 2004, pp. 580-586. doi:10.1111/j.1444-2906.2004.00844.x
- T. Watanabe, T. Takeuchi, A. Murakami and C. Ogino, “The Availability to Tilapia nilotica of Phosphorus in White Fish Meal,” Bulletin of the Japanese Society of Scientific Fisheries, Vol. 46, No. 7, 1980, pp. 897-899. doi:10.2331/suisan.46.897
- J. S. Haylor, M. C. M. Beveridge and K. Jauncey, “Phosphorus Nutrition of Juvenile Oreochromis niloticus,” In: R. S. V. Pullin, T. Bhukaswan, K. Tonguthai and J. L. Maclean, Eds., The Second International Symposium on Tilapia in Aquaculture, Bangkok and ICLARM, Manila, 1998.
- S. J. Kaushik, D. Covès, G. Dutto and D. Blanc, “Almost Total Replacement of Fish Meal by Plant Protein Sources in the Diet of a Marine Teleost, the European Seabass, Dicentrarchus labrax,” Aquaculture, Vol. 230, No. 1-4, 2004, pp. 391-404. doi:10.1016/S0044-8486(03)00422-8
- H. G. Ketola and B. F. Harland, “Influence of Phosphorus in Rainbow Trout Diets on Phosphorus Discharges in Effluent Water,” Transactions of the American Fisheries Society, Vol. 122, No. 6, 1993, pp. 1120-1126. doi:10.1577/1548-8659(1993)122<1120:IOPIRT>2.3.CO;2
- M. J. Phillips, M. C. M. Beveridge and L. G. Ross, “The Environmental Impact of Salmonid Cage Culture on Inland Fisheries: Present Status and Future Trends,” Journal of Fish Biology, Vol. 27, Suppl. A, 1985, pp. 123-127. doi:10.1111/j.1095-8649.1985.tb03236.x
- Y. Abou, E. D. Fiogbe, M. P. Aina, A. Buldgen and J.-C. Micha, “Evaluation of Nitrogen and Phosphorus Wastes Produced by Nile Tilapia (Oreochromis niloticus L.) Fed Azolla-Diets in Concrete Tanks,” International Journal of Biological and Chemical Sciences, Vol. 4, No. 1, 2010, pp. 42-50. doi:10.4314/ijbcs.v4i1.54229
NOTES
*Nitrogen and Phosphorus Wastes Produced by Nile tilapia Fed Azolla.
#Corresponding author.

