Green and Sustainable Chemistry
Vol.4 No.2(2014), Article ID:46436,7 pages DOI:10.4236/gsc.2014.42014
Nano-γ-Fe2O3: Efficient, Reusable and Green Catalyst for N-tert-Butoxycarbonylation of Amines in Water
Venkataramana Medisetti1, Umadevi Parimi1*, Ramesh Babu Anagani2, K. V. V. V. Satyanarayana1
1Department of Chemistry, Gitam University, Visakhapatnam, India
2Department of Chemistry, Acharya Nagarjuna University, Guntur, India
Email: *parimiumadevi@yahoo.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 20 December 2013; revised 19 February 2014; accepted 10 March 2014
ABSTRACT
An efficient and versatile practical protocol for the chemoselective N-tert-butoxycarbonylation of amines using Nano-γ-Fe2O3 and (BOC)2O. Nano-γ-Fe2O3 was applied as an efficient, green, heterogeneous and reusable catalyst at ambient temperature; the method is general for the preparation of N-Boc derivatives of aliphatic, heterocyclic, aromatic as well as amino acid derivatives.
Keywords:Nano-γ-Fe2O3, N-Boc Protection, Amine, Heterogeneous Catalyst, Water

1. Introduction
The protection and deportation of amines have become a very important and widely used method for many multi-step organic syntheses. Among the various groups reported for the protection of amines, the use of N-tert-butoxycarbonyl (N-Boc) group has become very popular [1] -[3] . Different groups are available for N-tert-butylcarbamate such as (Boc)2O, BocONH2, BocN3 and 1-(tert-butoxycarbonyl) benzotriazole [4] -[8] .
N-tert-Butoxycarbonylation of amines has received considerable attention in synthesis due to the stability of the N-tert-butoxycarbonyl group toward basic and nucleophilic attack and its labile nature in the presence of acid, commercial availability, low cost and efficiency. Various reagents and methods have been developed in the past years for the N-tert-butyloxycarbonylation of amines.
The conventional procedure employs di-tert-butyldicarbonate (Boc)2O and base catalysts such as 4-(N, N-dimethylamino) pyridine DMAP, [9] [10] , NaHMDS, [11] , K2CO3, [12] and Et3N, [13] , the last one being most commonly used.
Further, modified methods have been reported with amines and (Boc)2O in the presence of Lewis acids. Many of these procedures involve the use of corrosive and moisture-sensitive reagents like ZrCl4, [14] , LiClO4, [15] , Cu(BF4)2, HClO4, [16] and La(NO3) [17] .
In recent years, several new and efficient methods have been developed including the use of Montmorillonite K10, [18] , thiourea [19] , HFIP, sulfamicacid, Amberlyst 15.
However, most of these methods still have several drawbacks, such as the air-sensitive nature of the catalysts unpleasant smell, high toxicity, corrosiveness, and non-recyclability of these catalysts make the method objectionable, especially from the point of view of green chemistry.
Recently several methods for N-protection of amino groups have been reported with the “green chemistry” concept. Water and ionic liquids were used as solvents.
2. Results and Discussion
In our continuous research for green and eco friendly chemical methods, water is the key solvent, and there is increasing interest in using it as green solvent for organic conversions. However, reports about using water as a catalyst to promote organic reactions are very limited. Compared to conventional solvents water is preferred for organic reaction. Because of cheap, nontoxic, no explosive, and environmentally acceptable [20] . Thus, the use of water instead of organic solvents has gained much importance in the development of sustainable protection in generally chemistry.
In this paper, we report efficient and eco-friendly protocol for chemoselective N-tert-butyloxycarbonylation of various structurally amines in water-related system under room temperature conditions in the absence of any acid/base-catalyst.
Nano-γ-Fe2O3 particles are considered to be attractive as catalysts for their greater reactivity, due to high surface area, recovered easily from the reaction mixture and reusable for further reaction, therefore the method being more Efficient [21] .
The morphology and size of the Nano-γ-Fe2O3 particles were analyzed by X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) as shown in Figure 1 and Figure 2. The average grain size of zinc oxide nano particles was calculated using the Scherrer formula and was found to be about 15 nm indicating nano crystalline nature. No other metal phases were detected by XRD (Figure 2). The low magnification SEM and TEM images (Figure 1(a) and Figure 1(b)) shows small nano sized grains having spherical and hexagonal morphology nano particles. The presence of some larger particles attributes aggregating or overlapping of smaller particles.
In continuation of our search in organic synthesis by different methods with the use of nano catalyst, we report an easy and efficient method for the N-tert-butoxycarbonyl of various amines using Fe2O3 nanoparticle in water media (Scheme 1).
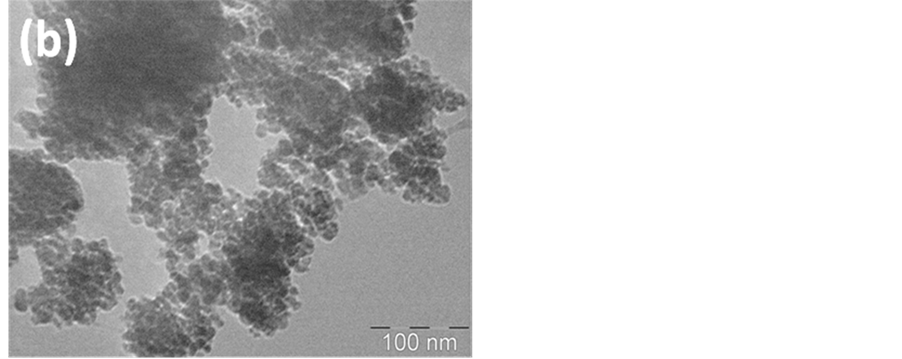
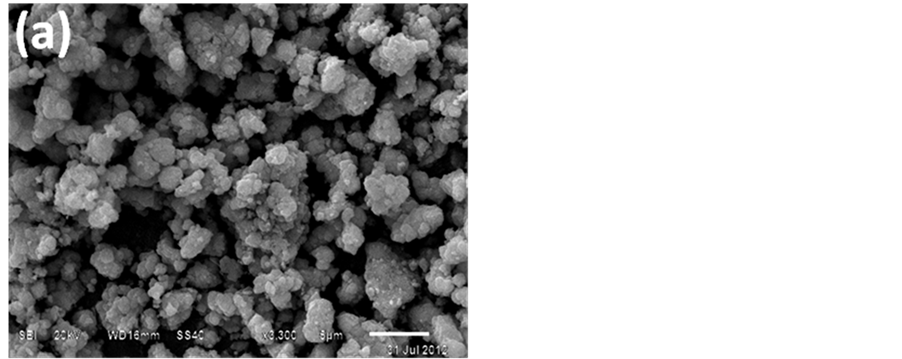
Figure 1. (a) SEM; (b) TEM images of Nano-Fe2O3 particles.
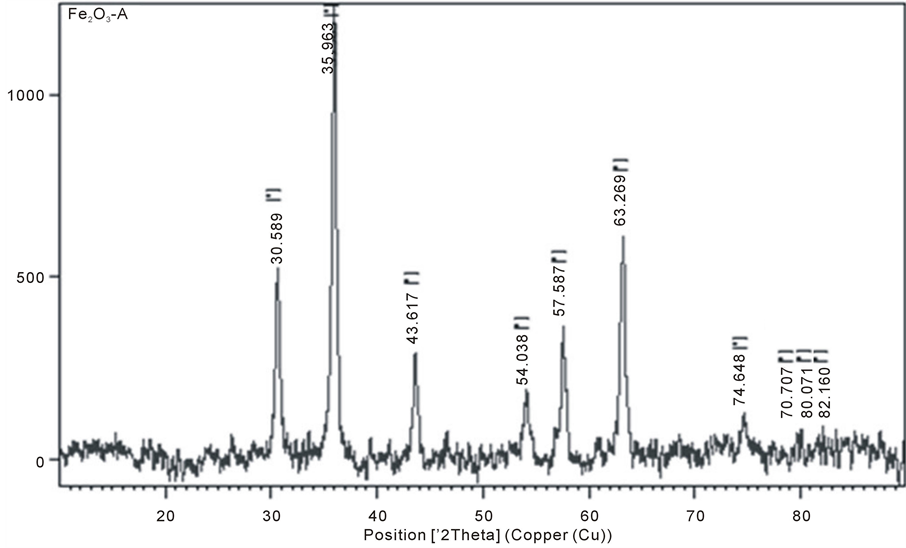
Figure 2. XRD Pattern for Nano-Fe2O3 particles.
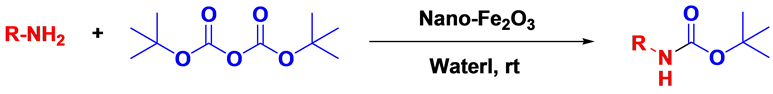
Scheme 1. Fe2O3 Mediated N-tert butoxycarbonylation.
To the best of our knowledge, the use of Nano-γ-Fe2O3 for the N-tert-butoxycarbonylation of amine in water has not been reported.
In our initial study (Scheme 1), aniline was reacted with di-tert-butyldicarbonate in the presence of Nano-γ- Fe2O3 in water at room temperature for 60 mins when the expected product was obtained in 95%. (Table 1, entry 1), While the same reaction was performed in presence of Nano-γ-Fe2O3 without solvent at room temperature afforded the N-tert-butoxycarbonylated compound in low yield with starting aniline remaining unreacted.
In presence of electron withdrawing or donating substituents on different aromatic amines the reactions proceeded efficiently to provide the desired products in good yields (Table 1, entries 2 - 7) Also, the method provides chemoselective process for the substrates with OH or SH group (Table 1, entries 10 and 11) giving N-Boc derivatives with good yields.
The diamine gave selective N-Boc protected compound with 1.0 equivalent of (Boc)2O in the presence another amino group, however, with 2.0 equivalent of (Boc)2O both the amino group were reacted giving excellent yield of the product (Table 1, entries 8 and 9).
In all cases, the expected N-Boc protected amines were obtained in excellent yield as summarized in Table 1 The N-Boc protected amines were fully characterized by recording their 1H, 13C NMR, IR spectroscopy and elemental analysis.
From these studies, Nano-γ-Fe2O3 has been proved as efficient reusable catalyst for N-tert-butoxycarbonylation of amines in water.
3. Experimental Section
General procedure for N-tert-butoxycarbonylation of amines:—To a stirred solution of amine (2 mmol) and (Boc)2O (2.1 mmol) in water was added Nano-γ-Fe2O3 (10 mol%) catalyst. The reaction mixture was stirred at
Table 1. N-tert-butoxycarbonylation of aromatic and aliphatic amines in presence of Nano-γ-Fe2O3 (Scheme 1).
room temperature (30˚C - 35˚C). On completion (indicated by thin layer chromatography), the reaction was filtered through a sintered funnel and washed thoroughly with water. The combined filtrate was extracted with ethyl acetate dried over anhydrous Na2SO4, then concentrated the solvent under reduced pressure to get the crude product. The pure products were obtained by column chromatography using Ethyl acetate and hexane as an eluents.
All commercial chemicals and solvents were without further purification. All reactions were carried out under inert argon atmosphere. Melting points were determined on a capillary melting point apparatus and are uncorrected. The 1H NMR was recorded in the indicated solvent on a Varian 300 MHz spectrometer with TMS as internal standard. All chemical shifts (δ) were reported in ppm from internal TMS. Mass spectra were measured on a Jeol JMS D-300 spectrometer. Infrared spectra were recorded in KBr on Brucher-IFS-66 FTIR spectrophotometer. The homogeneity of the compounds was checked using precoated TLC plates (E.Merk Kieselgel 60 F254).
4. Conclusions
In summary, we have developed a green, inexpensive and efficient method for water-mediated N-tert-butoxycarbonylation of amines at room temperature using Nano-Fe2O3. The absence of acid/base and the use of water make this procedure environmentally friendly.
Tert-butyl phenylcarbamate (01): 1H NMR (300 MHz, DMSO d6) δ = 1.50 (s, 9H), 7.68 - 7.37 (m, 5H), 8.30 (brs, 1H); 13C NMR (100 MHz, CDCl3): δ = 28.07, 80.7, 121.6, 124.4, 129.0, 134.07, 154.12.
Tert-butyl 2-bromophenylcarbamate (02): 1H NMR (300 MHz, CDCl3): δ = 1.45 (s, 9H), 7.61 (m, 2H), 7.12 (d, 1H), 7.35 (s, 1H); 13C NMR (100 MHz, CDCl3): δ = 28.07, 80.7, 112.18, 119.19, 123.62, 128.06, 132.01, 136.08, 146.57, 152.12, IR (KBr): 3415, 2979, 1735, 1517, 1433, 1158, 1119, 749.
Tert-butyl 4-chlorophenylcarbamate (03): 1H NMR (300 MHz, DMSO d6): δ = 1.48 (s, 9H), 7.36 (d, 2H J = 8.2 Hz), 7.71 (d, 2H J = 8.2 Hz); 13C NMR (100 MHz, CDCl3): d = 28.34, 80.19, 121.40, 128.66, 130.64, 136.69, 153.91.
Tert-butyl 4-(trifluoromethyl)phenylcarbamate (04): 1H NMR (300 MHz, DMSO d6):δ = 1.47 (s, 9H), 7.63 (d, 2H, j = 8Hz), 7.80 (d, 2H, J = 8 Hz), 8.10 (brs, 1H); 13C NMR (100 MHz, CDCl3): d = 28.34, 80.19, 120.90, 124.86, 125.66, 126.7, 138.91, 152.91.
Tert-butyl 4-tert-butylphenylcarbamate (05): 1H NMR (300 MHz, CDCl3): δ = 1.35 (s, 9H), 1.60 (m, 12H), 6.43 (s, 1H), 7.30 (m, 4H); 13C NMR (100 MHz, CDCl3): d = 27.34, 28.29, 31.33, 34.14, 80.19, 118.40, 125.66, 135.64, 146.69, 152.91. IR (KBr): 3443, 2963, 1703, 1526, 1396, 1235, 1075, 769.
Tert-butyl 3,5-dimethylphenylcarbamate (06): 1H NMR (300 MHz, CDCl3): δ = 1.50 (s, 9H), 2.27 ( s, 6H), 6.43 (s, 1H), 6.67 (s, 1H),6.98(s, 1H); 13C NMR (100 MHz, CDCl3): d = 21.23, 28.31, 80.24, 116.23, 124.73, 138.13, 138.60, 152.78; IR (KBr): 3359, 3010, 2987, 2917, 1694, 1523, 1435, 1278, 1159, 1075, 843, 617 cm−1.
Tert-butyl m-tolylcarbamate (07): 1H NMR (300 MHz, CDCl3): δ = 1.57 (s, 9H), 2.129 (s, 3H) 6.38 (brs, 1H), 6.58 (d, 1H ), 7.00 (d, 1H), 7.11 (s, 2H); 13C NMR (100 MHz, CDCl3): d = 24.51, 28.54, 80.10, 118.92, 121.72, 124.86, 128.96, 136.7, 139.87, 153.81.
Tert-butyl 2-aminophenylcarbamate (08): 1H NMR (300 MHz, DMSO d6): δ = 1.49 (s, 9H), 4.30 (brs, 1H), 7.50 (d, 2H), 7.64 (d, 2H), 8.01 (brs, 1H); 13C NMR (100 MHz, CDCl3): d = 28.56, 79.66, 116.45, 119.25, 122.65, 123.51, 126.35, 146.32, 154.23.
Tert-butyl 2-mercaptophenylcarbamate (10): 1H NMR (300 MHz, DMSO d6): δ = 1.47 (s, 9H), 6.69 (t, 1H), 6.83 (m, 2H ), 7.60 (d, 1H), 7.81 (brs, 1H) 9.78 (s, 1H); 13C NMR (100 MHz, CDCl3): d = 28.44, 81.10, 124.70, 124.86, 125.76, 129.7, 153.91.
Tert-butyl 2-hydroxyphenylcarbamate (11): 1H NMR (300 MHz, CDCl3): d = 1.45 (s, 9H), 6.72 (d, 1H), 6.82 (m, 2H), 7.59 (d, 1H), 7.75 (s, 1H), 9.70 (s, 1H); 13C NMR (100 MHz, CDCl3): d = 28.27, 82.08, 118.72, 120.78, 121.33, 125.53, 125.62, 147.33, 155.03; IR (KBr): 3426, 3293, 2979, 2933, 2561, 1692, 1526, 1454, 1153, 1051, 744, 614 cm−1.
References
- Ballini, R., Bigi, F., Bosica, G., Maggi, R., Satori, G. and Righi, P. (2004) Protection (and Deprotection) of Functional Groups in Organic Synthesis by Heterogeneous Catalysis. Chemical Reviews, 104, 199-250.
- Wuensch, E. (1974) Houben-Weyl Methods of Organic Chemistry. In: Mueller, E., Bayer, O. and Ziegler, K., Eds., Methods of Organic Chemistry, 4th Edition, George Thieme, Stuttgart, 46.
- Wuts, P.G.M. and Greene, T.W., Protective Groups in Organic Synthesis (1999) Greene’s Protective Groups in Organic Synthesis. 2nd Edition, Wiley, New York, 503.
- Yamamoto, Y., Tarbell, D.S. and Pope, B.M. (1972) New Method to Prepare N-t-Butoxycarbonyl Derivatives and the Corresponding Sulfur Analogs from di-t-Butyl Dicarbonate or di-t-Butyl Dithiol Dicarbonates and Amino Acids. Proceedings of the National Academy of Sciences of the USA, 69, 730-732. http://dx.doi.org/10.1073/pnas.69.3.730
- Itoh, M., Hagiwara, D. and Kamiya, T. (1977) Peptides. VI. Some Oxime Carbonates as Novel t-Butoxycarbonylating Reagents. Bulletin of the Chemical Society of Japan, 50, 718-721. http://dx.doi.org/10.1246/bcsj.50.718
- Wilson, I.B. and Harris, R.B. (1983) Synthesis of tert-Butyl Aminocarbonate, a New Type of Compound That Can Be Used to Acylate Amines. Tetrahedron Letters, 24, 231-232.
- Hansen, J.B., Nielsen, M.C., Ehrbar, U. and Buchradt, O. (1982) Partially Protected Polyamines. Synthesis, 1982, 404-405. http://dx.doi.org/10.1055/s-1982-29814
- Fali, C.N., Li, J., Katritzsky, A.R., Ager, D.J. and Prakash, I. (1997) Synthesis of 1-(T-Butoxycarbonyl)benzotriazole and 1-(p-Methoxybenzyloxycarbonyl)benzotriazole and Their Use in the Protection of Amino Acids. Synthetic Communications, 27, 1623-1630. http://dx.doi.org/10.1080/00397919708006101
- Hassner, A. and Basel, Y.J. (2000) Di-tert-Butyl Dicarbonate and 4-(Dimethylamino)pyridine Revisited. Their Reactions with Amines and Alcohols. Organic Chemistry, 65, 6368-6380.
- Burk, M.J. and Allen, J.G. (1997) A Mild Amide to Carbamate Transformation. The Journal of Organic Chemistry, 62, 7054-7057. http://dx.doi.org/10.1021/jo970903j
- Kelly, T.A. and McNeil, D.W. (1994) A Simple Method for the Protection of Aryl Amines as Their t-Butylcarbamoyl (Boc) Derivatives. Tetrahedron Letters, 35, 9003-9006. http://dx.doi.org/10.1016/0040-4039(94)88411-0
- Senet, J.-P., Barcelo, G. and Sennyey, G. (1986) Alkyl 1-Chloroalkyl Carbonates: Reagents for the Synthesis of Carbamates and Protection of Amino Group. Synthesis, 8, 627-632.
- Kamiya, T., Itoh, M. and Hagiwara, D. (1975) Synthesis of Cyclo(L-Pro-L-Tyr) from the t-BOC Derivative of L-Proline. Tetrahedron Letters, 49, 4393-4394.
- Reddy, J.J., Lakshmi, P.S., Sharma, G.V.S. and Krishna, P.R. (2004) Rapid and Facile Lewis Acid Catalysed Boc Protection of Amines. Tetrahedron Letters, 45, 6963-6965. http://dx.doi.org/10.1016/j.tetlet.2004.07.072
- Heydari, A. and Hosseini, S.E. (2005) Lithium Perchlorate-Catalyzed Boc Protection of Amines and Amine Derivatives. Advanced Synthesis & Catalysis, 347, 1929-1932. http://dx.doi.org/10.1002/adsc.200505218
- Chakraborti, A.K. and Chankeshwara, S.V. (2006) HClO4-SiO2 as a New, Highly Efficient, Inexpensive and Reusable Catalyst for N-tert-Butoxycarbonylation of Amines. Organic & Biomolecular Chemistry, 4, 2769-2771. http://dx.doi.org/10.1039/b605074c
- Prabhakar, P., Reddy, S.T., Rajesh, K., Suryakiran, N. and Venkateswarlu, Y. (2006) Facile N-tert-Butoxycarbonylation of Amines Using La(NO3)3·6H2O as a Mild and Efficient Catalyst under Solvent-Free Conditions. Tetrahedron Letters, 47, 8039-8042. http://dx.doi.org/10.1016/j.tetlet.2006.09.081
- Chankeshwara, S.V. and Chakraborti, A.K. (2006) Montmorillonite K 10 and Montmorillonite KSF as New and Reusable Catalysts for Conversion of Amines to N-tert-Butylcarbamates. Journal of Molecular Catalysis A: Chemical, 253, 198-202. http://dx.doi.org/10.1016/j.molcata.2006.03.042
- Heydari, A., Khaksar, S., Tajbakhsh, M. and Vahdat, S.M. (2008) Hydrogen Bond Catalyzed Chemoselective N-tertButoxycarbonylation of Amines. Tetrahedron Letters, 49, 3527-3529. http://dx.doi.org/10.1016/j.tetlet.2008.03.138
- Siskin, M., Katritzky, A.R. and Allin, S.M. (1996) Aquathermolysis: Reactions of Organic Compounds with Superheated Water. Accounts of Chemical Research, 29, 399-406. http://dx.doi.org/10.1021/ar950144w
NOTES

*Corresponding author.


