Green and Sustainable Chemistry
Vol.1 No.4(2011), Article ID:8503,10 pages DOI:10.4236/gsc.2011.14024
Cu(II) Extraction in Ionic Liquids and Chlorinated Solvents: Temperature Effect
1CEMIN, Center of Excellence on Innovative Nanostructured Materials, Department of Chemistry, University of Perugia, Perugia, Italy
2Department of Chemistry, Chemical Engineering and Materials, University of L’Aquila, L’Aquila, Italy
E-mail: *spreti@univaq.it
Received July 7, 2011; revised August 17, 2011; accepted August 28, 2011
Keywords: Chlorinated Solvents, Liquid-Liquid Extraction, Hydrophobic Ionic Liquids, Lipophilic Polyamine, Temperature Effect
Abstract
Room temperature ionic liquids have been currently used in liquid/liquid extraction processes in order to substitute conventional organic solvents. In this paper, a series of chlorinated solvents and 1-alkyl-3-methylimidazolium-based ionic liquids were selected to study the extraction efficiency of a lipophilic polyamine 1,1,7,7-tetraethyl-4-tetradecyldiethylenetriamine (TE14DT) towards the model ion Cu(II) in such different media. The effect of temperature on the extraction efficiency was also investigated. The metal ion partition was found to be strongly dependent on both the nature of the solvent and on the working temperature. The viscosity of ionic liquids and the water content in ionic liquid were found to affect the extraction efficiency of TE14DT. The chemical nature of the cation of ionic liquids, and in particular the alkyl chain length on imidazolium ring, also seemed to be important in determining the efficiency of the extraction process. Finally, preliminary experiments on back-extraction of Cu(II) ions from ionic liquid also revealed interesting hints to the development of a continuous transport process.
1. Introduction
In recent years, Room Temperature Ionic Liquids (RTILs) have been regarded as potential alternative media in a wide range of different applications. Their particular features, such as the very low volatility, non-flammability and ability to dissolve both organic and inorganic compounds, are important advantages over conventional organic solvents, the use of which has been increasingly recommended to be avoided. Moreover, RTILs can be made hydrophobic while retaining ionic features and this dual nature forms the basis for their use as novel separation media for the solvent extraction of ionic species.
In literature, extraction of a wide range of metal ions has been reported by using RTILs containing proper complexing agents and alkali and alkaline earth, heavy and even radioactive metal ions can be thus effectively removed from aqueous solutions [1]. It is noteworthy that significant differences both in efficiency and chemical equilibria can be found between RTILs and organic solvents when employed in biphasic systems [2]. Many efforts have been made to elucidate the mechanism of partitioning, and it was found to be dependent on the nature of the extractant molecule, the species in the aqueous phase and the hydrophobic/hydrophilic balance of the ionic liquid [3]. However, a thorough comparison among traditional molecular solvents and ionic liquids is not available yet, and only few attempts have been carried out to understand what kind of correlations can be set between specific chemico-physical properties and the observed partitioning effect [4,5].
In the past, our research group successfully achieved the transport of different metal ions through bulk liquid membranes in the presence of new polyamine carriers [6-9]. Briefly, in a bulk liquid membrane aqueous donor and acceptor phases are separated by an immiscible liquid membrane and suitable carrier molecules are solubilized in the membrane to achieve the transport process. So far, dichloromethane and chloroform were used as liquid membranes and therefore, in order to provide a great enhancement to one-step extraction/recovery sytems, the possibility to employ our chelating agents in ionic liquids was considered. This, in our present knowledge, is the first systematic survey performed to obtain comparative information on metal ion extraction by a chelating agent, as a function of temperature, both in organic solvents and in ionic liquids.
In this paper, Cu(II) was selected as model ion and results on its partitioning both in a series of chlorinated solvents and in 1-alkyl-3-methylimidazolium-based ionic liquids ([CnMIM][X]) are reported. The simple lipophilic polyamine 1,1,7,7-tetraethyl-4-tetradecyldiethylenetriamine (TE14DT, Scheme 1) [6,7] was used as chelating agent in water/organic solvents or RTILs biphasic systems and its extraction efficiency was evaluated. The structural features of TE14DT make it easily soluble in both organic solvents and ionic liquids at the same concentrations. Moreover, the temperature effect on the extraction process was assessed in both organic solventand RTILbased biphasic systems.
2. Experimental Section
2.1. Materials
All the materials were analytical grade, purchased from Aldrich (Milan, Italy) and used as received. Solvents, purchased from Carlo Erba (Milan, Italy), were ACScertified quality. Synthesis and purification procedures of 1,1,7,7-tetraethyl-4-tetradecyldiethylenetriamine (TE14DT) were achieved as previously reported [6]. Ionic liquids were synthesised as indicated below.
2.2. Metal Ion Analysis
The concentration of Cu(II) ions in the aqueous phase was measured following the sensitive spectrophotometric method described in an earlier publication [7]. A diode array Hewlett Packard 8452 A spectrophotometer was used for the quantitative determinations.
2.3. Extraction Experiments
In a screw cap, flat-bottomed vial, 1 mL of a 5 × 10−3 M
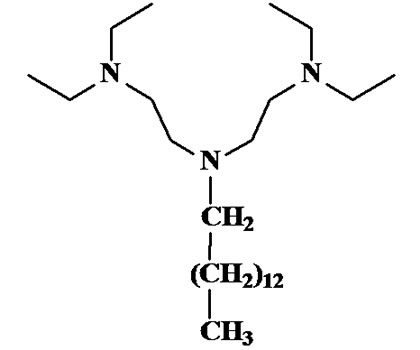
Scheme 1. Structure of 1,1,7,7-tetraethyl-4-tetradecyldiethylentriamine (TE14DT).
TE14DT solution in chlorinated solvent or ionic liquid and 1 mL of a 5 × 10−3 M CuCl2 solution in 0.15 M acetate buffer, pH 4.68 were added. They were kept under stirring in a glycerin/water bath at constant temperature and, if not otherwise described in the text, metal ion concentration in aqueous phase was measured after 24 h, to allow the equilibrium to be reached. Then, Cu(II) ion concentration in the organic phase was calculated as the difference. In back-extraction experiments, after partition at 333.15 K, the upper aqueous phase was removed and replaced with a 0.1 M HCl solution; the biphasic system was kept either at 298.15 or at 333.15 K and metal ion concentration was monitored as a function of time. Each experiment was done in at least triplicate and results agreed to within 5%.
2.4. Water Content Determination
The water amount in ionic liquids was determined using a Metrohm 684 Karl Fischer coulometer. Equal volumes of ionic liquid and 0.15 M acetate buffer were kept in a glycerin/water bath at constant temperature. After equilibration, a few drops of ionic liquid were weighed, poured into a 1 mL volumetric flask and dissolved in water-equilibrated chloroform. A fixed volume of solution was then injected into the Karl Fischer coulometer and a reading of water content was obtained.
2.5. Viscosity Measurements
The viscosity of ionic liquids was measured with a Brookfield Dial Reading viscosimeter RVT model. For each analysis, the sample was equilibrated with 0.15 M pH = 4.68 acetate buffer for 24 h and then kept in a glycerin/water bath at constant temperature; the measurements were performed in duplicate and results agreed to within 0.01 Pa∙s.
2.6. Synthesis of 1-Methyl-3-alkylimidazolium Bromide
In an oven-dried 500 mL-flask with ground glass stopper, 1-methylimidazole (freshly distilled), the proper alkyl bromide in 1:1.05 molar ratio and ethyl acetate (150 mL) were added. The reaction mixture, clear and colorless, was kept at T = (333.15 to 343.15) K in an oil bath under magnetic stirring. After nearly 30 min the mixture starts to become turbid and gradually, a second, pale yellow liquid phase formed. These conditions were kept until the upper liquid phase turned out perfectly clear (about 12 h). Then, it was removed and the remaining liquid phase was washed two times with ethyl acetate (100 mL). Sovent traces were removed under vacuum (6.5 Pa) at 353.15 K for the night. A quite viscous liquid was finally obtained (yields: 95% - 96%).
1H-NMR (CD3OD, 200 MHz)
[C6MIM] [Br] δ (ppm): 0.84 (t, J = 7.3 Hz, 3H, CH3); 1.11 - 1.30 (m, 6H, 3CH2); 1.83 - 1.88 (m, 2H, CH2); 3.87 (s, 3H, N-CH3); 4.16 (t, J = 7.4 Hz, 2H, N-CH2); 7.52 (d, J = 1.8 Hz, 1H, CH); 7.59 (d, J = 1.8 Hz, 1H, CH); 8.96 (s, 1H, N-CH-N).
[C8MIM] [Br] δ (ppm): 0.87 (t, J = 7.3 Hz, 3H, CH3); 1.09 - 1.29 (m, 10H, 5CH2); 1.81 - 1.87 (m, 2H, CH2); 3.85 (s, 3H, N-CH3); 4.15 (t, J = 7.4 Hz, 2H, N-CH2); 7.51 (d, J = 1.8 Hz, 1H, CH); 7.59 (d, J = 1.8 Hz, 1H, CH); 8.95 (s, 1H, N-CH-N).
2.7. Synthesis of 1-Methyl-3-alkylimidazolium Hexafluorophosphate
The obtained bromide (200 g) was dissolved in ultra pure water (the resistance was > 15 MW cm) (500 mL); the solution was then transferred in a 1 L separatory funnel and washed two times with ethyl ether (200 mL). After removal of ether, the solution was transferred in a 1 L round-bottomed flask equipped with magnetic stirrer and cooled in a water/ice bath. At r.t. aqueous solution of hexafluorophosporic acid (120 mL, 60% w/w) was then added to the stirred solution through a separatory funnel and the mixture was stirred at r. t. for about 2 h. The aqueous phase was extracted several times with dichloromethane and the product recovery was then obtained. The organic phases are then collected and washed with deionized water (100 mL) until a neutral pH was reached. Then, it was dried, kept at 373.15 K and under vacuum (6.5 Pa) overnight. A nearly colorless liquid was finally obtained (yields: 92% - 95%). A test for bromide presence gave a negative result.
2.8. Synthesis of 1-Methyl-3-octylimidazolium Bis[(Trifluoromethyl)Sulfonyl]imide
1-Octyl-3-methylimidazolium bromide (0.151 mol, 41.7 g) was dissolved in ultra pure water (200 mL), placed in a 1 L separatory funnel and washed three times with ethyl ether (200 mL). Ether was removed and decolorizing carbon was added to the clear, pale yellow aqueous solution; further filtration followed. The colorless aqueous phase was then transferred into a 1 L flask and a water solution of lithium bis[(trifluoromethyl) sulfonyl]imide (0.166 mol, 200 mL) was added. The formation of another liquid phase was then observed; the mixture was stirred for 1 h and the obtained 1-octyl-3-methylimidazolium bis [(trifluoromethyl)sulfonyl] imide was extracted with ethyl ether. The organic layer was washed with ultra pure water until test for bromide presence gave a negative result. The organic solution was then concentrated on a rotary evaporator until a constant weight was reached. Then, it was dried, kept at 373.15 K and under vacuum (6.5 Pa) overnight. Yield of this colorless liquid is 93%.
3. Results and Discussion
3.1 Cu(II) Extraction in Chlorinated Solvents
Extractive features of TE14DT were studied as a function of the working temperature and comparisons between molecular and ionic solvents could be accomplished. The high thermal stability and the negligible vapour pressure of ionic liquids allow such investigations in a wider temperature range as compared to dichloromethane (b.p. = 312.75 K) i.e. the solvent so far used in the membrane transport experiments with TE14DT [6,7]. Therefore, some chlorinated solvents were selected on the basis of their lower volatility: chloroform (b.p. = 334.35 K), 1,2-dichloroethane (b.p. = 356.65K), 1,1,1- trichloroethane (b.p. = 347.25 K) and tetrachloromethane (b.p. = 349.65 K) and they were used as solvents in biphasic extraction systems. In any cases, no extraction of Cu(II) was observed in the absence of the chelating agent. In order to preliminarily evaluate the partitioning capability of TE14DT towards Cu(II) ions in the different organic phases, experiments at 298.15 K were carried out by simply contacting equal volumes of chlorinated solvents containing TE14DT and a buffered CuCl2 aqueous solution. The results, expressed as percentages of Cu(II) ions transferred in the organic phase after 24 hours, are shown in Table 1.
As already reported [6], the nature of the organic phase strongly affected the extraction efficiency of the complexing agent and a rationale was provided by considering the different polarities of the solvents. As showed in Table 1, no partitioning of Cu(II) ions was achieved in tetrachloromethane while the TE14DT-mediated transfer in dichloromethane reached the highest value, i.e. 45%.
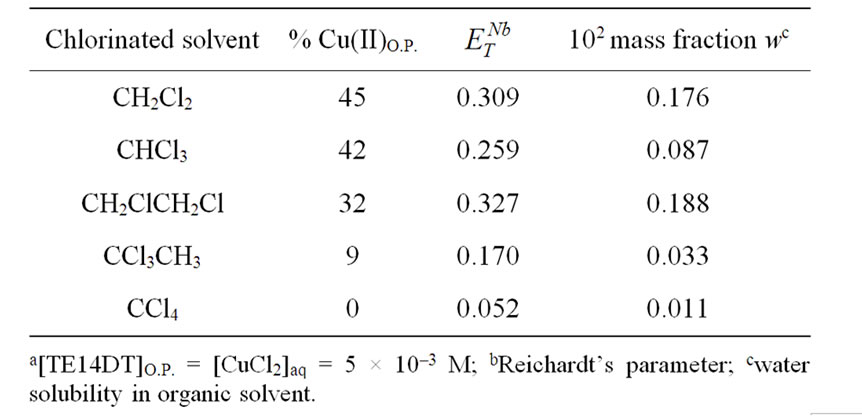
Table 1. Mol percentages of Cu(II) ions partitioned in the organic phase (O. P.) at 298.15 K after 24 h.a
To explain the observed trend the Reichardt’s parameter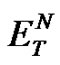 , the most widely used empirical scale of solvent polarity and the mass fraction w, indicating the water solubility in the organic solvent, were selected and their values are also reported in Table 1. Both the parameters likely play a relevant role in the metal ion extraction process and they could be correlated with the obtained data. In fact, the notable extraction efficiency of TE14DT towards Cu(II) ions in dichloromethane, chloroform and 1, 2-dichloroethane could be linked to their high values of
, the most widely used empirical scale of solvent polarity and the mass fraction w, indicating the water solubility in the organic solvent, were selected and their values are also reported in Table 1. Both the parameters likely play a relevant role in the metal ion extraction process and they could be correlated with the obtained data. In fact, the notable extraction efficiency of TE14DT towards Cu(II) ions in dichloromethane, chloroform and 1, 2-dichloroethane could be linked to their high values of 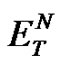 and w, while, in the case of the other two solvents, the low polarity and water solubility could explain the small extent of the metal ion partitioning. The polarity of the medium and the properties of dissolved water could allow the amphiphilic TE14DT-Cu(II) complex to interact more efficiently with the organic solvent and to partition more effectively in it.
and w, while, in the case of the other two solvents, the low polarity and water solubility could explain the small extent of the metal ion partitioning. The polarity of the medium and the properties of dissolved water could allow the amphiphilic TE14DT-Cu(II) complex to interact more efficiently with the organic solvent and to partition more effectively in it.
The extraction mechanism is an extremely complex issue and then a strict correlation between experimental data and a single chemico-physical solvent parameter could hardly be found. In fact, Cu(II) partitioning in the organic phase results from a number of chemical equilibria involving several different species. In detail, it should be considered in the process: i) the partitioning equilibria of both TE14DT and TE14DT-Cu(II) complex between the two phases, ii) the possible different stoichiometric ratios between the ligand and the metal ion and then the possibility of chelating multi-equilibria, and finally iii) the requirement of charge balance in the system. The latter involves the co-extraction of an accompanying anion, i.e. the chloride ion derived from the salt and/or the acetate from the buffer; the differences in organic solvents should affect the solubility of both the ionic species and the water.
On the basis of the above-reported results at 298.15 K, chloroform, 1,2-dichloroethane and 1,1,1-trichloroethane were selected to investigate the temperature effect on Cu(II) ions partitioning; dichloromethane was ruled out owing to its too low boiling point. In the examined temperature range, no partition of Cu(II) ions was observed in the absence of TE14DT and then the ligand-mediated transfer of Cu(II) ions in the organic phase was quantitatively determined, after 24 h, as a function of working temperature (Figure 1).
The features of chlorinated solvents deeply affected not only the partitioning extent at each temperature value, as already observed at 298.15 K, but also the relationship between the mol percentages of partitioned metal ion and the temperature itself. In 1,1,1-trichloroethane, the small extraction efficiency of TE14DT kept on diminishing as the temperature increased; partitioning of Cu(II) ions reached values less than 5% and it remained about constant in the range of T = (303.15 to 333.15) K. An in
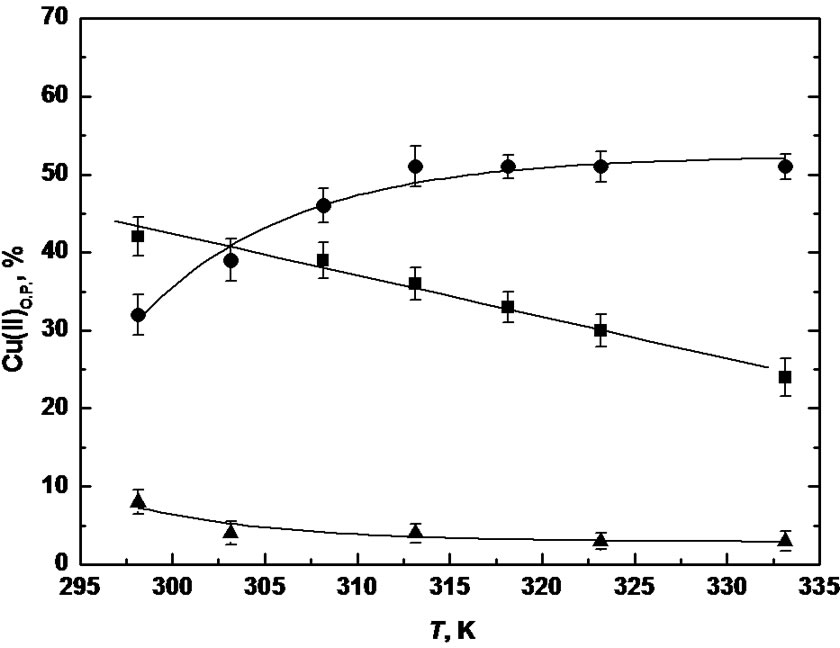
Figure 1. Mol percentages of Cu(II) ions in organic phase (O. P.) as a function of working temperature after 24 h; [CuCl2]aq = 5 × 10–3 M in 0.15 M pH 4.68 acetate buffer; [TE14DT] = 5 × 10–3 M in chloroform (■), 1,2-dichloroethane (●) and 1,1,1-trichloroethane (▲).
verse proportion between metal ion extraction and temperature was found also in chloroform and, in this case, the decrease in the metal ion partitioning extent with the temperature followed a linear trend. The extraction of Cu(II) ions turned out to be much different in 1, 2-dichloroethane instead: metal ion partitioning steadily increased in the range of T = (298.15 to 313.15) K up to 50% extraction; then a plateau was reached and no significant differences in the extraction percentages were observed with further increases in working temperature. So far, a 1:1 ligand metal ion molar ratio was employed; therefore, the lack of a temperature effect above 313.15 K was hypothesized to be due to an insufficient amount of the complexing agent in the organic phase. Then, TE14DT concentration in 1,2-dichloroethane was doubled and not only higher partitioning percentages were observed at all temperature values but a gradual increase in metal ion extraction was observed in the whole investigated temperature range (data not shown).
Parameters of organic solvents reported in Table 1 may be not adequate to explain the observed opposite trends in chloroform and in 1,2-dichloroethane, since, for example, a higher water solubility in all organic solvent could be recorded as temperature increased. The temperature rise, however, can lead to a different increase of water content in the various solvents, according to the nature of the solvent itself. Since the extraction efficiency has different trends for the various solvents as a function of temperature, it means that it is not simply water content, which controls the partitioning, but the way the water and the complex TE14DT-Cu(II) interact with solvent molecules.
Moreover, complex formation takes place in the aqueous phase, and then the temperature effect on the corresponding equilibrium should be the same in all considered systems; so, our results might be related to a different temperature effect on the partitioning equilibria of the ligand and its metal ion-complex in the various chlorinated phases.
At last, after partitioning at 333.15 K, biphasic systems were put at 298.15 K again and the extraction percentages reverted to the beginning values within 24 h, as it could be expected in the case of a reversible reaction.
3.2. Cu(II) Extraction in Ionic Liquids
In literature, Cu(II) extraction in ionic liquids by differrent chelating agents has been already reported [10,11]. In our previous study [12], concerning the extraction of Hg(II) ions in ionic liquids in absence of chelating agent, the key role of both working temperature and ionic liquid hydrophobicity has been pointed out. The aim of this paper is to investigate if, also in presence of a chelating agent, the above factors still play a significant role. Here, three imidazolium-based ionic liquids were used as “alternative” media in liquid/liquid extraction systems, i.e. 1-hexyl-3-methylimidazolium and 1-octyl-3-methylimidazolium hexafluorophosphate ([CnMIM] [PF6]) and 1-octyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl] imide ([C8MIM] [Tf2N]). They were selected so that the anionic components were hydrophobic enough to be water-immiscible and to obtain biphasic systems; moreover, imidazolium-based ionic liquids are by far the most employed in extraction processes [1]. Then, the use of such ionic liquids could allow us to evaluate both the role of the alkyl chain length on the imidazolium ring and of the anionic component; structures and abbreviations are reported in Scheme 2.
Preliminary partitioning tests in the absence of chelating agent revealed that no extraction of Cu(II) ions was obtained, despite of the similar ionic nature of both the extracting phase and the substrate. Then, the coordinating properties of TE14DT in ionic liquids were assayed; usually, in similar reported experiments, metal ion extraction was performed by mixing equal volumes of ionic liquid and an aqueous solution of metal ion at pH = 7, shaking for a few minutes and then centrifuging to separate the two phases [13,14]. In our procedure [12], liquid/liquid extraction was carried out by contacting equal volumes of ionic liquid and the CuCl2 buffered aqueous solution, kept under stirring at constant temperature. This method (the same used in the previous section) offered several advantages, including the possibility to control the working temperature. At first, the temperature effect on metal ion extraction by TE14DT was studied in [C8MIM] [PF6] and [C8MIM] [Tf2N] and the mol percentages of Cu(II) in the membrane after 24 h are shown in Figure 2.
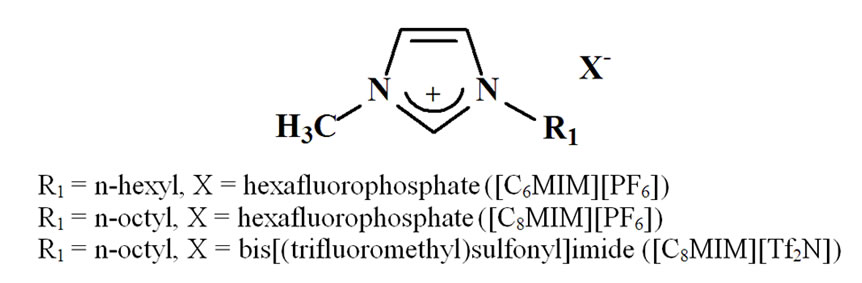
Scheme 2. Structure and abbreviations of the selected ionic liquids.

Figure 2. Mol percentages of Cu(II) as a function of working temperature in [C8MIM] [PF6] (●) and [Tf2N] (■) after 24 h. [TE14DT]I.L. = [CuCl2]aq. = 5×10–3 M in 0.15 M pH 4.68 acetate buffer.
A positive, non-linear relationship between extraction percentages in ionic liquids and temperature was observed, with quite different patterns as compared with those obtained in chlorinated solvents. In detail, at the lowest investigated temperatures (277.15 K in [C8MIM] [Tf2N] and 298.15 K in [C8MIM] [PF6]) Cu(II) ions partitioning was very low (less than 5% in both ionic liquids), comparable with the extraction in 1,1,1-trichloroethane, and then any rise in temperature produced a corresponding increase in metal ion partition; interestingly, at 333.15 K, the same extraction efficiency of TE14DT was gained in both ionic liquids, i.e. 45% of partitioned Cu(II) ions.
Similar trends were observed in both ionic liquids; despite they deeply differ not only in their chemicophysical properties but also in their airand water-stability [15]. Indeed, even water-immiscible RTILs are hygroscopic and usually contain some water. As regards hexafluorophosphate-containing ionic liquids, their hydrolysis instability has often been noted in literature [16-18] especially at high temperature and in the presence of strong acids. Hydrolysis of 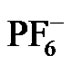 forms a mixture of products, including HF, POF3,
forms a mixture of products, including HF, POF3, 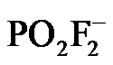 etc. [18], which could affect the extraction behaviour of TE14DT, with a clearly pH-sensitive chelating region. However, the salt aqueous solution used in the extraction experiments was buffered at pH 4.68 and even if the buffer solution/[C8MIM] [PF6] biphasic system was kept at 333.15 K no variation in pH value of the upper aqueous solution was detected. Moreover, the extraction efficiency of TE14DT should decrease at higher temperature, as a consequence of the protonation of N-atoms in its chelating region, while an opposite trend was actually observed. Furthermore, the same positive relationship between partition extent and working temperature was also found in a water-stable ionic liquid as [C8MIM] [Tf2N].
etc. [18], which could affect the extraction behaviour of TE14DT, with a clearly pH-sensitive chelating region. However, the salt aqueous solution used in the extraction experiments was buffered at pH 4.68 and even if the buffer solution/[C8MIM] [PF6] biphasic system was kept at 333.15 K no variation in pH value of the upper aqueous solution was detected. Moreover, the extraction efficiency of TE14DT should decrease at higher temperature, as a consequence of the protonation of N-atoms in its chelating region, while an opposite trend was actually observed. Furthermore, the same positive relationship between partition extent and working temperature was also found in a water-stable ionic liquid as [C8MIM] [Tf2N].
After the partitioning of the metal ion in both ionic liquids at 333.15 K, a thermal-reversible process was also tested by keeping the biphasic systems at room temperature. In [C8MIM] [PF6], only a slow transfer of the metal ions from the ionic liquid to the aqueous phase was observed and even after a week Cu(II) ion percenttage in the ionic liquid was still noticeably higher than the expected value at 298.15 K (about 15% versus 3%); then in [C8MIM] [PF6] the extraction process has a trend to hysteresis. On the contrary, complete back-extraction from [C8MIM] [Tf2N] to the aqueous solution was achieved in 24 h, being the metal ion partition in the ionic liquid equal to 12% again. Anyway, in both cases, if temperature was once more raised up to 333.15 K, extraction percentages get back to the initial value, i.e. 45, in only 24 h. On the basis of these initial observations, it is possible to assert that, at low temperatures, the nature of the anion of the ionic liquid further controls the extraction process in relation to its different way of interacting with the imidazolium cation and water molecules dissolved in it.
In order to explain such experimental data, some physico-chemical parameters of ionic liquids were taken into account, in particular the mutual water/ionic liquid miscibility and the viscosity. Moreover, the hydrophilic/ hydrophobic behavior is also important for the solvation properties of ILs, since it can affect their ability to dissolve species of different nature, and investigation was extended to a comparison between [C8MIM] [PF6] and [C6MIM] [PF6].
Several researches were carried out on the water solubility in ionic liquids [19]; in particular, voltammetric studies have suggested that the addition of controlled amounts of water to water-immiscible ILs can produce nano-dishomogeneity [20,21]. Therefore, wet ionic liquids should not be considered as homogeneous structures (isotropic medium) but have to be regarded as nanostructures with polar and non-polar regions. This could strongly affect the solvation not only of an amphiphilic molecule as the chelating agent TE14DT (with a long, hydrophobic alkyl chain and a more polar region containing the aminic groups) but also of its metal ions complex. NOEs experiments on 1-butyl-3-methylimidazolium tetrafluoroborate also revealed a progressive change in the IL structure with the increase of water content: cation–anion interactions are probably progressively replaced with hydrogen bonds involving water as acceptor towards the cation and as donor towards the anion [22]. Moreover, in the presence of water, the IL has a different organization characterized by a looser imidazolium–imidazolium association. An increase in water content could aid the partitioning of the TE14DT-Cu(II) complex thanks to a more favorable environment, where the above mentioned looser cation–cation association could enlarge the complex solubility in the ionic liquid phase. Water content in [C8MIM] [PF6] and in [C8MIM] [Tf2N] was measured (data not shown) and it was found that the higher the temperature, the higher the water amount solubilized in both ILs. In particular, not only the water content in [C8MIM] [PF6] was higher than that of [C8MIM] [Tf2N] at any investigated temperature, but also the effect of temperature on water percentages was more marked in [C8MIM] [PF6] than in [Tf2N]-derivative, being the relative increase of about 50% and 30% respecttively from 303.15 to 333.15 K. The different hydrophobicity and ability to form hydrogen bond of the anionic component of ionic liquids could be responsible for the just described results.
To date, temperature-dependence of viscosity of ionic liquids has been extensively investigated. In fact, the knowledge of viscosity is of prime importance in a wide range of different applications and especially in liquid/ liquid extraction systems: it can have a great concern in diffusion-controlled processes and in the mass transport of ionic species through the phases. In literature, viscosity data on 1-alkyl-3-methylimidazoliun hexafluorophosphate ionic liquids display a broad dishomogeneity [23-31]. In particular, the water content or simply the wetting or drying conditions seem to greatly influence the viscosity [30,32]; as regards [C6MIM] and [C8MIM] [PF6], it has been reported that their viscosity was about 25% higher in dried than in water equilibrated states [23]. Most data refer to dried conditions and mean values at 298.15 K vary in the range of η = (0.550 to 0.600) Pa∙s and η = (0.600 to 0.700) Pa∙s for [C6MIM] and [C8MIM] [PF6], respectively. Conversely, literature data on [C8MIM] [Tf2N] are much more consistent, being viscosity measurements steadily about 0.09 Pa∙s at 298.15 K [25,26,31]. In fact, water content in this ionic liquid is always lower than the corresponding hexafluorophosphate-derivative and then a looser influence of wetting or drying conditions is reported. Viscosity of [C8MIM] [PF6] and [C8MIM] [Tf2N] was measured at different temperatures and under the same operating conditions employed in the partitioning experiments, i.e. after equilibration of the ionic liquids with the buffered solution used to solubilize the metal salt; the obtained results are shown in Figure 3. Viscosity of both ionic liquids significantly decreased with increasing temperature. Viscosity–temperature dependence is commonly described by the Arrhenius equation (for non-associating electrolytes) [33] or by the Vogel-Tamman-Fulcher (VTF) equation (commonly used for glass forming RTILs) [34,35]. In our case, obtained plots were not accurately described by either Arrhenius or VTF models and, as already reported in other papers, such behaviour is usual for ionic liquids whose cations are less symmetric and have high molar mass [36].
Furthermore, as expected on the basis of literature data [30], a [Tf2N]-derivative ionic liquid has much lower viscosity than the [PF6]-salt at any investigated temperature values. As is known, the charge delocalization on the anion aids lower viscosity by weakening hydrogen bonding with the cation [37]. For instance, at 298.15 K, viscosity of [C8MIM] [PF6] is five-fold higher than [C8MIM] [Tf2N] while at higher temperatures (around 335 K) the viscosity tends to reach similar values.
Therefore, temperature effects on two chemicophysical properties, i.e. viscosity and water content, and on Cu(II) extraction efficiency of TE14DT, previously reported in Figure 2, are much more evident in the presence of PF6 anion with respect to Tf2N. In both systems water content and viscosity affect complex partition that became similar as viscosity also became similar. In detail, viscosity measurements indicate that an increase of both temperature and water content cause a change of the degree of ionic liquid isotropy/anisotropy. When viscosity decreased, it can be assumed that the water confined into polar nano-domains, changes its properties, with a consequent increase of ionic liquid nano-heterogeneity. Moreover, the higher the water content, the less the interaction

Figure 3. Temperature effect on [C8MIM] [PF6] (●) and [C8MIM] [Tf2N] (■) viscosity.
between the cation and the anion and such effect will be more evident for  than for Tf2N–. The lower extraction capability of TE14DT in [C8MIM] [PF6] with respect to [Tf2N]-derivative, at least at lower temperatures, could be explained by assuming that it is not simply the absolute water content that controls the efficiency of extraction. At 333.15 K, i.e. when the viscosity of the two ionic liquids became similar, owing to both the increase in temperature and the concomitant increase in water content, the ligand extraction capability is almost similar. The nature of the anion thus became less and less important and it can therefore hypothesize that both ionic liquids produce very similar polar nano-domains, i.e. similar chemical and physical properties, and then similar interactions with both the chelating agent and the complex.
than for Tf2N–. The lower extraction capability of TE14DT in [C8MIM] [PF6] with respect to [Tf2N]-derivative, at least at lower temperatures, could be explained by assuming that it is not simply the absolute water content that controls the efficiency of extraction. At 333.15 K, i.e. when the viscosity of the two ionic liquids became similar, owing to both the increase in temperature and the concomitant increase in water content, the ligand extraction capability is almost similar. The nature of the anion thus became less and less important and it can therefore hypothesize that both ionic liquids produce very similar polar nano-domains, i.e. similar chemical and physical properties, and then similar interactions with both the chelating agent and the complex.
The viscosity of the organic phase does not affect the amount of extracted metal ion at equilibrium, but the kinetics of process. Therefore, at room temperature, diffusive processes and mass transport through the phases turned out to be much more hindered in [C8MIM] [PF6] and longer time was required to reach the equilibrium condition, thus explaining the previously described differences in the back-extraction behaviour.
In order to elucidate also the role of idrophobicity of cationic component, [C6MIM] [PF6] was used in liquid/ liquid extraction experiments at different working temperatures; data, along with the viscosity measurements, are shown in Table 2, where they are compared with data of [C8MIM] [PF6].
It should be noted that viscosity values of [C6MIM] [PF6] were considerably lower than the literature ones [23,26]. It has been reported that the presence of contaminants such as chloride and/or water could signifycantly alter RTILs viscosity and, in particular, chloride contaminated RTILs show higher viscosities, attributed to hydrogen bonding between the cation of ionic liquid and chloride anion, while water contaminated RTILs usually have lower viscosity [32]. In our case, the especially

Table 2. Mol percentages of Cu(II) ions partitioned in [C6MIM] [PF6] after 24 h and IL viscosity measurements as a function of working temperature.b
bExtraction conditions: [TE14DT]I.L. = [CuCl2]aq. = 5 × 10–3 M in 0.15 M pH 4.68 acetate buffer (data referred to [C8MIM] [PF6] are displayed only for comparison purpose).
low viscosity obtained in [C6MIM] [PF6] is probably due both to the presence of trace of water and to the absence of chloride impurities (see Experimental Section).
At 298.15 K very low partition of metal ions was observed also in [C6MIM] [PF6], despite its viscosity was about half of the viscosity of the octyl chain homologue. On the contrary, at high temperature, viscosities of ionic liquids were basically the same, while extraction efficiency of TE14DT towards Cu(II) ions was significantly different and higher in [C8MIM]- than in [C6MIM]-based ionic liquid. In this case, being the anion the same, the hydrophobicity of the cation plays a major role in controlling the characteristics and the properties of nanodomains, almost at the highest temperature.
Therefore, a role of alkyl chain length on imidazolium ring should be assumed in the extraction process: hydrophobic interactions probably occurred between such alkyl chains and the lipophilic region of the chelating agent, aiding the extraction process itself. These results seemed to suggest that, being the cation the same, a very striking relationship exists between a physical parameter of ionic liquids and the metal ion extraction capability of a chelating agent. A main role in affecting the extraction efficiency is probably played by water into the ionic liquid, not merely by the amount of water but by its properties and its ability in structuring the medium. A further experimental evidence in favour of this hypothesis is that percentages of Cu(II) at 298.15 K in [C8MIM] [PF6] depend on the “history” of the sample.
Finally, in view of a continuous transport process, the back stripping of Cu(II) ions partitioned in the ionic liquid phase was attempted. After the extraction of the metal ion in [C8MIM] [PF6] at 333.15 K, the aqueous phases were removed and replaced with a 0.1 M HCl solution to promote the protonation of the ligand N-atoms and the subsequent release of metal ion from the ionic liquid. Then, the biphasic system was kept at two differrent temperature values, 298.15 and 333.15 K, and metal ion concentration in the aqueous phase was measured at different times; results are reported in Figure 4 where Cu(II) ion percentages are referred to the amount of the metal ion partitioned in the ionic liquid. In both experimental conditions, the complete back-extraction of Cu(II) ions was achieved, even if process was strongly dependent on the temperature.
Indeed, at 298.15 K a slow increase in metal ion concentration in the aqueous phase was attained, being the whole extraction performed in two days, and the high viscosity of [C8MIM] [PF6] at room temperature could explain this result. On the contrary, 100% of Cu(II) ions was stripped in only 4 h when the higher working temperature was adopted, providing very interesting perspectives to the development of a continuous transport
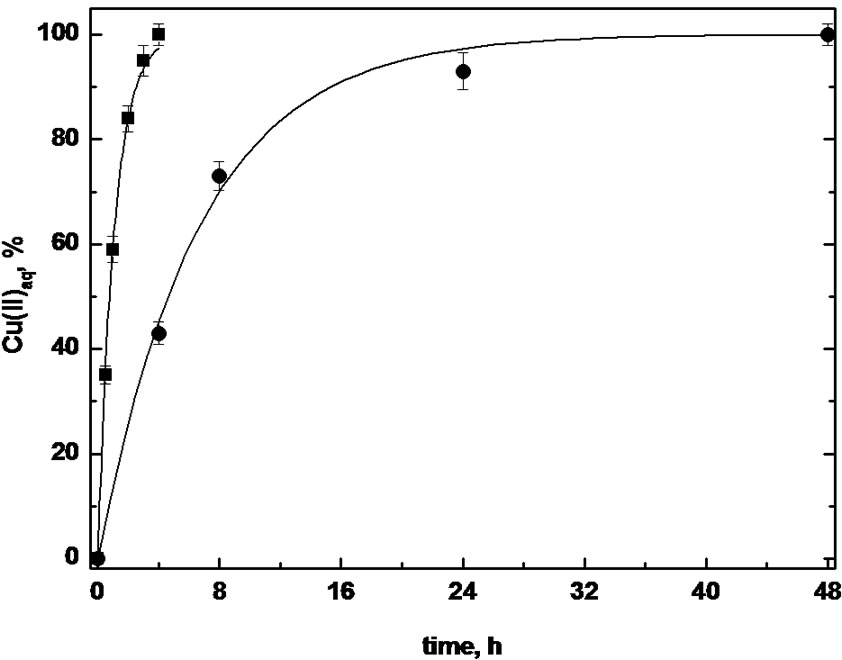
Figure 4. Mol percentages of back-stripped Cu(II) ions from [C8MIM] [PF6] in the pH = 1 aqueous phase at 298.15 (●) and at 333.15 K (■) as a function of time.
process by using an ionic liquid as membrane.
4. Conclusions
The feasibility of biphasic systems based on imidazolium-type room temperature ionic liquids has been here displayed. The chelating properties of the lipophilic polyamine TE14DT towards Cu(II) ions were assessed both in chlorinated solvents and in ionic liquids and the comparison highlighted deep differences not only in the partitioning extent under the same conditions but also in the temperature effect on extraction efficiency. Some features of the chlorinated solvents were found to be correlated with the achieved results: in particular, the higher the values of the Reichardt’s parameter and the water solubility in the organic phase, the larger the metal ion partitioning extent. Relationships between extraction percentages in ionic liquids and temperature were instead quite different with respect to chlorinated solvents. When the cation is the same, the viscosity of ionic liquids, the water content and the metal ion extraction capability of the chelating agent were found to be related; moreover, the chemical nature of the IL cation also seemed to be important in determining the efficiency of the extraction process and in particular the alkyl chain length on imidazolium ring seemed to play a relevant role. Water content in chlorinated solvent and in ionic liquids is very important and plays a crucial role in Cu(II) partioning efficiency, although there is no simple correlation.
Finally, stripping tests from ionic liquids showed the possibility of a back-extraction process with simple variations in the operating conditions and the possibility to effectively substitute bulk organic solvents with the “novel” media ionic liquids was then displayed, with many advantages in terms of recyclability, thermal stability and poor biodegradability. The presented results provided attractive insights for the development of one-step extraction/recovery systems based on ionic liquids along with a rational comparison with conventional molecular solvents.
5. Acknowledgements
Support of the Ministero dell’Università e Ricerca (MIUR), (PRIN 2008, grant number 2008AZT7RK_002).
6. References
[1] R. Liu, J. F. Liu, Y. G. Yin, X. L. Hu and G. B. Jiang, “Ionic Liquids in Sample Preparation,” Analytical and Bioanalytical Chemistry, Vol. 393, No. 3, 2009, pp. 871-883. doi:10.1007/s00216-008-2445-6
[2] H. Zhao, S. Xia and P. Ma, “Use of Ionic Liquids as ‘Green’ Solvents for Extractions,” Journal of Chemical Technology & Biotechnology, Vol. 80, No. 10, 2005, pp. 1089-1096. doi:10.1002/jctb.1333
[3] V. A. Cocalia, J. D. Holbrey, K. E. Gutowski, N. J. Bridges and R. D. Rogers, “Separations of Metal Ions Using Ionic Liquids: The Challenges of Multiple Mechanisms,” Tsinghua Science & Technology, Vol. 11, No. 2, 2006, pp. 188-193. doi:10.1016/S1007-0214(06)70174-2
[4] G.-T. Wei, J.-C. Chen and Z. Yang, “Studies on Liquid/ Liquid Extraction of Copper Ion with Room Temperature Ionic Liquid,” Journal of the Chinese Chemical Society, Vol. 50, No. 6, 2003, pp. 1123-1130.
[5] M. L. Dietz, “Ionic Liquids as Extraction Solvents: Where Do We Stand?” Separation Science and Technology, Vol. 41, No. 10, 2006, pp. 2047-2063. doi:10.1080/01496390600743144
[6] L. Brinchi, P. Di Profio, R. Germani, G. Savelli and N. Spreti, “Structurally Simple Lipophilic Polyamines as Carriers of Cupric Ions in Bulk Liquid Membranes,” European Journal of Organic Chemistry, Vol. 2002, No. 5, 2002, pp. 930-937. doi:10.1002/1099-0690(200203)2002:5<930::AID-EJOC930>3.0.CO;2-I
[7] L. Brinchi, R. Germani, M. V. Mancini, G. Savelli and N. Spreti, “Carrier-Mediated Transport of Toxic Heavy Metal Ions in Bulk Liquid Membranes,” European Journal of Organic Chemistry, Vol. 2004, No. 6, 2004, pp. 1330- 1335. doi:10.1002/ejoc.200300622
[8] N. Spreti, L. Brinchi, R. Germani, M. V. Mancini and G. Savelli, “A New Carrier for Selective Removal of Heavy Metal Ions from Aqueous Solutions through Bulk Liquid Membranes,” European Journal of Organic Chemistry, Vol. 2004, No. 18, 2004, pp. 3865-3871. doi:10.1002/ejoc.200400222
[9] N. Spreti, L. Brinchi, R. Germani, M. V. Mancini and G. Savelli, “Quantitative Removal of Mercury(II) from Water by Lipophilic Polyamines through Bulk Liquid Membranes,” European Journal of Organic Chemistry, Vol. 2006, No. 19, 2006, pp. 4379-4384. doi:10.1002/ejoc.200600249
[10] S. C. N. Hsu, C.-J. Su, F.-L. Yu, W.-J. Chen, D.-X. Zhuang, M.-J. Deng, I.-W. Sun and P.-Y. Chen, “Extracting Cu(II) from Aqueous Solutions with Hydrophobic Room-Temperature Ionic Liquid in the Presence of a Pyridine-Based Ionophore to Aattempt Cu Recovery: A Laboratory Study,” Electrochimica Act, Vol. 54, No. 6, 2009, pp. 1744-1751. doi:10.1016/j.electacta.2008.09.068
[11] K. Kidani, N. Hirayama and H. Imura, “Extraction Behavior of Divalent Metal Cations in Ionic Liquid Chelate Extraction Systems Using 1-Alkyl-3-methylimidazolium bis (trifluoromethansulfonyl) imides and thenoyltrifluoroacetone”, Analytical Sciences, Vol. 24, No. 10, 2008, pp. 1251-1254. doi:10.2116/analsci.24.1251
[12] R. Germani, M. V. Mancini, G. Savelli and N. Spreti, “Mercury Extraction by Ionic Liquids: Temperature and Alkyl Chain Length Effect,” Tetrahedron Letters, Vol. 48, No. 10, 2007, pp. 1767-1769. doi:10.1016/j.tetlet.2007.01.038
[13] E. Visser, R. P. Swatloski, W. M. Reichert, R. Mayton, S. Sheff, A. Wierzbicki, J. H. Davis and R. D. Rogers, “TaskSpecific Ionic Liquids Incorporating Novel Cations for the Coordination and Extraction of Hg2+ and Cd2+: Synthesis, Characterization, and Extraction Studies,” Environmental Science & Technology, Vol. 36, No. 11, 2002, pp. 2523-2529. doi:10.1021/es0158004
[14] E. Visser, R. P. Swatloski, W. M. Reichert, R. D. Rogers, R. Mayton, S. Sheff, A. Wierzbicki and J. H. Davis, “Task-Specific Ionic Liquids for the Extraction of Metal Ions from Aqueous Solutions,” Chemical Communications, No. 1, 2001, pp. 135-136. doi:10.1039/b008041l
[15] S. Keskin, D. Kayrak-Talay, U. Akman and O. Hortaçsu, “A Review of Ionic Liquids towards Supercritical Fluid Applications,” Journal of Supercritical Fluids, Vol. 43, No. 1, 2007, pp. 150-180. doi:10.1016/j.supflu.2007.05.013
[16] S. I. Nikitenko and P. Moisy, “Formation of Higher Chloride Complexes of Np(IV) and Pu(IV) in Water-Stable Room-Temperature Ionic Liquid [BuMelm][Tf2N],” Inorganic Chemistry, Vol. 45, No. 3, 2006, pp. 1235-1242.
[17] S. I. Nikitenko, C. Cannes, C. Le Naour, P. Moisy and D. Truber, “Spectroscopic and Electrochemical Studies of U(IV)-Hexachloro Complexes in Hydrophobic Room Temperature Ionic Liquids [BuMeIm][Tf2N] and [MeBu3N] [Tf2N],” Inorganic Chemistry, Vol. 44, No. 25, 2005, pp. 9497-9505. doi:10.1021/ic051065b
[18] R. Fernandez-Galan, B. R. Manzano, A. Otero, M. Lanfranchi and M. A. Pellinghelli, “19F and 31P NMR Evidence for Silver Hexafluorophosphate Hydrolysis in Solution. New Palladium Difluorophosphate Complexes and X-ray Structure Determination of [Pd(η3-2-Me-C3H4)(PO2F2) (PCy3)],” Inorganic Chemistry, Vol. 33, No. 10, 1994, pp. 2309-2312. doi:10.1021/ic00088a039
[19] C. Chiappe and D. Pieraccini, “Ionic Liquids: Solvent Properties and Organic Reactivity,” Journal of Physical Organic Chemistry, Vol. 18, No. 4, 2005, pp. 275-297. doi:10.1002/poc.863
[20] J. Dupont, R. F. de-Souza and P. A. Z. Suarez, “Ionic Liquid (molten salt) Phase Organometallic Catalysis,” Chemical Reviews, Vol. 102, No. 10, 2002, pp. 3667-3692. doi:10.1021/cr010338r
[21] J. Dupont, “On the Solid, Liquid and Solution Structural Organization of Imidazolium Ionic Liquids,” Journal of the Brazilian Chemical Society, Vol. 15, No. 3, 2004, pp. 341-350. doi:10.1590/S0103-50532004000300002
[22] A. Mele, C. D. Tran and S. H. De Paoli-Lacerda, “The Structure of a Room-Temperature Ionic Liquid with and without Trace Amounts of Water: The Role of C-H···O and C-H···F interactions in 1-N-butyl-3-me-thylimidazolium Tetrafluoroborate,” Angewandte Chemie International Edition, Vol. 42, No. 36, 2003, pp. 4364-4366. doi:10.1002/anie.200351783
[23] J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, “Characterization and Comparison of Hydrophilic and Hydrophobic Room Temperature Ionic Liquids Incorporating the Imidazolium Cation,” Green Chemistry, Vol. 3, No. 4, 2001, pp. 156-164. doi:10.1039/b103275p
[24] G. Fadeev and M. M. Meagher, “Opportunities for Ionic Liquids in Recovery of Biofuels,” Chemical Communications, Vol. 3, 2001, pp. 295-296.
[25] J. McLean, M. J. Muldoon, C. M. Gordon and I. R. Dunkin, “Bimolecular Rate Constants for Diffusion in Ionic Liquids,” Chemical Communications, No. 17, 2002, pp. 1880-1881. doi:10.1039/b202944h
[26] S. V. Dzyuba and R. A. Bartsch, “Influence of Structural Variations in 1-Alkyl(aralkyl)-3-methylimidazolium HexaFluorophosphates and Bis(trifluoromethyl-sulfonyl)imides on Physical Properties of the Ionic Liquids,” Chemical Physics, Vol. 3, No. 2, 2002, pp. 161-166. doi:10.1002/1439-7641(20020215)3:2<161::AID-CPHC161>3.0.CO;2-3
[27] K. R. Seddon, A. Stark and M. J. Torres, “Viscosity and Density of 1-Alkyl-3-methylimidazolium Ionic Liquids,” In: M. A. Abraham and L. Moens, Eds., Clean Solvents: Alternative Media for Chemical Reactions and Processing, American Chemical Society, Washington, 2002, pp. 34-49. doi:10.1021/bk-2002-0819.ch004
[28] C. F. Poole, “Chromatographic and Spectroscopic Methods for the Determination of Solvent Properties of Room Temperature Ionic Liquids”, Journal of Chromatography A, Vol. 1037, No. 1-2, 2004, pp. 49-82. doi:10.1016/j.chroma.2003.10.127
[29] J. F. Liu, J. A. Jonsson and G. B. Jiang, “Application of Ionic Liquids in Analytical Chemistry,” Trends in Analytical Chemistry, Vol. 24, No. 1, 2005, pp. 20-27. doi:10.1016/j.trac.2004.09.005
[30] J. Jacquemin, P. Husson, A. A. H. Padua and V. Majer, “Density and Viscosity of Several Pure and Water-Saturated Ionic Liquids,” Green Chemistry, Vol. 8, No. 2, 2006, pp. 172-180. doi:10.1039/b513231b
[31] P. S. Kulkarni, L. C. Branco, J. G. Crespo, M. C. Nunes, A. Raymundo and C. A. M. Afonso, “Comparison of Physicochemical Properties of New Ionic Lliquids Based on Imidazolium, Quaternary Ammonium, and Guanidinium Cations,” Chemistry—A European Journal, Vol. 13, No. 30, 2007, pp. 8478-8488. doi:10.1002/chem.200700965
[32] K. R. Seddon, A. Stark and M. Torres, “Influence of Chloride, Water, and Organic Solvents on the Physical Properties of Ionic Liquids,” Pure and Applied Chemistry, Vol. 72, No. 12, 2000, pp. 2275-2287. doi:10.1351/pac200072122275
[33] P. Bonhöte, A. Dias, N. Papageorgiou, K. Kalyanasundaran and M. Gratzel, “Hydrophobic, Highly Conductive Ambient-Temperature Molten Salts,” Inorganic Chemistry, Vol. 35, No. 5, 1996, pp. 1168-1178. doi:10.1021/ic951325x
[34] R. A. Carpio, L. A. King, R. E. Lindstram, J. C. Nardi and C. L. Hussey, “Density Electric Conductivity, and Viscosity of Several N-alkylpyridinium Halides and Their Mixtures with Aluminium Chloride,” Journal of the Electrochemical Society, Vol. 126, No. 10, 1979, pp. 1644- 1650. doi:10.1149/1.2128768
[35] J. R. Sanders, E. H. Ward and C. L. Hussey, “Aluminium Bromide-1-methyl-3-ethylimidazolium Bromide Ionic Liquids,” Journal of the Electrochemical Society, Vol. 133, No. 2, 1986, pp. 325-330. doi:10.1149/1.2108570
[36] O. O. Okoturo and T. J. Vander-Noot, “Temperature Dependence of Viscosity for Room Temperature Ionic Liquids”, Journal of the Electrochemical Society, Vol. 568, No. 1-2, 2004, pp. 167-181.
[37] Z. B. Zhou, H. Matsumoto and K. Tatsumi, “Low-Melting, Low-Viscous, Hydrophobic Ionic Liquids: 1-Aalkyl (alkylether)-3-methylimidazolium Perfluoroalkyl-tri-fluoroborate,” Chemistry—A European Journal, Vol. 10, No. 24, 2004, pp. 6581-6591. doi:10.1002/chem.200400533

