Soft Nanoscience Letters
Vol.3 No.4A(2013), Article ID:40418,3 pages DOI:10.4236/snl.2013.34A006
Electrochemical Synthesis of Titanium Nano Particles at Carbon Paste Electrodes and Its Applications as an Electrochemical Sensor for the Determination of Acetaminophen in Paracetamol Tablets
![]()
Department of P.G. Studies and Research in Industrial Chemistry, Kuvempu University, Shimoga, India.
Email: *utpalchemiitkgp@yahoo.com
Copyright © 2013 K. J. Gururaj, B. E. Kumara Swamy. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received October 1st, 2013; revised November 6th, 2013; accepted November 13th, 2013
Keywords: Titania Nano Particles; Electrochemical Sensor; Electrocatalysis; Cyclic Voltammetry; Electrochemical Impedance Spectroscopy
ABSTRACT
Titania nano particles were synthesized at carbon paste electrode by cyclic voltammetry and then it was employed for the determination of acetaminophen in phosphate buffer at pH 7.4. Carbon paste electrode with titania nano particle displayed excellent electrochemically catalytic activities by shifting the oxidation potential of acetaminophen towards the negative side. The mass transfer process at electrochemical interface was diffusion controlled. Electrochemical techniques such as, electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization methods were used to measure the resistance of the electrodes. The resistance of the titanium electrode decreased in two orders when compared to the bare carbon paste electrode; the decrease in the resistance of the electrode and increase in the surface area of the electrode are responsible for the negative shifting of the oxidation potential of acetaminophen. The present method was applied to the determination of actetaminophen in paracetamol tablet, urine and blood sample by using standard addition method and the obtained results were satisfactory with a good recovery of 98%.
1. Introduction
Acetaminophen/paracetamol (ACOP) is an active ingredient of several pharmaceutical products, widely used as an analgesic [1]. Acetaminophen overdose is a frequent cause of fulminating hepatic failure in Europe and the USA [2]. However, recent studies have shown that paracetamol is associated to hepatic toxicity and renal failure despite of its apparent innocuous character [3]. Voltammetric techniques are more selective, less timeconsuming and widely used for the determination of acetaminophen [4]. Carbon paste electrode (CPE), which is made up of carbon particles and silicon oil binder, has been widely applied in the electroanalytical community due to its low cost, ease of fabrication, high sensitivity for detection and renewable surface. To further improve the sensing properties of CPE they are modified by surfactants and nanoparticles. In this paper, a novel, simple, precise and sensitive, cyclic voltammetric method utilizing a tiatania nanoparticle modified carbon paste electrode for the detection of acetaminophen was reported. The method was applied successfully for the detection of acetaminophen in paracetamol tablets, urine and serum.
2. Experimental Part
2.1. Materials
0.05 g TiO2 dispersed in 25 ml of dimethylformamide in (Merck made), 0.04 M of glycine solution prepared in aqueous solution for modification of carbon electrode. ACOP was prepared 1 × 10−5 M stock solution was prepared by dissolving in distilled water solution. Phosphate buffer solution (PBS) was prepared sodium dihydrogen phosphate monohydrate and disodium hydrogen phosphate. Chemicals mentioned above were all of analytical grade. The water used in the preparation of solutions was double distilled water.
2.2. Apparatus
The electrochemical experiments were carried out using a CHI 660C. All experiments were carried out in a conventional three-electrode system.
2.3. Preparation of Bare Carbon Paste Electrode and Polyglycine TiO2 Carbon Paste Electrode (MCPE)
Bare carbon paste electrode was prepared as described [5], polyglycine TiO2 modified carbon paste electrode was prepared by dispersing TiO2 solution with 0.04 M glycine in pH7.4 PBS. Electropolymerisation was achieved by the formation of film that grew between −0.5 V and 1.8 V at the scan rate of 50 mV/s for 10 cycles using cyclic voltammetry as shown in Figure 1(a).
3. Results and Discussion
3.1. Cyclic Voltammetric Investigation of ACOP at MCPE
The voltammetric behavior of ACOP was examined using cyclic voltammetry. Figure 1 shows typical cyclic voltammograms of 1.0 × 10−5 M of ACOP, in PBS pH 7.4 at scan rate 50 mV·s−1 recorded at two different working electrodes BCPE (solid line) and MCPE (dashed). The MCPE shows significant increase in current (almost 9 folds) compare to BCPE and potential of
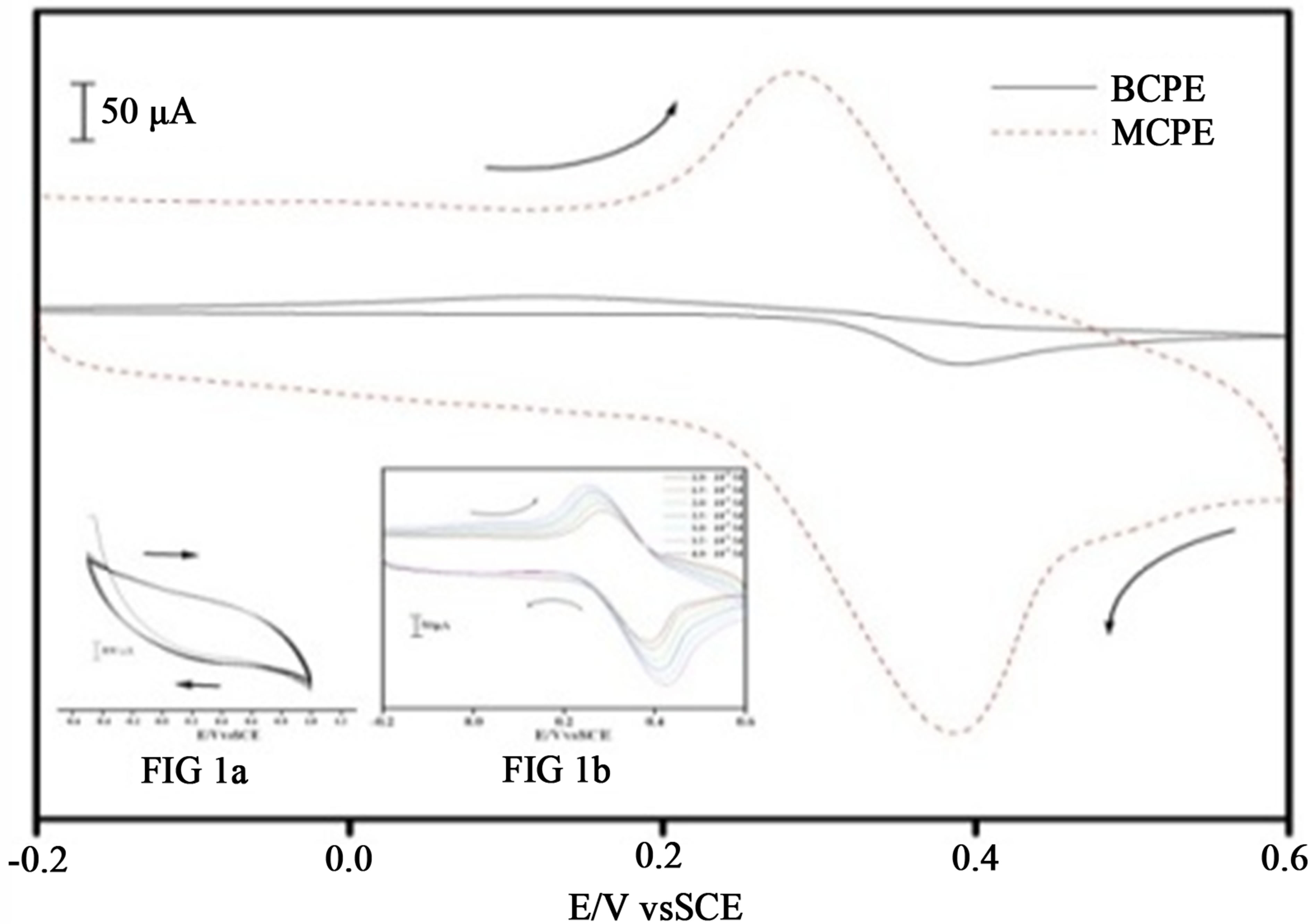
Figure 1. Cyclic voltammogram of 10 mM acetaminophen at BCPE (solid line) and TiO2/glycine MCPE (dotted line) in 0.1 M PBS pH 7.4 scan rate 50 mV·s−1 (a) Figure 1(a) Cyclic voltammogram for the electrochemical polymerisation of TiO2 dispersed glycine at carbon paste electrode (b) Figure 1(b) Cyclic voltammogram of acetaminophen at different concentration at TiO2/glycine MCPE in 0.1 M PBS of pH 7.
MCPE shifted to less positive when compare to BCPE, due to the improvement in the reversibility of the electron transfer process and improvement of surface area of the modified electrode. The MCPE resulted in an observable increase in the peak current, which indicated an improvement in the electrode kinetics and a decrease in the potential of oxidation substantially. The inset Figure 1(b) shows the effect of concentration variation of ACOP at MCPE.
3.2. Effect of Scan Rate on the Peak Currents of ACOP
The effect of scan rate for ACOP was studied by CV at MCPE (shown in Figure 2). MCPE showed increase in the redox peak current with increase in scan rate (50 - 100 mV·s−1) (Inset figure). The graph of current (Ipa) vs square root of scan rate (ν) was plotted. The graph obtained was good linearity between the scan rate and Ipa (Figure 3). In the range from 50 to 100 mV·s−1 the anodic peak currents were proportional to the scan rate (ν). The correlation coefficient was 0.9942, which indicate the electrode reaction was adsorption controlled (5).
3.3. Electrochemical Impedance Spectroscopy
Electrochemical impedance spectroscopy is considered to be an effective tool for investigating the property of electrode interface. Figure 3 shows the complex plane diagram (Nyquist plot, Z' versus Z''). The impedance behavior of the electrochemical impedance spectroscopy of BCPE and MCPE was studied in PBS of pH 7.4. The equivalent circuit used analyze the impedance behavior is shown in the inset figure. A semicircle with a large diameter is observed at the unmodified electrode, indicating the high charge resistance of BCPE (14.8 KΩ) and it
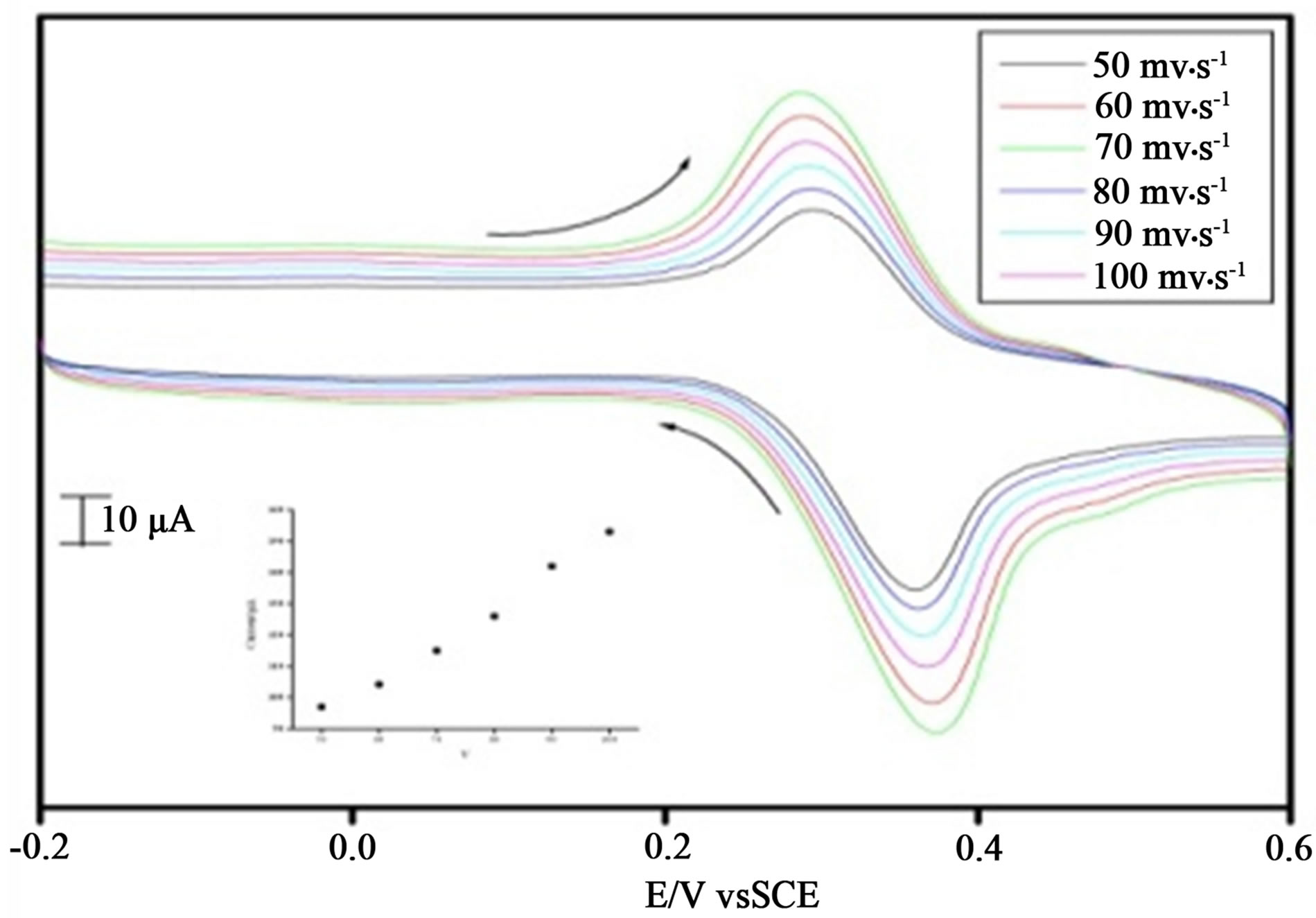
Figure 2. Cyclic voltammogram of different scan rate in the presence of 10 mM acetaminophen in PBS at pH 7.4, scan rate 50 mV·s−1 100 mV·s−1 inset figure Graph of anodic peak current v/s scan rate.
is difficult for the electron transfer at electrode surface. However, the diameter of semicircle diminishes significantly at MCPE. It was obvious that charge transfer resistance (6.7 KΩ) of the electrode surface decreased and the charge transfer rate sped up. Overall impedance study suggests on the surface of MCPE electron transfer is easy when compare to BCPE.
3.4. Detection of Acetaminophen in Tablets, Urine and Serum
In order to testify the performance of this sensor in real sample analysis, it was used to determine the content of ACOP in tablets. Then commercial pharmaceutical samples (tablets) containing ACOP was analyzed to evaluate the validity of the proposed method (results tabulated in Table 1), analysis of ACOP in urine was done by using procedure of [6] and in the same manner detection of ACOP in serum carried out (Table 2).
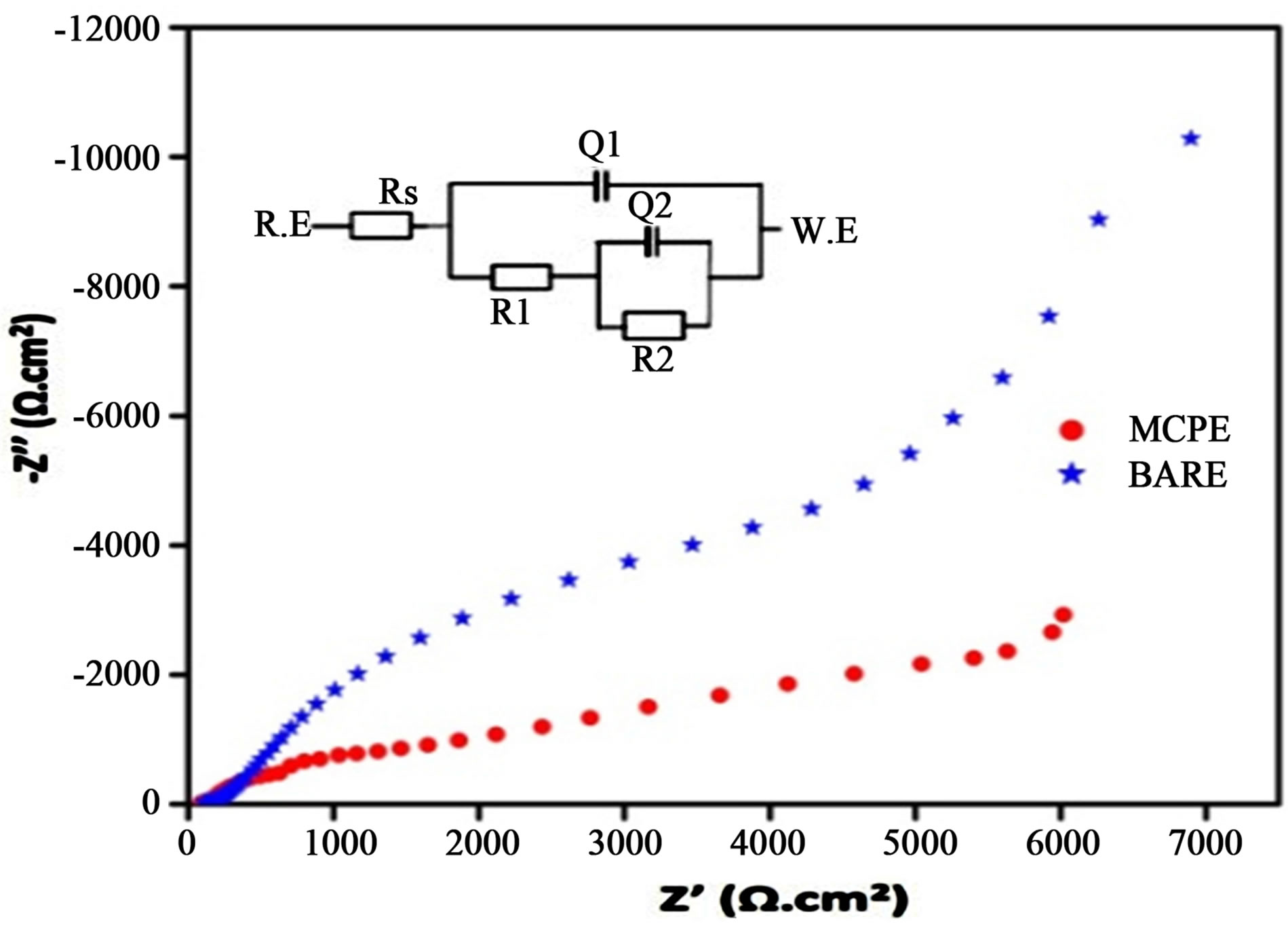
Figure 3. Electrochemical impedance spectroscopy (Nyquist plot) of BCPE and TiO2/glycine MCPE in PBS at pH 7.4.
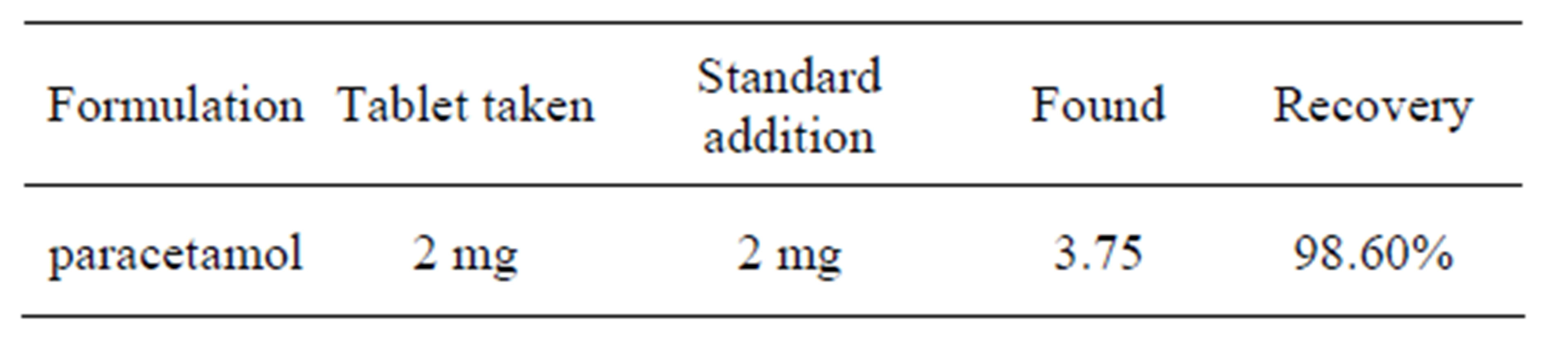
Table 1. Commercial pharmaceutical samples (1).
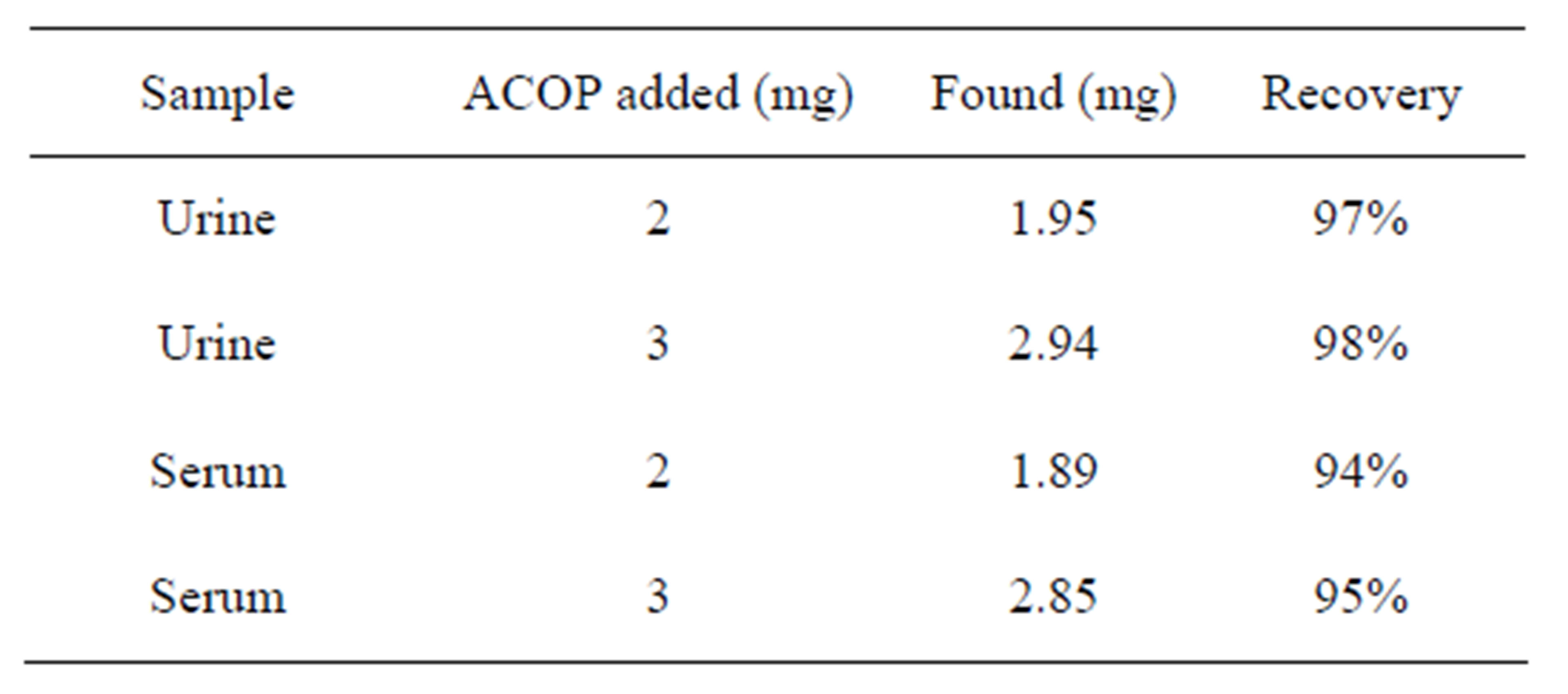
Table 2. Commercial pharmaceutical samples (2).
4. Conclusion
The poly glycine TiO2 was synthesized, and then it was employed for detection of ACOP electrochemically in serum, tablets and urine. The study reveals that, the MCPE shows as an excellent electrochemical sensor and the same method can be applied for organic and inorganic neurotransmitters.
5. Acknowledgements
We express our sincere thanks to Dr. B. N. Chandrashekar and Mr. C. C. Vishwanath research scholars Dept of industrial chemistry Kuvempu University for their constant support and suggestions.
REFERENCES
- R. Sandulescu, S. Mirel and R. Oprean, “The Development of Spectrophotometric Andelectroanalytical Methods for Ascorbic Acid Andacetaminophen and Their Applications in the Analysis Ofeffervescent Dosage Forms,” Journal of Pharmaceutical and Biomedical Analysis, Vol. 23, No. 1, 2000, pp. 77-87. http://dx.doi.org/10.1016/S0731-7085(00)00277-6
- X. D. ShangGuan, H. F. Zhang and J. B. Zheng, “Electrochemical Behavior and Differential Pulse Voltammetric Determination of Paracetamol at a Carbon Ionic Liquid Electrode,” Analytical Bioanalytical Chemistry, Vol. 391, No. 3, 2008, pp. 1049-1055. http://dx.doi.org/10.1007/s00216-008-2096-7
- I. Noviandri and R. Rakhmana, “Carbon Paste Electrode Modified with Carbon Nanotubes and Poly(3-aminophenol) for Voltammetric Determination of Paracetamol,” International Journal of Electrochemical Science, Vol. 7, No. 5, 2012, pp. 4479-4487.
- C. Y. Li, G. Q. Zhan, Q. D. Yang and J. J. Lu, “Electrochemical Investigation of Acetaminophen with a Carbon Nano-Tube Composite Film Electrode,” Bullatin of Korean Chemical Society, Vol. 27, No. 11, 2006, pp. 1854-1860.
- O. Gilbert, B. E. Kumara Swamy, U. Chandra and B. S. Sherigara, “Simultaneous Detection of Dopamine and Ascorbic Acid Using Polyglycine Modified Carbon Paste Electrode: A Cyclic Voltammetric Study,” Journal of Electroanalytical Chemistry, Vol. 636. No. 1-2, 2009, pp. 80-85. http://dx.doi.org/10.1016/j.jelechem.2009.09.016
- F. N. Atta, A. Galal and S. M. Azab, “Electrochemical Determination of Paracetamol Using Gold Nanoparticles —Application in Tablets and Human Fluids,” International Journal of Electrochemical Science, Vol. 6, No. 10, 2011, pp. 5082-5096.
NOTES
*Corresponding author.

