Journal of Diabetes Mellitus
Vol. 3 No. 2 (2013) , Article ID: 31380 , 7 pages DOI:10.4236/jdm.2013.32012
Use of pocket pulse oximeters for detecting peripheral arterial disease in patients with diabetes mellitus*
![]()
1Division of General Internal Medicine, Hospital Marina Baixa, Villajoyosa, Alicante, Spain; #Corresponding Authors: ena_jav@gva.es
2Division of Endocrinology and Metabolism, Hospital Marina Baixa, Villajoyosa, Alicante, Spain
3Division of Cardiology, Hospital Marina Baixa, Villajoyosa, Alicante, Spain
Copyright © 2013 Javier Ena et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 9 February 2013; revised 10 March 2013; accepted 18 March 2013
Keywords: Ankle-Brachial Pressure Index; Doppler Ultrasound; Pulse Oximetry; Comparative Study; Sensitivity; Specificity; Likelihood Ratio
ABSTRACT
Recent Aims: New diagnostic methods are needed to detect peripheral arterial disease easier than using the ankle-brachial index measured by Doppler devices. We investigated whether the use of pocket pulse oximeters could meet sensitivity and specificity criteria as screening method to detect significant peripheral arterial perfusion deficits. Methods: We measured oxygen saturation (SaO2) at index fingers and great toes (on horizontal and elevated 30˚) by a pocket pulse oximeter in 250 subjects with diabetes mellitus attending the outpatient clinic. A finger-to-toe SaO2 gradient greater than 2% was considered abnormal. Ankle-brachial index was measured by a hand held Doppler device. Peripheral arterial disease was defined as an ankle-brachial index less than 0.9. Results: A total of 1392 (93%) valid SaO2 readings were obtained. Twenty-seven (11%) patients were excluded due to not having measurable SaO2 finger-to-toe gradients. A total of 223 patients were analyzed. Peripheral arterial disease was detected in 47 (21%) patients. A finger-to-toe SaO2 gradient greater than 2% had sensitivity 42.6% (95% CI 30.0% - 55.3%), specificity 79.1% (95% CI 75.7% - 82.6%), positive predictive value 35.7% (95% CI 25.2% - 46.4%), negative predictive value 83.4% (95% CI 79.8 - 87.1), positive likelihood ratio 2.03 (95% CI 1.23 - 3.17) and negative likelihood ratio 0.73 (95% CI 0.54 - 0.93) to detect peripheral arterial disease. The area under the receiving operating characteristic curve was 0.69 (95% CI 0.62 - 0.77). Conclusion: Pocket pulse oximeters showed insufficient sensitivity as screening method for detecting peripheral arterial disease in patients with diabetes mellitus.
1. INTRODUCTION
Peripheral arterial disease is a leading cause of limb amputations in patients with diabetes [1]. Early identification of at-risk patients consists on foot examination and screening for neuropathy and peripheral arterial disease [2]. The ankle-brachial index is considered the screening method in the evaluation at office for peripheral arterial disease [3,4]. Measuring an ankle-brachial index requires a continuous-wave Doppler machine, ultrasonic gel, and a sphygmomanometer with a bloodpressure cuff. Systolic blood pressure is recorded at both brachial arteries and at both dorsalis pedis and posterior tibial arteries. While the methods for calculating the ankle-brachial index can vary, one commonly accepted calculation is the ratio of the highest ankle systolic pressure divided by the highest brachial systolic pressure.
Although ankle-brachial index is considered as the standard method for the diagnosis of lower extremity peripheral arterial disease in field epidemiological surveys, in vascular laboratories, and in office practice, the procedure is cumbersome since it needs a dedicated device, it is time consuming and it requires technical skills. These shortcomings may explain why peripheral arterial disease remains largely underdiagnosed in general practice [5]. Additional limitations include inaccurate measurements as a result of calcified or incompressible vessels (which would produce falsely elevated readings) and the presence of a subclavian-artery stenosis (which could also falsely elevate the ankle-brachial index on the side of the stenosis) [6].
Pulse oximetry has been developed as a non invasive screening method to detect low oxygen haemoglobin saturation in finger and toe tips. The rationale for using fingertip pulse oximetry as screening for peripheral arterial disease is based on the hypothesis that there would be a gradient of oxygen saturation between upper and lower limbs in patients with significant arterial perfusion defects [7]. Previous studies have shown conflicting results when this method is applied in different clinical settings. Therefore we aimed to assess the performance of pocket fingertip pulse oximeters to detect peripheral vascular disease in patients with diabetes.
2. MATERIALS AND METHODS
The study was carried out and reported according to the Standards for Reporting of Diagnostic Accuracy criteria (STARD Initiative) [8].
2.1. Study Population
Patients with diabetes mellitus were considered for enrollment in the study. Eligibility criteria included: 1) age equal or greater than 50 years old and diagnosed of diabetes mellitus; 2) able to walk; 3) given informed consent. The exclusion criteria used were: 1) obese individual requiring special cuffs; 2) presence of painful inflammatory processes, wounds, phlebitis or extreme edema; 3) presence of revascularization procedures or amputation in any of the limbs. Patients were prospectively enrolled during times the investigators were available. Data collection started in February 2011 and finished in December 2011.
2.2. Data Collection
Index test and reference standard were carried out sequentially during the same observation period after resting in supine position for 5 min in a room at 24˚C. Nail polish was removed before pulse oximetry was carriedout. Data collection was planned before the index test and reference standard was performed. Data included age, sex, duration of diabetes mellitus since diagnosis, smoking habit, diagnosis of hypertension or hyperlipidemia and past history of coronary artery disease or cerebrovascular disease. Laboratory data obtained were fasting blood glucose, hemoglobinA1c, total cholesterol, HDL cholesterol, triglycerides, creatinine, glomerular filtration rate estimated by means of Modification of Diet in Renal Disease (MDRD-4) formula. In addition, patients were assessed for symptomatic peripheral artery disease by means of the Edinburgh claudication questionnaire [9].
2.3. Index Test
We used the Oxym6000 pocket-size fingertip pulse oximeter (Quirumed Health & Care, Beijing, China). These devices have a measurement range from 70 to 99%, and an accuracy of ± 2% on the stage of 70% - 99%. Pulse oximetry of the toes was considered abnormal if there was a decrease of more than 2% in arterial oxygen saturation (SaO2) at the toe from the finger or a decrease of more than 2% on elevation of the foot by 30 cm at each side (SaO2 gradient > 2%).
2.4. Reference Standard
The ankle-brachial index was used as the reference standard to identify patients with peripheral artery disease. Ankle-brachial index (ABI) was calculated in every patient after collection of all data. We used the ratio of the highest registered measurement of ankle and brachial blood pressure.
We used the definitions of normal and abnormal ABI values provided by the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines last review [4]. This includes a normal ABI range of 1.00 to 1.40, and abnormal values continue to be defined as those less than 0.90. ABI values of 0.91 to 0.99 are considered “borderline” and values greater than 1.40 indicate noncompressible arteries.
A handheld Doppler device with an 8 MHz continuous wave probe (Minidop ES-100 VX, Hadeco Inc., Japan) was used to assess the systolic blood pressure at the brachial, dorsalis pedis and posterior tibial arteries at each limb. Once the pulse sound was located by the handheld Doppler probe, a 24 - 32 cm. cuff of an aneroid sphygmomanometer (Riester Minimus III, Germany) was inflated until the signal disappeared. The cuff was then slowly deflated and the pressure at which the signal reappeared was recorded. Examination was repeated up to three times if no recording was obtained.
2.5. Sample Size Estimation
We would recommend pulse oximetry as an alternative diagnostic test if there were 80% certainty (power = 0.80) that its sensitivity was not more than 20% less than that for the ankle-brachial pressure index measured by handheld Doppler. On the basis of literature data, the estimated sensitivity for ankle-brachial index measured by Doppler is 95% [10]; therefore the expected sensitivity of pulse oximetry was set at 75% or more. We used as rejection limit for type I error 0.05 (one-tailed). According to these assumptions, a total of 38 consecutive patients with peripheral vascular disease were needed to test our hypothesis [11].
2.6. Statistical Analysis
Continuous variables are summarized as mean (standard deviation) when normally distributed and median (interquartile range) when asymmetrically distributed. Categorical variables are presented as numbers (percentage). We analyzed the data using the handheld Doppler as the reference standard. Sensitivity, specificity, likelihood ratios and area under the receiving operating characteristic curve were derived for abnormal pulse oximetry with 95% confidence intervals (CIs). An analysis of receiving operating characteristic curve was used to select the SaO2 gradient that maximized sensitivity without compromising specificity (MedCalc Software version 12.3.0, Mariakerke, Belgium). Statistical analysis was carried out using 2-way contingency table analysis and paired T-test (SPSS version 15.0, Chicago, USA).
3. RESULTS
3.1. Clinical and Demographic Characteristics
We screened a total of 250 patients. Twenty-seven (11%) patients were excluded due to not having measurable SaO2 finger-to-toe gradients. A total of 223 patients were analyzed. Patients entering the study had a median age of 65 years (interquartile range from 59 to 71). Fiftynine percent of patients were male. The median time since the diagnosis of diabetes was 10 years (interquartile range from 5 to 17 years). There was no difference between paired systolic blood pressure measurements in right arm and left arm (136.37 ± 24.28 mmHg vs. 136.16 ± 22.90 mmHg; P = 0.79).
As shown in Table 1, among 223 patients, 171 (76.7%) had hypertension, 176 (78.9%) hyperlipidemia, 55 (24.7%) were smokers, and 86 (38.6%) had a previous cardiovascular event. A total of 48 (21.5%) patients had a reduction of the estimated glomerular filtration rate below 60 mL/min/1.73 m2.
A total of 47 (21.0%) patients had ankle-brachial index values less than 0.90, with a mean (SD) value of 0.70 (0.10). According Edinburgh questionnaire 27 (57.4%) had symptomatic peripheral arterial disease.
3.2. Pulse Oximetry Values
A total of 1392 (93%) valid SaO2 readings were obtained. Patients with ankle-brachial index less than 0.90 showed statistically significant reductions in SaO2 values taken at feet when they were compared to patients with
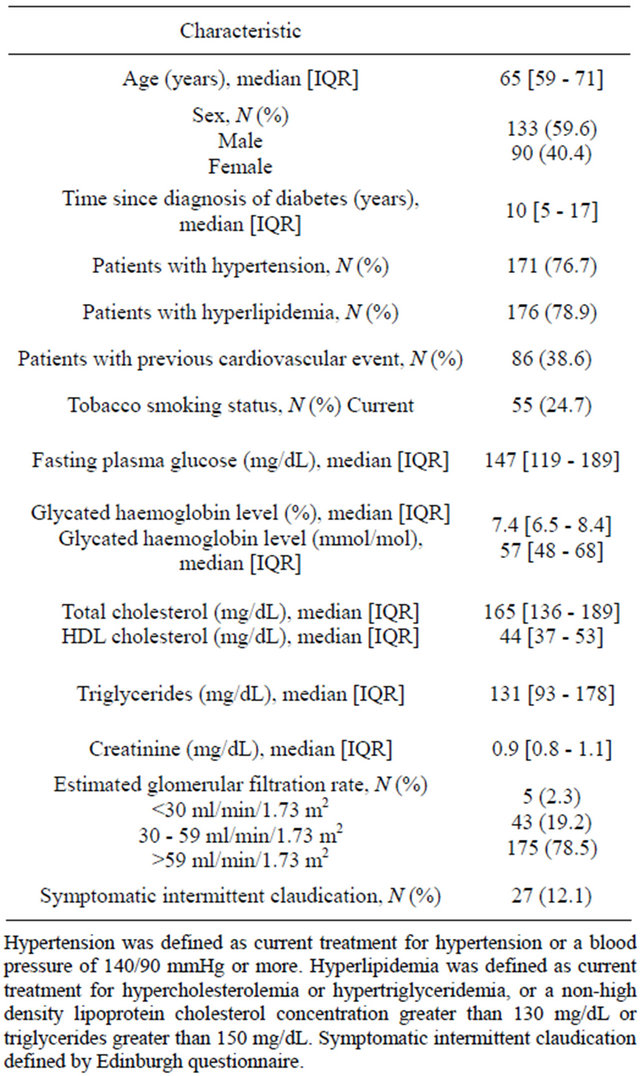
Table 1. Characteristics of the patients entering the study (N = 223).
normal ankle-brachial index (Table 2). We did not observe significant differences in SaO2 values taken at feet compared to those taken at fingers within the ankle-brachial index categories considered (low, normal, or high). There were no significant differences in SaO2 values taken at feet on horizontal or after 30˚ elevation. Table 3 shows the distribution of finger-to-toe gradients according several intervals of ankle-brachial index. There was a significant association (P = 0.013) among ankle-brachial index intervals and finger-to-toe gradient categories.
3.3. Overall Performance of Fingertip Pulse Oximetry
Figure 1 described the flowchart of patients entering the study. Twenty-seven patients had not measure SaO2 at toes, among them 17 (63%) patients had ankle-brachial index less than 0.90, 8 (30%) had normal anklebrachial index values and, 2 (7%) had ankle-brachial
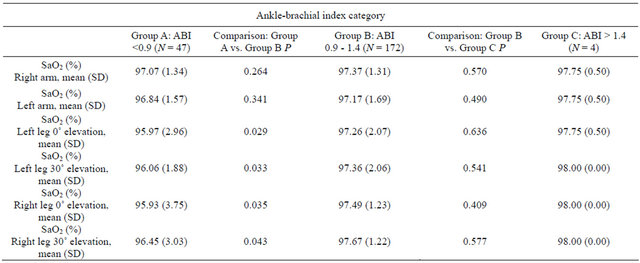
Table 2. SaO2 at fingerand toe-tips according ankle-brachial index categories.
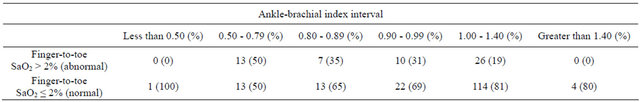
Table 3. Distribution of finger-to-toe gradient greater than 2% according different groups of ankle-brachial index values.
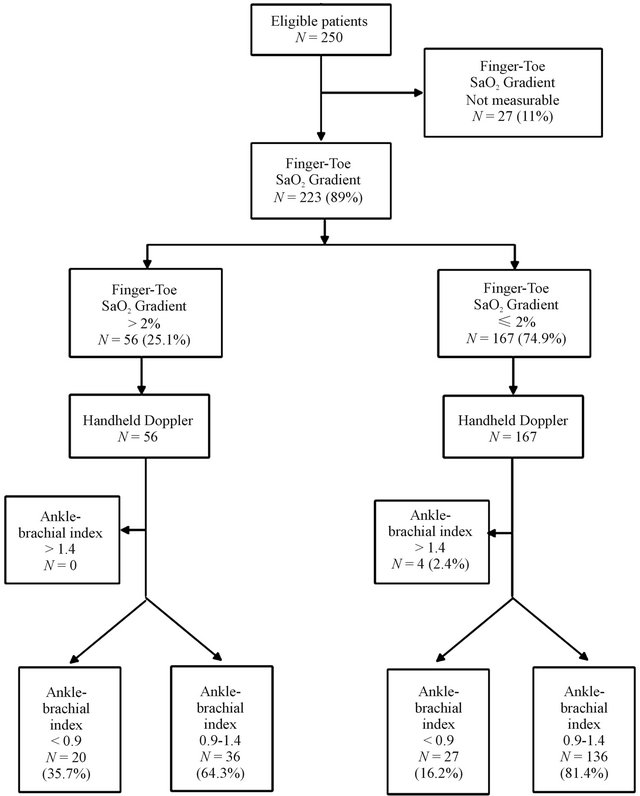
Figure 1. Flow diagram of the finger-toe pulse oximetry accu racy compared with handheld Doppler to estimate the presence of peripheral arterial disease.
values greater than 1.40.
Among 223 patients analyzed, 56 (25.1%) had a finger-to-toe SaO2 gradient greater than 2% suggesting peripheral arterial disease. In this group, 20 (35.7%) patients had true positive results with a mean ( ± SD) ankle-brachial index 0.66 ( ± 0.14), and 36 (64.3%) patients had false positive results with a mean ankle-brachial index 1.07 ( ± 0.12) (P < 0.001).
A total of 167 patients (74.9%) had normal values of finger-to-toe SaO2 gradient, suggesting absence of peripheral arterial disease. In this group, 136 (81.4%) patients had true negative results with mean ( ± SD) ankle-brachial index 1.08 ( ± 0.11) and 31 (18.6%) patients had false negative results with mean ( ± SD) anklebrachial index 0.78 ( ± 0.22) (P < 0.001).
The overall performance of fingertip pulse oximetry was: sensitivity 42.6% (95% CI 30.0% - 55.3%), specificity 79.1% (95% CI 75.7% - 82.6%), positive predictive value 35.7% (95% CI 25.2% - 46.4%), negative predictive value 83.4% (95% CI 79.8 - 87.1), positive likelihood ratio of 2.03 (95% CI 1.23 - 3.17) and, a negative likelihood ratio of 0.73 (95% CI 0.54 - 0.93) (Table 2).
The area under the receiving operating characteristic (ROC) curve was 0.69 (95% CI 0.62 to 0.77) (Figure 2). In order to assess the consistency of the results, we carried out a subgroup analysis. The subgroups analyzed were: sex, male vs. female; time since diagnosis of dia-
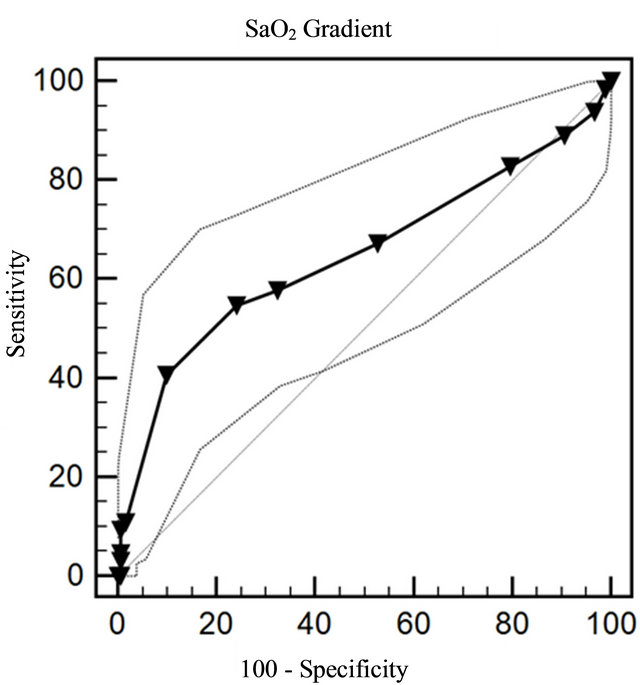
Figure 2. Area under the receiver operator characteristic curve and 95% confidence intervals for SaO2 gradient measured by pocket pulse oximeter for the diagnosis of peripheral arterial disease.
betes, <10 years vs. ≥10 years; presence of intermittent claudication, yes vs. no; past history of cardiovascular disease yes vs. no. Subgroup analysis showed a poor sensitivity and fair specificity of the pocket pulse oximetry for every category of patients considered (Table 4).
4. DISCUSSION
We confirmed the hypothesis that patients with anklebrachial index less than 0.90 showed significant decrease in SaO2 values taken at feet when they were compared to those obtained from patients with normal ankle-brachial index. However, the discriminative power of finger-totoe SaO2 to diagnose peripheral arterial disease was poor. Moreover, in 11% of patients the fingertip device was not able to assess SaO2 at toes.
Our study population accumulated significant cardiovascular risk factors with a high proportion of hypertension, hyperlipidemia and a long duration of diabetes. The prevalence of peripheral arterial disease defined by an ankle-brachial index less than 0.90 was 21.6%, a value similar than that reported in other population studies of patients with diabetes that varied between 19% and 31% [12,13].
In our experience the use of fingertip pulse oximetry as screening tool for peripheral arterial disease had a sensitivity value of 42.6%, with a positive predictive value of 37.5%. On the other hand, pulse oximetry showed a specificity of 77.2% with a negative predictive value of 83.4%, meaning that it had better performance for ruling out the disease.
The use of fingertip pulse oximetry for detecting peripheral arterial disease has been evaluated in a number of studies. Parameswaran el al. studied 57 asymptomatic patients with mean age 63 years and 9 years of known duration of diabetes in whom peripheral arterial disease was confirmed in 31% by Doppler waveform analysis [7]. The study showed that pulse oximetry had a sensitiveity of 77% and a specificity of 97%. Joyce et al. [14] and Ignjatović et al. [15] used pulse oximetry to evaluate degree of limb ischemia in patients attending two surgi
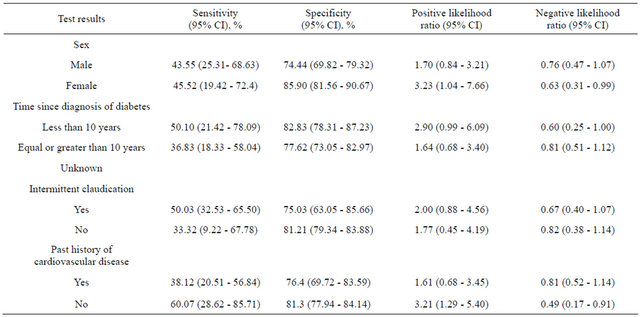
Table 4. Results for the pulse oximetry test according different categories of risk.
cal vascular units. They found that SaO2 assessed by pulse oximetry was useful to determine the stage of functional ischemia and the success of revascularization.
On the contrary, a poor sensitivity of pulse oximetry has been observed in some other studies. Jawahar et al. [16] studied a total of 51 legs with moderate and severe peripheral arterial disease and the sensitivity was 53%. Couse et al. [17] analyzed arterial oxygen saturation at the big toe in fourteen symptomatic patients with exercise induced leg pain, seven elderly men admitted to the hospital for a variety of reasons unrelated to vascular disease and six young men with no symptoms. Surprisingly, arterial oxygen saturation at rest was similar among three groups. They did not evaluate the performance characteristics of the test. Reasons for such discrepancies in test performance lie mainly in the type of population examined. Patients with symptoms of peripheral arterial disease or those suffering revascularization procedures are those with greater arterial perfusion defects, thus increasing the sensitivity of diagnostics tests. On the other hand, patients entering screening programs have lower probability of finding significant perfusion defects, and will require more sensitive tests to detect the disease. It had been argued that ankle-brachial index may not be the best reference method for diagnosis of peripheral vascular disease in patients with longer duration of diabetes mellitus or suffering from chronic kidney disease. The reasons are related with falsely elevated values of ankle-brachial index due to vascular calcification and noncompressible vessels [18,19]. A recent study confirmed the association between diabetes, regular hemodialysis and presence of arterial calcification in 269 patients with critical limb ischemia, but neither ankle systolic blood pressure nor ankle-brachial index were affected by the presence of vessel calcification [20]. Regarding our data, if long standing diabetes would have produced falsely elevated values of ankle-brachial index it should have been expected that pulse oximetry had greater sensitivity to detect significant arterial perfusion defects. From our data, and the literature reviewed pulse oximetry showed better performance to rule out significant peripheral arterial disease.
Our study included as strengths 1) a large sample size, big enough to assess test performance; 2) comparison with a known reference standard for office diagnosis; and 3) appropriate spectrum of patients in which the diagnostic test should apply in clinical practice. Although we found the use of pulse oximeter devices useful to rule out significant peripheral arterial disease, our study had some limitations: 1) we did not evaluate the interobserver variability, nevertheless all physicians had a training period before performing the test; 2) the sequence of measurements, pulse oximetry followed by ankle-brachial index may have biased the results, but the bias should have increase the agreement between two methods; and 3) there was no confirmatory test such as lower limb angiography to assess the presence of peripheral arterial disease, however, several societies consider that resting ankle-brachial index has enough sensitivity and specificity to be used a reference method [3,4].
In conclusion, pocket pulse oximeters showed insufficient sensitivity as screening method for peripheral arterial disease in patients with diabetes mellitus. Anklebrachial index measured by Doppler remains as the reference method for diagnosing peripheral arterial disease in clinical practice.
5. DECLARATION
Funding: The project was funded by Fundación Mutua Madrileña para la Investigación. Fundación Mutua Madrileña para la Investigación had no role in study design, data collection, data analysis, data interpretation, or writing the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Etical approval: The study comply with the principles laid down in the Declaration of Helsinki “Recommendations guiding physicians in biomedical research involving human subjects”, adopted by the 18th World Medical Assembly, Helsinki, Finland, June 1964 (and its successive amendments), and the requirements of Spanish law in the field of biomedical research, data protection and bioethics. The study was approved by the institutional review board of our hospital. All patients provided informed consent.
REFERENCES
- Global Lower Extremity Amputation Study Group. (2000) Epidemiology of lower extremity amputation in centres in Europe, North America and East Asia. British Journal of Surgery, 87, 328-337. doi:10.1046/j.1365-2168.2000.01344.x
- Boulton, A.J., Armstrong, D.G., Albert, S.F., Frykberg, R.G., Hellman, R., Kirkman, M.S., Lavery, L.A., LeMaster, J.W., Mills Sr., J.L., Mueller, M.J., Sheehan, P. and Wukich, D.K. (2008) Comprehensive foot examination and risk assessment. A report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Physical Therapy, 88, 1436- 1443.
- Tendera, M., Aboyans, V., Bartelink, M.L., Baumgartner, I., Clément, D., Collet, J.P., Cremonesi, A., De Carlo, M., Erbel, R., Fowkes, F.G., Heras, M., Kownator, S., Minar, E., Ostergren, J., Poldermans, D., Riambau, V., Roffi, M., Röther, J., Sievert, H., van Sambeek, M., Zeller, T. (2011) Esc guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: The Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). European Heart Journal, 32, 2851-906. doi:10.1093/eurheartj/ehr211
- Rooke, T.W., Hirsch, A.T., Misra, S., Sidawy, A.N., Beckman, J.A., Findeiss, L.K., Golzarian, J., Gornik, H.L., Halperin, J.L., Jaff, M.R., Moneta, G.L., Olin, J.W., Stanley, J.C., White, C.J., White, J.V., Zierler, R.E. (2011) 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of American College of Cardiologiy, 58, 2020- 2045. doi:10.1016/j.jacc.2011.08.023
- Gregg, E.W., Sorlie, P., Paulose-Ram, R., Gu, Q., Eberhardt, M.S., Wolz, M., Burt, V., Curtin, L., Engelgau, M. and Geiss, L. (2004) Prevalence of lower-extremity disease in the US adult population ≥40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care, 27, 1591-1597. doi:10.2337/diacare.27.7.1591
- Grenon, S.M., Gagnon, J. and Hsiang, Y. (2009) Anklebrachial index for assessment of peripheral arterial disease. New England Journal of Medicine, 361, e40. doi:10.1056/NEJMvcm0807012
- Parameswaran, G.I., Brand, K. and Dolan, J. (2005) Pulse oximetry as a potential screening tool for lower extremity arterial disease in asymptomatic patients with diabetes mellitus. Archives of Internal Medicine, 165, 442-446. doi:10.1001/archinte.165.4.442
- Bossuyt, P.M., Reitsma, J.B., Bruns, D.E., Gatsonis, C.A., Glasziou, P.P., Irwig, L.M., Moher, D., Rennie, D., de Vet, H.C. and Lijmer, J.G. (2003) The STARD statement for reporting studies of diagnostic accuracy: Explanation and elaboration. Clinical Chemistry, 49, 7-18. doi:10.1373/49.1.7
- Leng, G.C. and Fowkes, F.G. (1992) The Edinburgh Claudication Questionnaire: An improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. Journal of Clinical Epidemiology, 45, 1101-1109. doi:10.1016/0895-4356(92)90150-L
- Fowkes, F.G. (1988) The measurement of atherosclerotic peripheral arterial disease in epidemiological surveys. International Journal of Epidemiology, 17, 248-254. doi:10.1093/ije/17.2.248
- Arkin, C.F. and Wachtel, M.S. (1990) How many patients are necessary to assess test performance. Journal of American Medical Association, 263, 275-278.
- Akram, J., Aamir, A.U., Basit, A., Qureshi, M.S., Mehmood, T., Shahid, S.K., Khoso, I.A., Ebrahim, M.A. and Omair. A. (2011) Prevalence of peripheral arterial disease in type 2 diabetics in Pakistan. Journal of Pakistan Medical Association, 61, 644-648.
- Walters, D.P., Gatling, W., Mullee, M.A. and Hill, R.D. (1992) The prevalence, detection, and epidemiological correlates of peripheral vascular disease: A comparison of diabetic and non-diabetic subjects in an English community. Diabetic Medicine, 9, 710-715. doi:10.1111/j.1464-5491.1992.tb01878.x
- Joyce, W.P., Walsh, K., Gough, D.B., Gorey, T.F. and Fitzpatrick, J.M. (1990) Pulse oximetry: A new non-invasive assessment of peripheral arterial occlusive disease. British Journal of Surgery, 77, 1115-1117. doi:10.1002/bjs.1800771013
- Ignjatović, N., Vasiljević, M., Milić, D., Stefanović, J., Stojanović, M., Karanikolić, A., Zlatić, A., Djordjević, G., Zivić, S., Jeremić, L., Djordjević, I. and Janković, R. (2010) Diagnostic importance of pulse oximetry in the determination of the stage of chronic arterial insufficiency of lower extremities. Srpski Arhiv Za Celokupno Lekarstvo, 138, 300-304. doi:10.2298/SARH1006300I
- Jawahar, D., Rachamalla, H.R., Rafalowski, A., Ilkhani, R., Bharathan, T. and Anandarao, N. (1997) Pulse oximetry in the evaluation of peripheral vascular disease. Angiology, 48, 721-724. doi:10.1177/000331979704800808
- Couse, N.F., Delaney, C.P., Horgan, P.G., O’Keeffe, J., Joyce, W.P., Gorey, T.F., Fitzpatrick, J.M. (1994) Pulse oximetry in the diagnosis of non-critical peripheral vascular insufficiency. Journal of the Royal Society of Medicine, 87, 511-512.
- Jude, E.B., Eleftheriadou. I. and Tentolouris. N. (2010) Peripheral arterial disease in diabetes—A review. Diabetic Medicine, 27, 4-14. doi:10.1111/j.1464-5491.2009.02866.x
- Leskinen, Y., Salenius, J.P., Letimäki, T., Huhtala, H. and Saha, H. (2002) The prevalence of peripheral arterial disease and medial arterial calcification in patients with chronic renal failure: Requirements for diagnostics. American Journal of Kidney Diseases, 40, 472-479. doi:10.1053/ajkd.2002.34885
- Takahara, M., Kaneto, H., Iida, O., Katakami, N., Matsuoka, T., Ikeda, M., et al. (2012) Association of diabetes and hemodialysis with ankle pressure and ankle-brachial index in Japanese patients with critical limb ischemia. Diabetes Care, 35, 2000-2004. doi:10.2337/dc11-1636
NOTES
*Conflict of interest: None.

