Agricultural Sciences
Vol. 4 No. 12 (2013) , Article ID: 40840 , 12 pages DOI:10.4236/as.2013.412088
Incidence evaluation of Crypticerya multicicatrices and Maconellicoccus hirsutus in Colombian Seaflower Biosphere Reserve
![]()
1Universidad Nacional de Colombia, Sede Medellín, Medellín, Colombia; limhoyosca@unal.edu.co
2Jardín Botánico de San Andrés, Universidad Nacional de Colombia, Sede Caribe, San Andrés, Colombia
Copyright © 2013 Marcela Silva-Gomez et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 20 March 2013; revised 6 November 2013; accepted 26 November 2013
Keywords: Colombia’s Caribbean Insular; Pink Hibiscus Mealybug; Multicicatrices Fluted Scale; Sap Sucking Insects; Honey Dew; Sooty Mold
ABSTRACT
An evaluation of the incidence of Crypticerya multicicatrices and Maconellicoccus hirsutus in Colombian Seaflower Biosphere Reserve in San Andrés, Providence and Santa Catalina islands, from mid-August to mid-November 2012 was made. 38 locations were sampled, and 34% of sites tested show an incidence of C. multicicatrices oscillating between a range of 80% - 100%, 24% with 40% - 80% of incidence, 18% between 10% - 40% and 24% with a range of 0% - 10%. The occurrence of M. hirsutus fluctuated between 0.5% and 17%. There were seven genera of ants associated C. multicicatrices and 94 hosts of this agriculturally important insect and we found high incidence of associated sooty mold in C. multicicatrices hosts. Observations also contained some developmental stages of C. multicatrices.
1. INTRODUCTION
The Seaflower Biosphere Reserve is situated at the Archipelago of San Andrés, Providencia and Santa Catalina, at the southwestern Caribbean, off the east coast of Nicaragua, halfway between Colombia and Jamaica. As a marine biosphere reserve, it covers approximately 10% of the Caribbean Sea, with three main islands, surrounded by coastal mangroves swamps and highly intact and productive associated coral reef ecosystems. The old Providence barrier reef alone is 32 km long and covers an area of 255 km2 making it one of the largest coral reefs in the Americas. It is identified as a major site of coral and fish diversity and is considered a biodiversity “hotspot” [1].
The pink hibiscus mealybug (PHM) Maconellicoccus hirsutus (Green, 1908) (Hemiptera: Pseudococcidae) is a pest subject of official control in Colombia (ICA Resolution 2895, 2010), and in consequence it is a network from constant monitoring and site inspections by the Instituto Colombiano Agropecuario (ICA), across the country. In 2010, it was reported that PHM infested fruit, ornamental and wild weeds, in 37.2% of the total area of the island of Providence island and 62% of remaining vegetation area with less infestation [2].
Multicicatrices fluted scale (MFS), Crypticerya multicicatrices (Hemiptera: Monophlebidae), was described as a new species for Colombia by Kondo and Unruh (2009), infesting various plants in the islands San Andrés, Providencia and Santa Catalina, in quantitatively unknown magnitude. MFS, a polyphagous species, has been reported in 95 hosts by [3] and for control. There is a single report with an entomopathogenic fungus (Isaria sp.) [4]. [5] claim that the spread of the PHM and MFS is a serious problem as invasive species, mainly by the apparent absence of natural enemies on the island of San Andres.
Studies were conducted on the incidence and geographical distribution of PHM And MFS, in the Islands of San Andrés, Providencia and Santa Catalina and completed a host record for C. multicatrices.
2. Materials and Methods
2.1. Location of Study Area
The research was carried out in different zones (Table 1), located in the islands of San Andrés, Providencia and Santa Catalina, part of Colombian Seaflower Biosphere in the southwestern Caribbean Sea. Bordered on the east
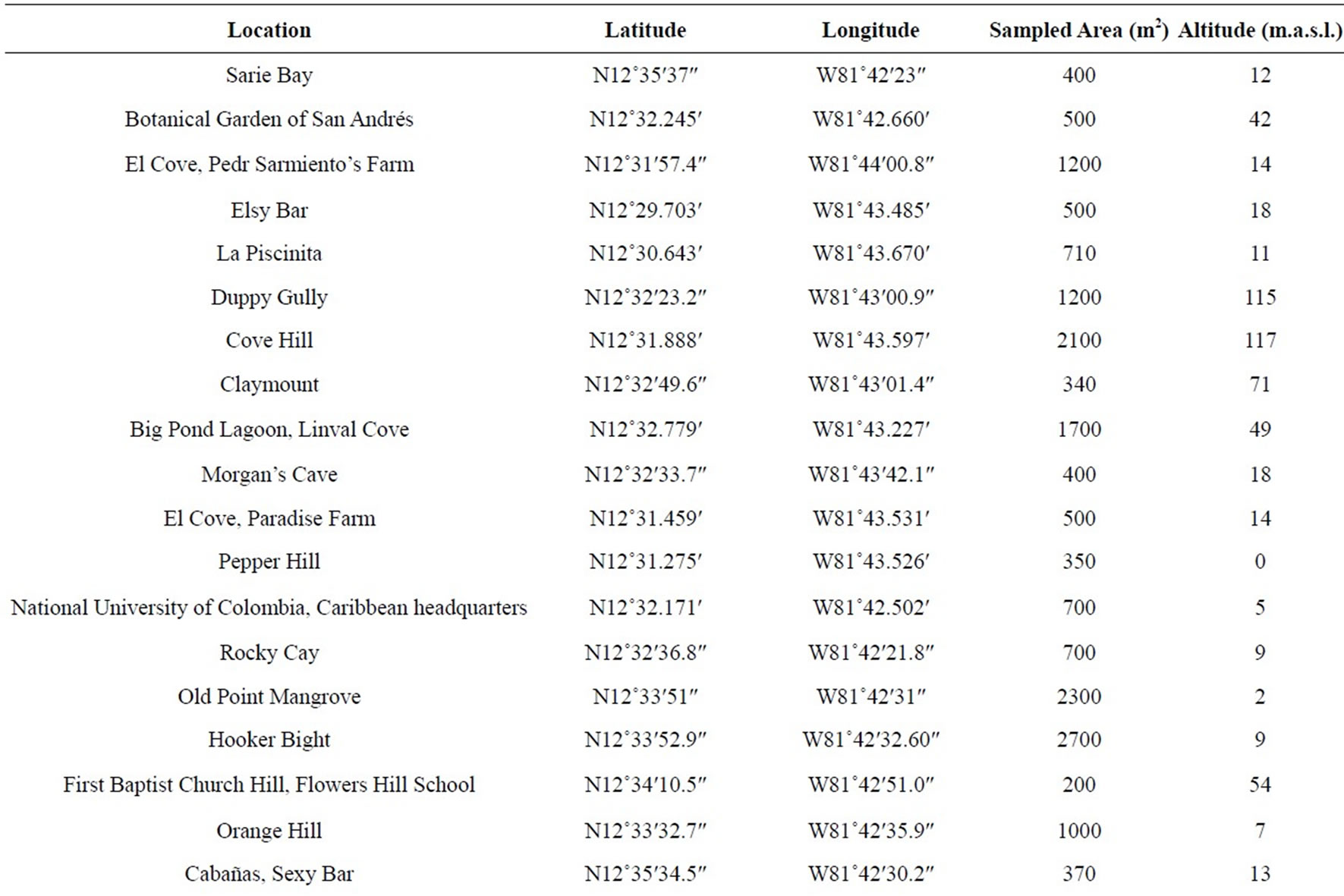
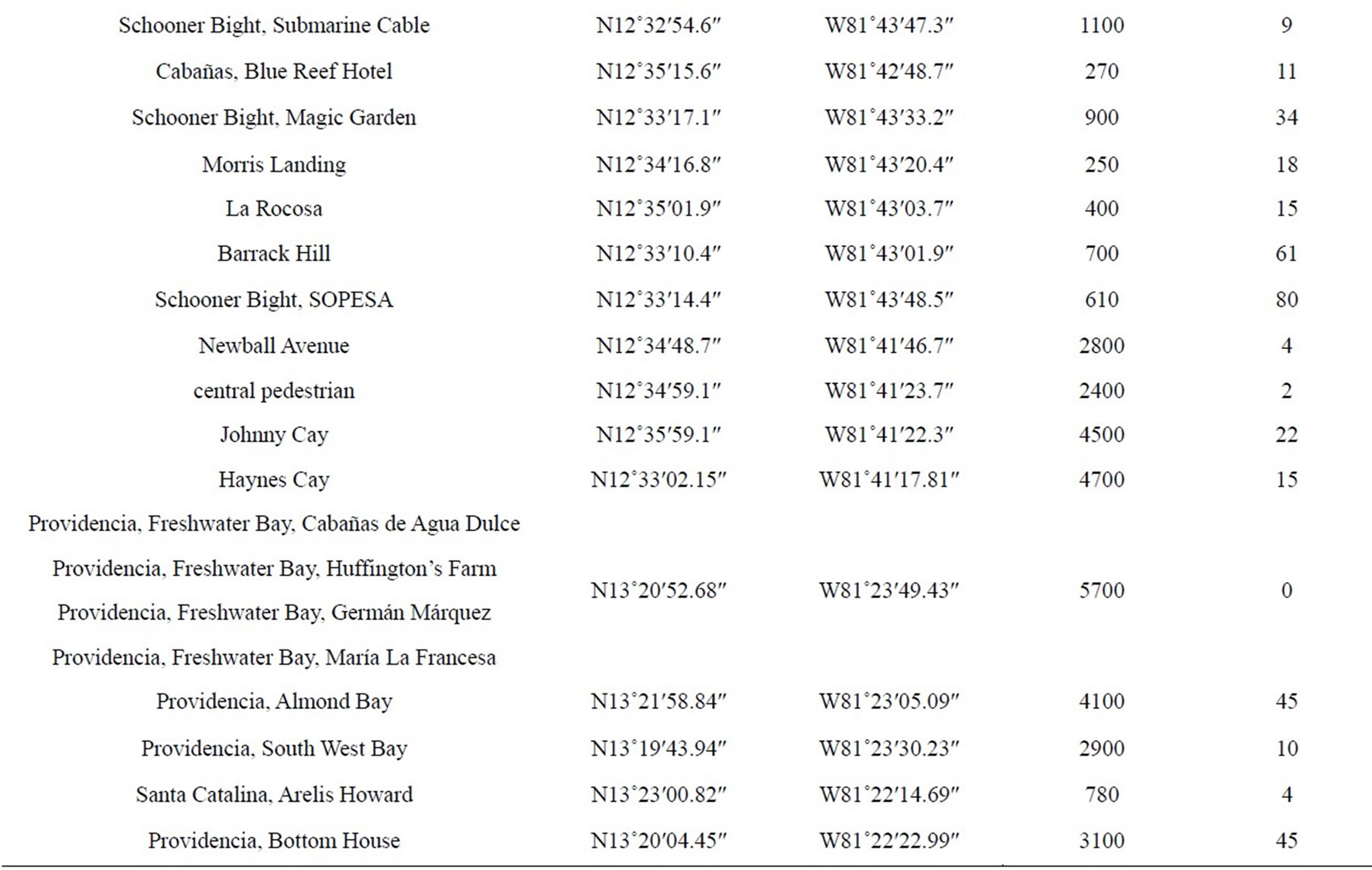
Table 1. Locations evaluated for monitoring the mealybug incidence in the Islands of San Andrés, Providencia and Santa Catalina, in August and November 2012.
by the Caribbean islands (islands of the Greater and Lesser Antilles), with Jamaica in the north and the northwest, west and south with the continental states of Honduras, Nicaragua, Costa Rica, Panama and the Colombian mainland. Between the meridians 78˚ and 82˚ longitude west and between 12˚ and 16˚ latitude north [6].
As a whole, the islands has an area of 52.5 km2 island, formed by the three largest islands (26 km2 San Andres, 17.2 km2 Providencia and 1 km2 Santa Catalina) and a set of keys with a length of 8.3 km2 (Viloria, 1998, Quoted by [6]).
The island of San Andrés is located south-west of the islands, between 12˚28ʹ and 12˚36ʹN and 81˚40ʹ and 81˚44'W, about 240 km from Central America coast [7]. Mean annual temperature is 27.4˚C, maximum values between 29˚C and 30˚C (May-June) and minimum values between 25.5˚C and 26.0˚C (December-February). Mean annual precipitation is 1797.8 mm distributed irregularly, in a dry period (January-April), with strong winds and a wet period (October-December), which concentrate an 80% of annual rainfall [8].
2.2. Sampling Design and Site Selection
Observations were made in the field, in order to establish the incidence of PHM and MFS in the Islands of San Andrés, Providencia and Santa Catalina. Sampling was conducted from mid-August to mid-November 2012 in 28 locations on the island of San Andres, on 2 cays adjacent to the island of San Andrés (Johnny Cay and Haynes cay) (Figure 1) and in 8 locations in the islands of Providence and Santa Catalina.
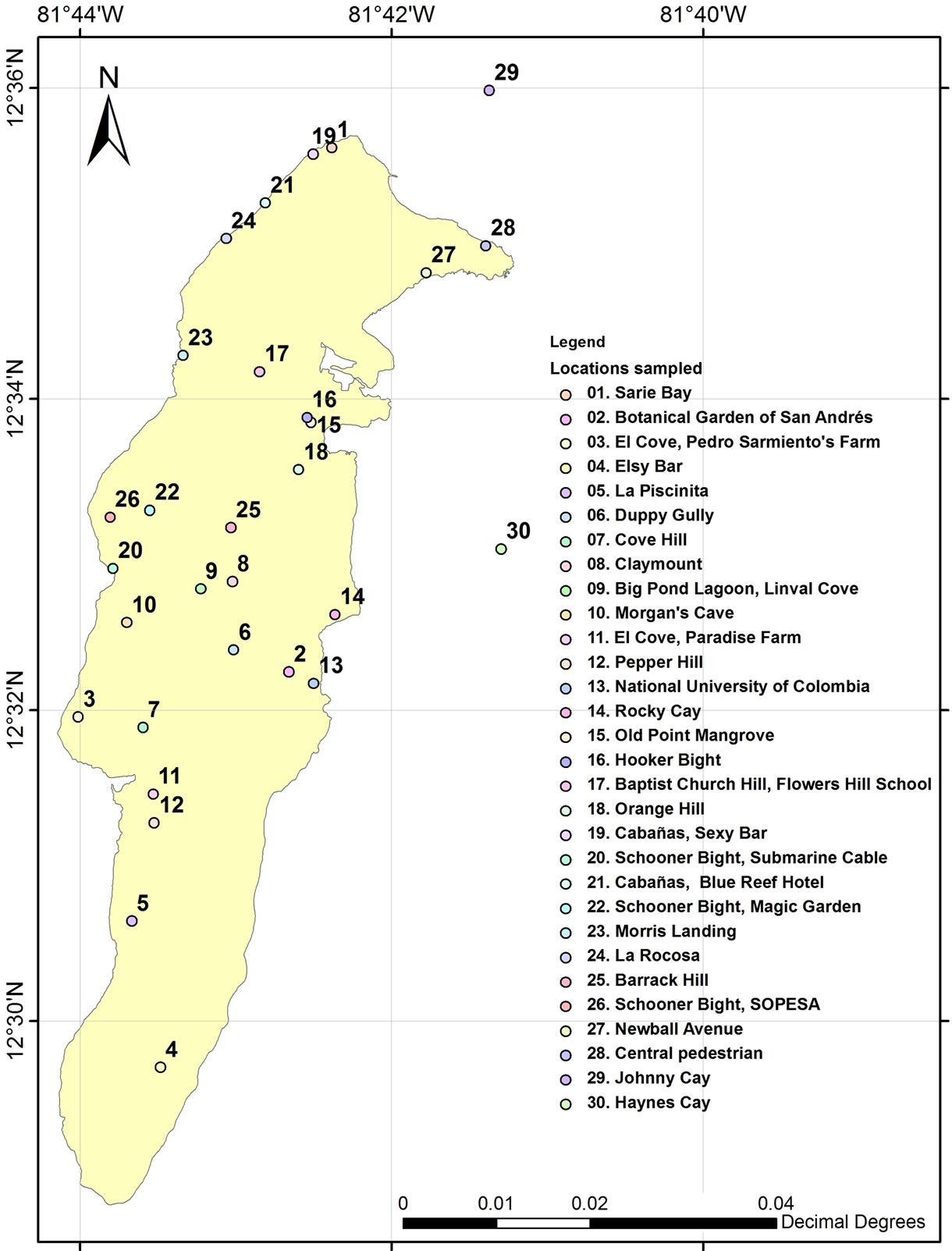
Figure 1. Locations evaluated in San Andres Island and surrounding cays, during August and November 2012. Map by Sebastián Giraldo Grisales.
In each sector, was taken because are representations of transept ecological use (mangroves, farms, pastures, gardens, etc.), recording the geo reference and determining the area sampled in each of these, using a GPS Garmin Oregon 550 and a dekameter (Table 1). A total area of 53,080 m2 was sampled during the research. From the field evaluation, a table was compiled in the list of host species of MFS (Table 2).
Randomly, 20 plants were taken, for each one, 10 branches were sampled, three (3) of the upper three (3) of the middle third and four (4) of the lower third, trying to cover the entire perimeter of the plant. In the case of plants with higher than 4 m, were evaluated 10 lower branches. The occurrence of both PHM and MFS were estimated, defining the total branches with presence of each insect, calculated using the next equation.
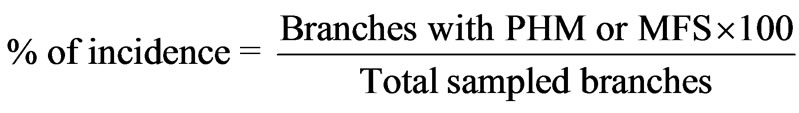
Information was recorded in formats for assessing PHM and MFS, adapted from assessing format of Tetranychus urticae and its predator [9].
In all locations were observed and collected the fauna ants, associated with MFS. Entomological material that was collected, and preserved as specimens in the Entomological Museum Francisco Luis Gallego (MEFLG) of the Nacional University of Co lombia, in Medellin.
3. Results Obtained
For purposes of discussion have been considered four intervals of incidence in Figure 1: 0% - 10% delimitated by black dotted line, 10% - 40% between black and green dotted lines, 40% - 80% between green and red dotted lines and 80% - 100% up dotted red line.
Incidence of C. multicicatrices MFS: 34% of locations shown the highest incidence of C. multicicatrices (80% - 100%), in sectors Sarie Bay, Botanical Garden of San Andres, El Cove Hill, Claymount, Big Pond Lagoon, Morgan’s Cave, El Cove-Paradise Farm, Pepper Hill, Rocky Cay, Orange Hill, Schooner Bigth-Submarine Cable, Schooner Bigth-SOPESA and Johnny Cay (Figures 2(a) and (b)).
24% of the locations evaluated (9 sites), presented incidences between 40% - 80% in El Cove-Pedro Sarmiento’s Farm, Elsy Bar, La Piscinita, Duppy Gully, National University of Colombia-Caribbean Headquarters, Cabañas-Sexy Bar, Cabanas-Blue Reef Hotel, Newball Avenue and Haynes Cay. Also, in 18% of the locations evaluated (7 sites) were 10% - 40% incidence in Baptist Church Hill, La Rocosa, Barrrack Hill and central pedestrain and in Freshwater Bay and Bottom House, in Providencia.
Additionally, 24% of the locations evaluated (9 sites)
 (a)
(a) (b)
(b)
Figure 2. Infested plants with MFS in Rocky Cay (a) and El Cove (b). Photo by M. Silva.
had an incidence between 0% - 10% in Old Point Mangrove, Hooker Bight, Schooner Bigth-Magic Garden, Morris Landing, Freshwater Bay, Almond Bay, South West Bay and Santa Catalina (Graphic 1).
It was found that muticicatrices has about 94 hosts in the islands (Table 2, Figures 3 and 4), mainly belonging to the family Fabaceae (10.64%); Arecaceae, Malvaceae and Rutaceae (5.32%), Euphorbiaceae, Lamiaceae and Solanaceae (4.26%) (Figure 3).





Table 2. Hosts found during monitoring of MFS incidence in the Islands of San Andrés, Providencia and Santa Catalina, in August and November 2012.
 (a)
(a) (b)
(b) (c)
(c) (d)
(d) (e)
(e)
Figure 3. MFS hosts. (a) Flemingia strobilifera; (b) Gliricidia sepium; (c) Mangifera indica; (d) Pritchardia pacifica; (e) Annona muricata. Photo by M. Silva.
The main hosts of MFS are the following species: Persea americana, Artocarpus altilis, Chrysophyllum cainito, Cocos nucifera, Guazuma ulmifolia, Annona muricata,
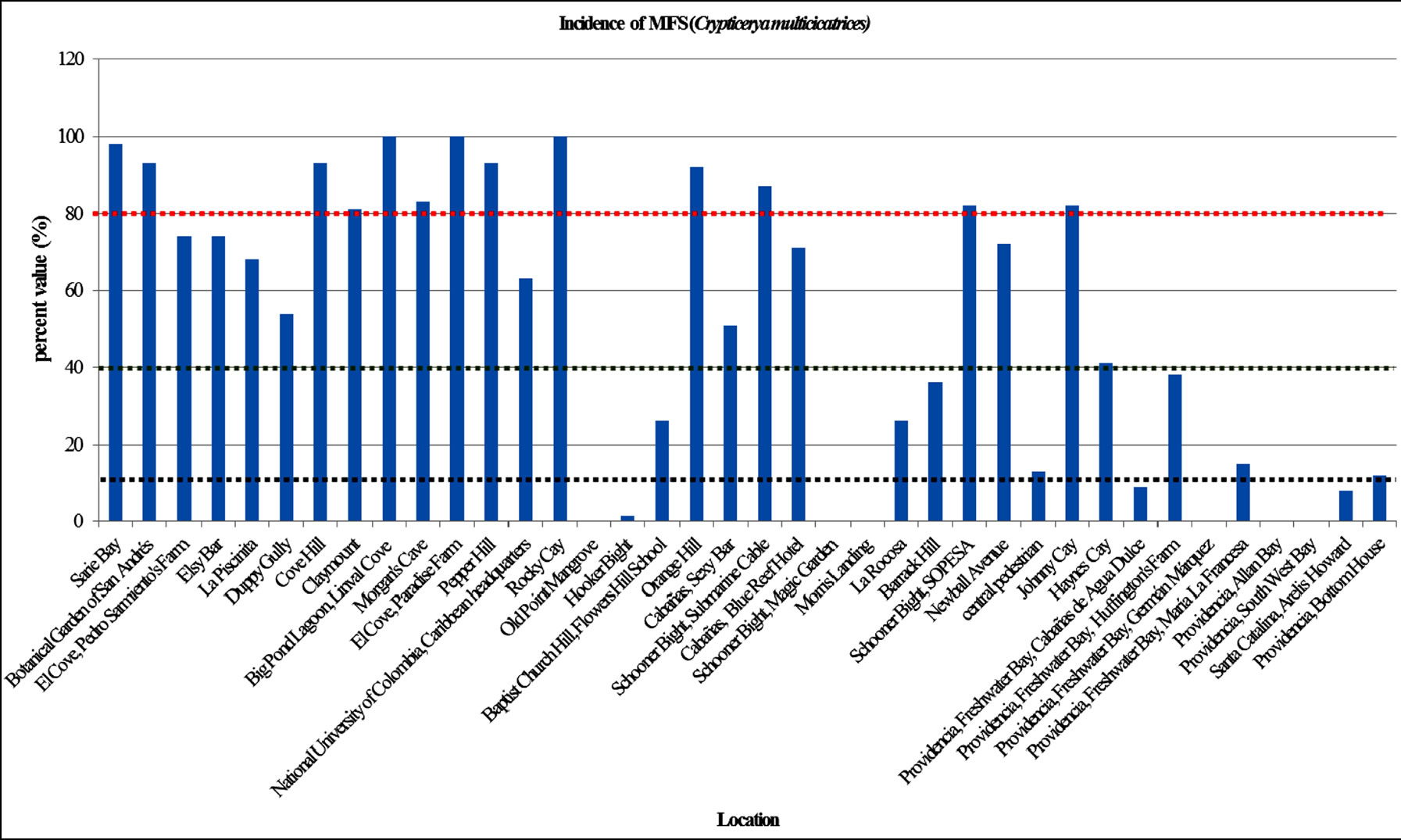
Graphic 1. Incidence evaluation of MFS (C. multicicatrices) in the islands of San Andrés, Providencia and Santa Catalina, in August and November 2012. Incidence ranges: 0% - 10% delimitated by black dotted line, 10% - 40% between black and green dotted lines, 40% - 80% between green and red dotted lines and 80% - 100% up dotted red line.
 (a)
(a) (b)
(b) (c)
(c) (d)
(d) (e)
(e)
Figure 4. MFS hosts. (a)-(c) Cocos nucifera; (d) Psidium guajava; (e) Artocarpus altilis. Photo by M. Silva.
Psidium guajava, Spondias mombin, Spondias purpurea, Citrus limon, Melicoccus bijugatus, Mangifera indica, Gliricidia sepium, Citrus sinensis, Citrus aurantium, Manilkara zapota, Pritchardia pacifica, Dypsis lutescens, Adonidia merrillii, Attalea butyracea, Carica papaya, Musa sp., Syzygium jambos, Tamarindus indica, Bougainvillea sp. and Cecropia schreberiana.
In all the sampling, locations were found high incidence of sooty mold (Figure 5).
During assessment, seven ant genera were found associated to MFS: Crematogaster, Monomorium, Paratrechina, Camponotus, Dorymirmex, Ectatomma and Dolichoderus. They were located in four subfamilies (Table 3). It was observed that a higher incidence of sooty mold, lower or complete absence of myrmecofauna (Figure 6).
High presence was evidenced, naturally, an entomopathogenic fungus, Isaria sp. whose species is in description process, affecting MFS, insects looks mummified and covered with white or lilac mycelium in Loma localities Barrack, Big Lagoon Pond, Hill First Baptist Church, Schooner Bight, Pepper—The Radar Hill, Orange Hill and Loma The Cove (Figure 7).
 (a)
(a) (b)
(b)
Figure 5. Sooty mold associated with MFS in different locations. (a) Big Pond Lagoon; (b) Cove Hill. Photo by M. Silva.
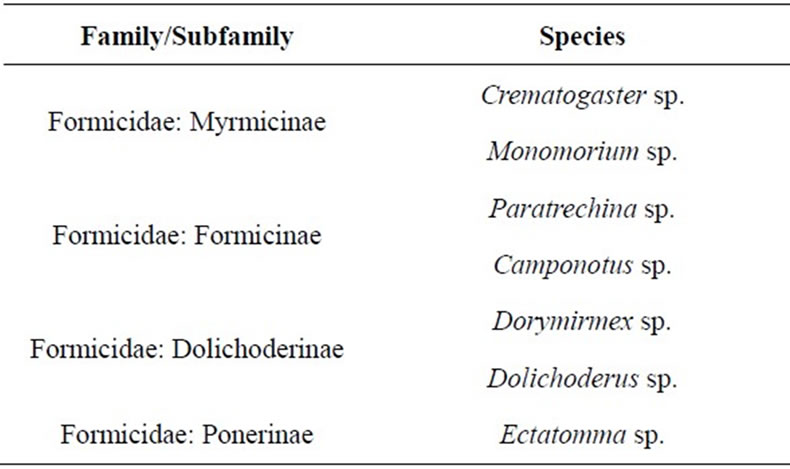
Table 3. Ants associated with MFS, found during monitoring of MFS incidence in the islands of San Andrés, Providencia and Santa Catalina, in August and November 2012.
Incidence of M. hirsutus-PHM-: On the island of San Andrés insect pest incidence in localities tested was 5% in the Botanical Garden, 15% at the Universidad Nacional de Colombia in Caribbean and 0.5% in the central pedestrian while in Providencia, was found incidence of this insect in Freshwater Bay (14%), South West Bay (17%) and Bottom House (5%) (Figure 2). Moreover, M. hirsutus was found primarily affecting Cocos nucifera, Hibiscus rosa-sinensis and Cordia sebestena (Graphic 2).
Additional Observations
This work allowed to make observations about developmental stages of C. multicicatrices (Figure 8). In the laboratory, emerged two male individuals of this mealybug.
4. DISCUSSION
In a total area of 53,080 m2 that was sampled during the research, it was found that 13.300 m2 (25% of the total sampled area) had higher values of incidence (between 80% and 100%), presented at The localities Cove Hill, Claymount, Big Pond Lagoon, Morgan’s Cave, El Cove-Paradise Farm, Pepper Hill, Rocky Cay, Orange Hill, Schooner Bigth-Submarine Cable, Schooner BigthSOPESA and Johnny Cay. This may be because in such sites predominate cultivated and ornamental species that have been observed as a primary host or more preferably by MFS as Cocos nucifera, Annona muricata, Psidium guajava, Melicoccus bijugatus, Mangifera indica, Pritchardia pacifica, Dypsis lutescens and Adonidia merrillii.
The lowest incidence (0% - 10%) occurred in an area of 18.930 m2 (36% of the total sampled area), in the localities Old Point Mangrove, Hooker Bight, Schooner BigthMagic Garden, Morris Landing, Freshwater Bay Cabañas de Agua Dulce, Almond Bay, Freshwater Bay-Germán Márquez, South West Bay and Santa Catalina. In these
 (a)
(a) (b)
(b) (c)
(c)
Figure 6. Ants associated with MFS in different locations and hosts, during monitoring of MFS incidence in the islands of San Andrés, Providencia and Santa Catalina, in August and November 2012. Photo by M. Silva.
 (a)
(a) (b)
(b) (c)
(c) (e)
(e) (f)
(f)
Figure 7. Entomopathogenic fungus, Isaria sp., affecting MFS in different locations. (a) Big Pond Lagoon; (b) Cove Hill; (c) Pepper Hill; (d) First Baptist Church Hill; (e) Barrack Hill. Photo by M. Silva.
locations, the species composition was predominantly composed of Terminalia catappa and Conocarpus erectus, species for which there was no incidence of MFS during evaluations.
Moreover in 39% (20.850 m2) of the total sampled area, there ocurred an incidence between 10% - 80%.
Carballo y Meneses, 2011, report applications with Black Mangovés extract (Avicenia nitida Jacq) to control insect pests of the Superfamily Coccoidea [10]. In the Old Mangrove Point, where there was no presence of C. multicicatrices could consider the existence of a secondary metabolite that is present in plants of this town, dominated by the red mangrove (Rhizophora mangle), white mangrove (Laguncularia racemosa) and black mangrove (Avicennia germinans), species for which there was no incidence of C. multicicatrices except L. racemosa, which was observed in several individuals infesting Dorna Laguna Pond, in the area of San Luis (Murcia, 2013, Pers.com.)
Results obtained samples MFS is comparatively more aggressive than PHM in Colombian Seaflower Biosphere Reserve. According to data, MFS has 94 hosts and PHM
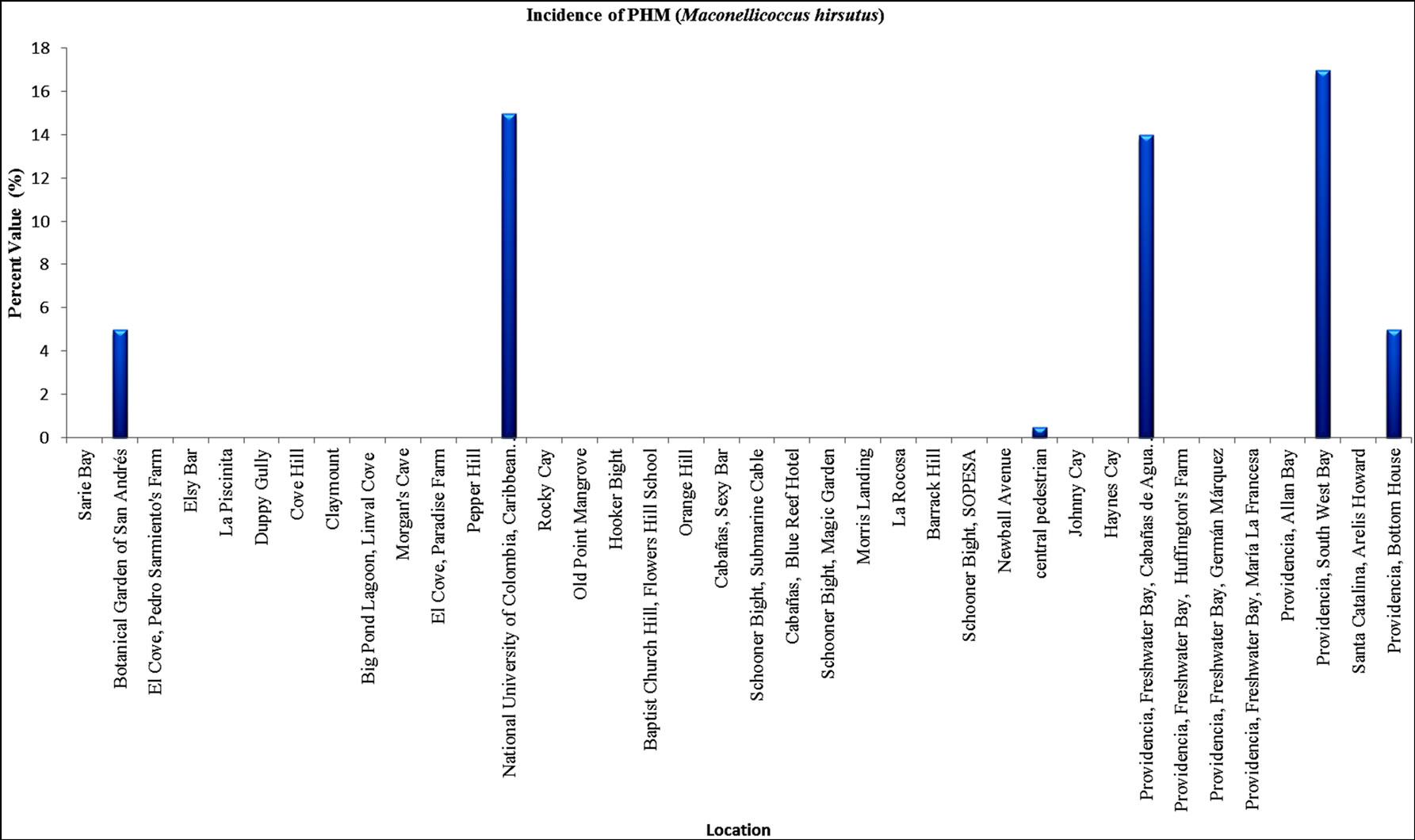
Graphic 2. Incidence evaluation of PHM (M. hirsutus) in the islands of San Andrés, Providencia and Santa Catalina, in August and November 2012.
 (a)
(a) (b)
(b) (c)
(c) (c)
(c) (e)
(e) (f)
(f) (g)
(g)
Figure 8. Developmental stages of MFS. (a) Eggs; (b)-(e) Nymphs; (f) Adult female; (g) Male. Photo by M. Silva.
3, the latest number is probably higher, but still more plants infested by MFS. For instance in San Andres 34% of sites tested, which showed higher incidences of 80%, are located in San Andres, in contrast the islands of Providencia and Santa Catalina, where the incidence in 7 of the 8 points evaluated ranged between 0% and 15% and only in one locality reached a value of 38% (Freshwater Bay-lot of Huffington).
The low incidence of PHM in San Andrés could be by the action of the natural enemy Anagyrus kamali (Hymenoptera: Encyrtidae) [11] and Allotropa utilis (Hymenoptera: Platygastridae) (Silva et al., 2013. Unpublished data). Both parasitoids were collected in the island of San Andres.
Anagyrus kamalii and Gyranusoidea indica (Hymenoptera: Encyrtidae) were reported by the first time in Colombia, in the urban area of the municipality of Cúcuta and Los Patios, in the Department of Norte de Santander [12].
It’s also important to note that where there is little incidence of MFS, Islands of Providencia and Santa Catalina, it is observed little development of sooty mold and ants are inusual, comparatively with San Andres; and is remarkable for the appearance of populations of other scales and mealybugs, mainly individuals of the Ortheziidae family.
In contrast to [3], who reported 95 host plants of C. multicicatrices, during the evaluation of host in present work, we found 94 hosts of the insect, of which, 51 plant species were recorded as new hosts, and 43 others were reported previously by the authors mentioned. New hosts are: Abelmoschus esculentus, Adonidia merrillii, Alibertia edulis, Allium cepa, Allium fistulosum, Alocasia macrorrhiza, Alpinia purpurata, Annona squamosa, Artocarpus heterophyllus, Attalea butyracea, Bauhinia monandra, Cassia fistula, Casuarina equisetifolia, Catharanthus roseus, Cecropia schreberiana, Chrysophyllum cainito, Citrus aurantium, Citrus sinensis, Cordia sebestena, Cordyline sp., Cucumis sativus, Cyperus ligularis, Dracaena sp., Euphorbia pulcherrima, Gossypium barbadense, Hibiscus sabdariffa, Hymenocallis caribaea, Ipomea batatas, Kalanchoe pinnata, Laguncularia racemosa, Luffa cylindrica, Lycopersicum esculentum, Nerium oleander, Passiflora edulis f. Flavicarpa, Phaseolus vulgaris, Phyllanthus acidus, Plectranthus amboinicus, Plumeria sp., Polyscias sp., Pseuderanthemum reticulatum, Punica granatum, Quadrella odoratissima, Ravenala madagascariensis, Salvia sp., Sansevieria fasciata, Schefflera sp., Spathodea campanulata, Spinacia oleracea, Syzygium jambos, Tecoma stans, Zea mays.
Moreover, during the evaluation period, there was no incidence of MFS in the following species reported as hosts by [3]: Acalypha wilkesiana, Achyranthes sp., Albizia lebbeck, Anthurium cúbense, Arachis pintoi, Averrhoa carambola, Avicennia germinans, Brassavola nodosa, Caesalpinia peltophoroides, Calliandra pittieri, Calliandra sp., Caryota sp., Cassia fistula, Cassia grandis, Cecropia peltata, Ceiba pentandra, Cestrum nocturnum, Citrus × aurantiifolia, Citrus grandis, Citrus latifolia, Cordyline terminalis, Delonix regia, Dicliptera assurgens, Emilia sonchifolia, Epipremnum aureum, Erythrina variegata, Erythrina sp., Euphorbia aphylla, Euphorbia hirta, Ficus benjamina, Ficus elástica, Ficus lyrata, Ficus microcarpa, Ficus sp., Graptophyllum pictum, Hibiscus sp., Jatropha gossypiifolia, Jatropha integérrima, Lagerstroemia indica, Licania tomentosa, Malvaviscus arboreus, Nandina domestica, Ocimum sanctum, Phoenix roebelenii, Pimenta dioica, Poa sp., Psidium sp., Rheedia madruno, Smilax spinosa, Syzygium samarangense, Thespesia populnea. Veitchia merrillii, Veitchia sp.
In all locations were presented high levels of incidence of sooty mold, found ants associated with MFS. This could be because the honey dew excreted by the MFS (Figure 9), colonized by sooty mold, is no longer attractive to ants (Quiroz-Gamboa, Pers. Com). The ants feed on the sugary excretions of MFS, those carry and protect the natural enemy attack (Cenicafe, 2001; Guharay et al., 2000, quoted by [13]).
According to [14], sucking insects produces large levels of honeydew, which stimulate development of sooty mold, which gives an appearance of soot to the leaves. In emerging attacks of this fungus, it is observed as waxy substance, gelatinous balls later appreciating syrup and cottony filaments, the effect of abundant honeydew, sooty appears many, looking like powder, which reduces the plant photosynthetic capacity. When a larger number of ants, which have a beneficial role for sucking insects, feed on the honey they secrete, there is an environment with low formation of sooty mold. This complex includes Capnodium and can be found associated with other genus such as Meliola, Scorias, Phragmocapnias, Microxyphium, Leptoxyphium, Tripospermum, Trichomerium and others [15].
5. ACKNOWLEDGEMENTS
This project was sponsored by the administrative agreement for technical cooperation to assembly scientific research laboratory. No 058, 2011, between Gobernación del Departamento Archipiélago and National University of Colombia, Sede Caribe. Thanks to Entomo-

Figure 9. Honey dew excreted by a nymph of MFS. Photo by M. Silva.
logical Museum Francisco Luis Gallego (MEFLG), National University of Colombia, Sede Medellin, Camilo Rodriguez Reeves, Godfray Peterson, Gloria Andrea Murcia Quimbayo and Sebastián Giraldo Grisales.
REFERENCES
- UNESCO (2011) MAB-biosphere reserves directory. http://www.unesco.org/mabdb/br/brdir/directory/biores.asp?mode=all&code=COL+05
- The Colombian Agricultural Institute (ICA) (2010) Plan for managing and mitigation caused by mealybug (Maconellicocus hirsutus) and corrugated bug (Crypticerya multicicatrices) on the islands of San Andres and Providencia. San Andrés, Colombia: Submanagement technical direction plant protection epidemiology and phytosanitary surveillance. Bogotá, Colombia.
- Kondo, T., Gullan, P. and Ramos, A.A. (2012) Report of new invasive scale insects (Hemiptera: Coccoidea), Crypticerya multicicatrices Kondo and Unruh (Monophlebidae) and Maconellicoccus hirsutus (Green) (Pseudococcidae), on the islands of San Andres and Providencia, Colombia, with an updated taxonomic key to iceryine scale insects of South America. Insecta Mundi 0265, 1- 17.
- Quiroga, I.A., Maya, M.F., Santos, A. and Hoyos, L.M. (2011) Paecilomyces sp como alternativa de control de la Cochinilla Acanalada (Crypticerya multicicatrices, Kondo y Unruh, 2009), (Hemíptera: Monophlebidae), en San Andrés, Colombia. Boletín Museo Entomológico Francisco Luis Gallego, 3, 13-17.
- Alterio, M.A. and Ramos, A. (2011) Informe de visita de diagnóstico de la situación sanitaria en el Archipiélago de San Andrés, Providencia y Santa Catalina. Instituto Colombiano Agropecuario (ICA). http://elisleño.com/index.php?option=com_content&view=article&id=2464:la-cochinilla-ide-que-se-trata&catid=41:ambiental&Itemid=83
- Aguilera, M. (2010) Geografía económica del Archipié- lago de San Andrés, Providencia y Santa Catalina. Documentos e trabajo sobre economía regional. Banco de la República, Centro de Estudios econÓmicos Regionales (CEER), 7-8. http://www.banrep.gov.co/documentos/publicaciones/regional/documentos/DTSER-33.pdf
- IGAC (1986) San Andrés y Providencia: Aspectos geográficos. Instituto Geográfico Agustín Codazzi, Bogotá.
- Garay, J., Castillo, F., Andrade, C., Aguilera, J., Niño, L., De la Pava, M., López, W. and Márquez, G. (1988) Estudio oceanográfico del área insular y oceánica del Caribe Colombiano-archipiélago de San Andrés y Providencia y cayos vecinos. Boletín Científico CIOH, 9, 3-73.
- Larral, P. and Ripa, R. (2005) Monitoreo para evaluar el control biológico de insectos y Ácaros plaga. Especial Control Biológico, Tierra Adentro. http://www.inia.cl/medios/biblioteca/ta/NR32968.pdf
- Carballo, L. and Meneses, R. (2011) Control de cochinilla con mangle negro (Avicennia nitida Jacq). Red Interactiva de Agricultura Orgánica (Redi-AO), San José de Costa Rica, Costa Rica. http://www.ruta.org/rediao/node/1540
- Evans, G., Takumasa, K., Maya, M.F., Hoyos, L.M., Quiroz, J.A. and Silva, M. (2013) First report of Anagyrus kamali Moursi and Gyranusoidea indica Shafee, Alam and Agarwal (Hymenoptera: Encyrtidae), parasitoids of the pink hibiscus mealybug Maconellicoccus hirsutus (Green) (Hemiptera: Pseudococcidae), on San Andres Island, Colombia. In Press.
- Montes, J.M. (2012) Primer registro de parasitoides de la cochinilla rosada del hibisco, Maconellicoccus hirsutus (Hemiptera: Pseudococcidae), en Colombia. Revista Colombiana de Entomología, 38, 274-275.
- Villegas-García, C., Zabala-Echavarria, G.A., Ramos-Portilla, A.A. and Benavides-Machado, P. (2009) Identificación y hábitos de cochinillas harinosas asociadas a raíces del café en Quindío. Cenicafé, 6, 354-365.
- FAO (Organización de las Naciones Unidas para la Agricultura y la Alimentación)-Ministerio de Ganadería, Agricultura y Pesca de la República Oriental del Uruguay (2006) Apoyo a la defensa y protección de las plantaciones forestales en el Uruguay. http://www.fagro.edu.uy/~forestal/cursos/proteccion/Fao%20Manual%20de%20Campo.pdf
- Kwee, L.T. (1988) Studies on some sooty moulds on guava in Malaysia. Pertanika, 11, 349-355.

