Case Reports in Clinical Medicine
Vol.3 No.3(2014), Article ID:43506,4 pages DOI:10.4236/crcm.2014.33031
Primary Malignant Lymphoma of Prostate with Silent MYD88 Mutation
Keisuke Yoshida, Riko Kitazawa, Munenori Komoda, Chihiro Ito, Ryuma Haraguchi, Sohei Kitazawa*
Department of Molecular Pathology, Ehime University Graduate School of Medicine, Toon City, Japan
Email: *kitazawa@m.ehime-u.ac.jp
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 31 January 2014; revised 25 February 2014; accepted 2 March 2014
ABSTRACT
We present a case of primary malignant lymphoma of the prostate in a 77-year-old Japanese man. Immunohistochemical examinations revealed the presence of a non-GCB subtype of DLBCL (CD10 (-), Bcl-6 (-), MUM1 (+)), and genetic analysis disclosed a lack of typical codon 206 or 265 missense mutation in MYD88, suggesting that the case was of type3 (non-GCB andnon-ABC), a subtype of DLBCL. Three courses of chemotherapy (rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP)) were effective, and the patient has been free of the disease with no local or systemic recurrence for three years. Although the significance of silent mutation at hot spot of the highly oncogenic MYD88 gene in this case is unclear, a minute increase in translational efficiency by silent mutation may have contributed to the activation of MYD88.
Keywords:Malignant Lymphoma; Prostate; MYD88

1. Introduction
Primary lymphoma of the prostate is extremely rare, representing approximately 0.2% to 0.8% of extra nodal lymphoma and less than 0.1% of all prostate neoplasms [1] . Here, we describe a case of prostatic diffuse large B-cell lymphoma (DLBCL) carrying silent MYD88 mutation in a 77-year-old man, which was successfully managed with rituximab-based chemotherapy.
2. Case Presentation
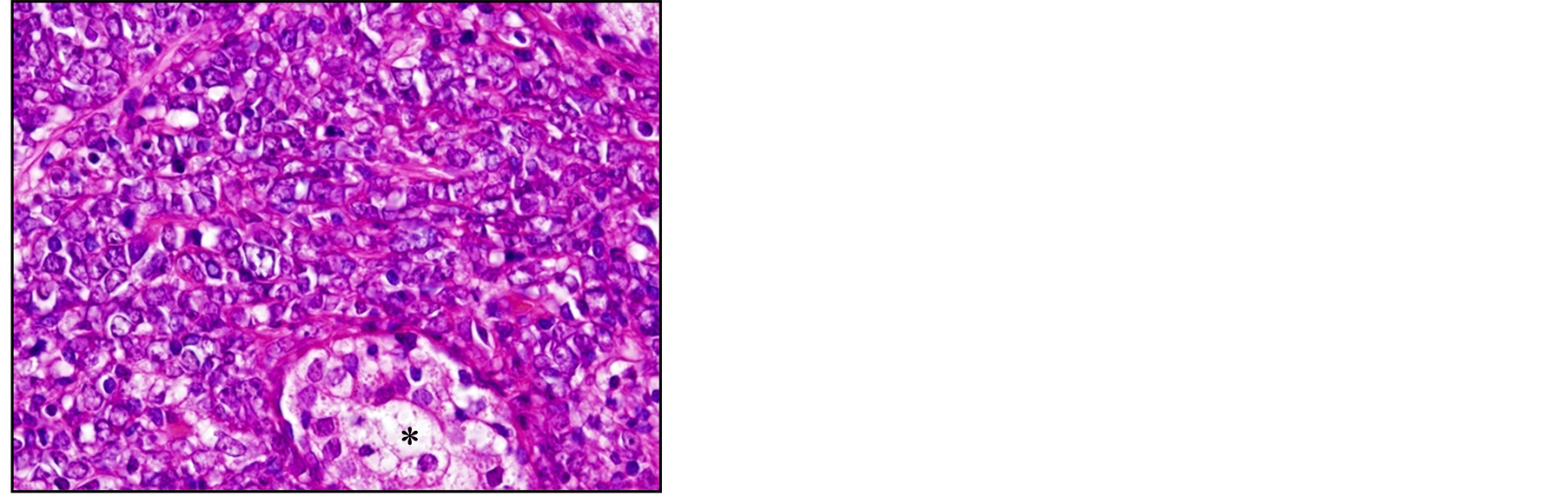
A 77-year-old man presented with acute urinary retention without hematuria lasting two days. Digital examination revealed an enlarged prostate in both lobes. Laboratory data including PSA gave normal values. After having urine drained through catheters, the patient underwent transurethral resection of the prostate. Pathological diagnosis of the resected specimen revealed diffuse infiltration of round cells with scant cytoplasm (Figure 1(a)). Immunohistochemical examination disclosed cells positive for CD20 (Figure 1(b)), MUM1 (data not shown) and Bcl-6 (Figure 1(c)), and negative for CD10 (data not shown). Five years earlier, the patient had visited a community hospital with the chief complaint of prolonged micturition, had been clinically diagnosed with benign prostate hypertrophy, had undergone partial transurethral resection of the prostate. Pathological examination had confirmed benign prostate hypertrophy with proliferative change in prostatic glands and stromal cells.
Systemic screening by CT-scan and MRI revealed a mass confined to the prostate without swelling of regional and systemic lymph nodes. Under the diagnosis of primary malignant lymphoma (DLBCL, non-germinal center-like cell (non-GCB) type [2] ) of the prostate, the patient received three course of chemotherapy (rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP)), and has been free of the disease with no local or systemic recurrence for three years.
To determine whether the non-GCB type of DLBCL detected at biopsy was activated B-cell like (ABC) [2] or of type3 (non-GCB and non-ABC) [2] , we checked for the presence of MYD88 mutation in lymphoma cells. Sections (5-μm thick) from formalin-fixed paraffin-embedded specimens taken at biopsy and DNA samples selectively prepared from microdissected lymphoma cells by the agarose-bead mediated technique were subjected to PCR. Since codons 206 and 265 are the two hot spots of somatic MYD88 mutations [3] , two sets of primers were used for polymerase chain reaction (PCR) to cover these two genomic regions: sense, 5’-CCGTGGCCTT-
 (a)
(a)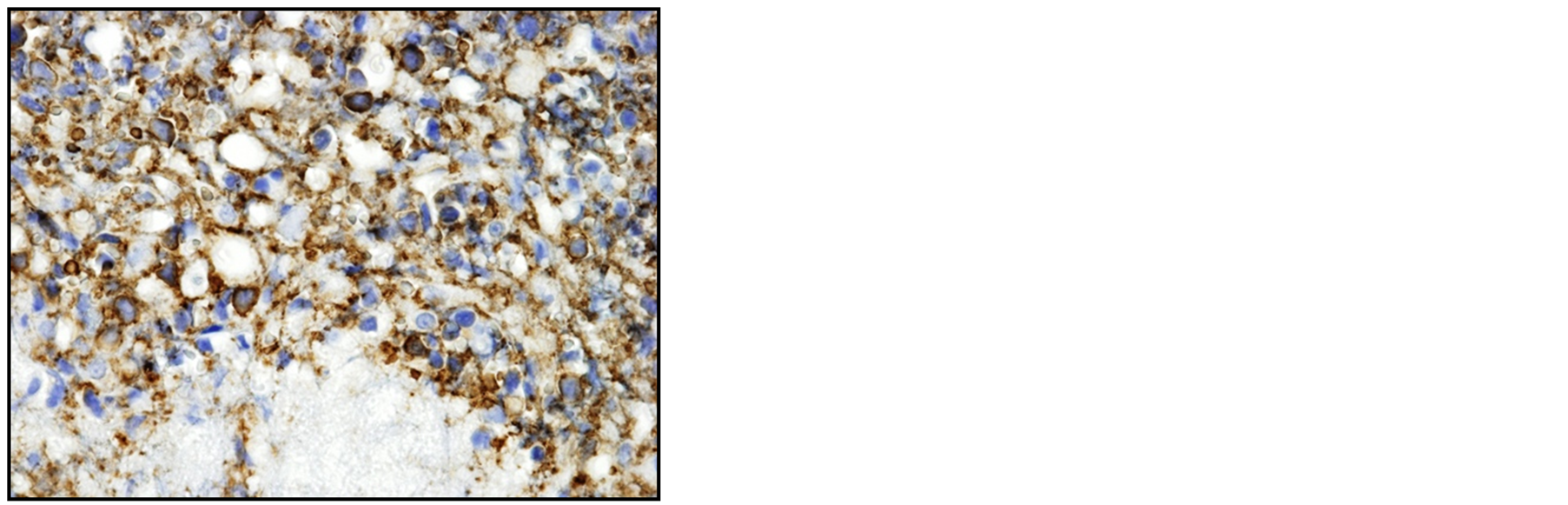 (b)
(b)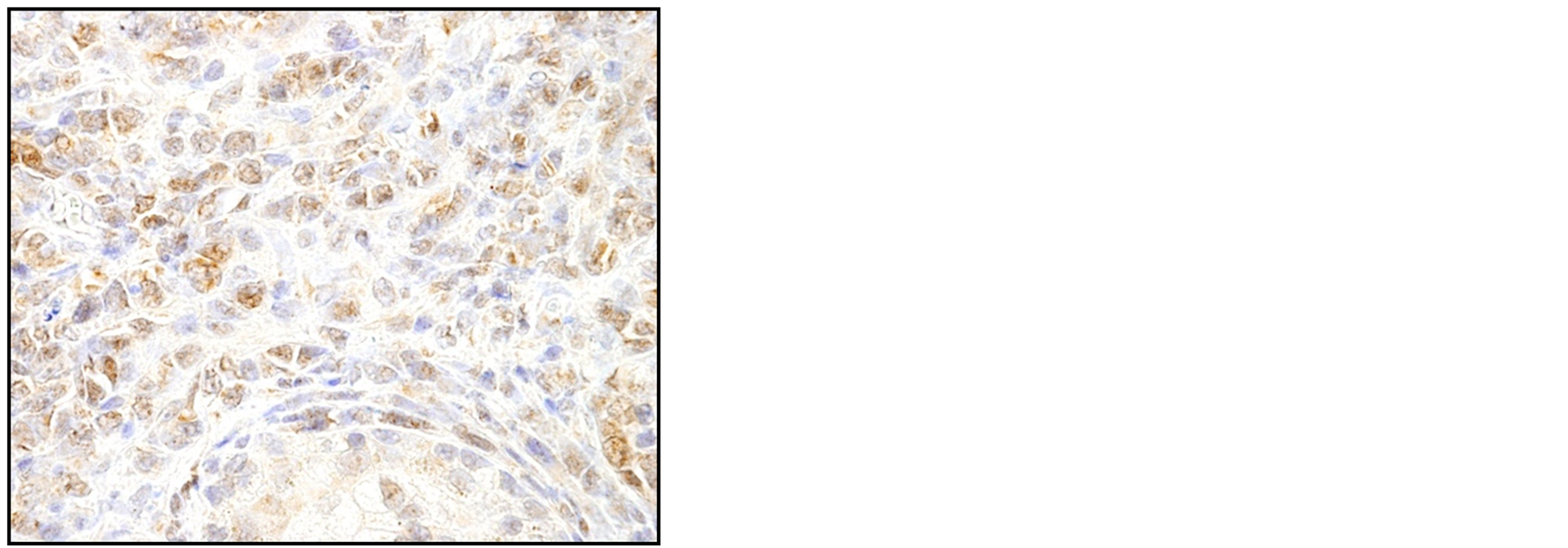 (c)
(c)
Figure 1. Histopathological analysis of the resected specimen shows diffuse infiltration of the round cells with scant cytoplasm. The atrophic and destroyed prostatic gland is seen at the bottom (*) (a, HE, ×400). Immunohistochemical examination shows infiltrating cells positive for CD20 (b, ×400) and Bcl-6 (c, ×400), confirming the diagnosis of primary malignant lymphoma (DLBCL, non-GCB type).


Figure 2. PCR was conducted with the use of formalin-fixed paraffin-embedded samples. The PCR products were ligated and cloned into T-vector, and sequenced. No somatic mutation is seen in samples of non-lymphoma tissue (a, c.618T, p.206S). On the other hand, a c.618T>A mutation causing p.S206S silent mutation is observed in a sample with abundant lymphoma cells (b).
CTAGCCAAC-3’ and antisense, 5’-GATGTCCTGCCTGGCACCTG-3’ for codon 206; and sense, 5’-TCTCTCCAGGTGCCCATCAGA-3’ and antisense, 5’-CAAGTACAAGGCAATGAAGAA-3’ for codon 265. PCR products were ligated and cloned into T-vector, and sequenced. No somatic mutation was observed in samples from non-lymphoma tissues (Figure 2(a)). On the other hand, while no typical c.794T>C mutation causing p.L256P mutation was found in 24 independent clones, c.618T>A mutation causing p.S206S silent mutation was observed in 6 of the 24 independent clones (Figure 2(b)). Since p.L256P mutation in MYD88, recurrently observed among specific subsets of B-cell malignancies, especially in the ABC type of DLBCL, was absent in the present case, the final diagnosis was primary prostatic malignant lymphoma, DLBCL type 3 (non-ABC and non-GCB).
3. Discussion
Compared with secondary systemic involvement as a manifestation of advanced stage, primary malignant lymphoma of the prostate is extremely rare and is defined on the basis of several criteria [1] [4] . The main symptom is urine retention, and the disease occurs predominantly in the prostate, with or without spreading to adjacent tissues and does not involve lymph nodes, liver, spleen or blood for up to one month after diagnosis. To date, fewer than 150 cases have been described in the literature, among which DLBCL is the most common type, followed by small lymphocytic lymphoma, follicular lymphoma, Burkitt’s lymphoma, MALT lymphoma, and mantle cell lymphoma [4] .
Since the first report of highly oncogenic MYD88 p.L265P mutation in the ABC subtype of DLBCL [3] , L265P mutation has been detected in primary central nervous system lymphoma, in primary cutaneous leg type DLBCL [5] and in Waldenstrom’s macroglobulinemia [6] , indicating that MYD88 is one of the target genes of certain subsets of B-cell malignancies at an early stage of oncogenesis. In the present case, while immunohistochemical examination of the biopsy revealed the presence of the non-GCB subtype of DLBCL (CD10 (-), Bcl-6 (−), MUM1 (+)), genetic analysis showed a lack of typical codon 206 or 265 missense mutation. We therefore speculate that this case was of type3 (non-GCB and non-ABC) subtype of DLBCL.
Regarding the silent mutation seen at codon 206 (S206S) of MYD88, we have ruled out the possibility of mere polymorphism, especially that silent mutation in codon 206 is present in a tumor specific manner (sequence obtained from non-neoplastic tissue showed no such silent mutation at codon 206, Figure 2(a)). In particular cases, silent mutations affect splicing patterns, translation efficiency and protein folding [7] . Although the significance of silent mutation in this case is unclear, minute increases in translational efficiency by silent mutation may have contributed to the activation of highly oncogenic MYD88.
References
- Sarris, A., Dimopoulos, M., Pugh, W. and Cabanillas, F. (1995) Primary Lymphoma of the Prostate: Good Outcome with Doxorubicin-Based Combination Chemotherapy. The Journal of Urology, 153, 1852-1854. http://dx.doi.org/10.1016/S0022-5347(01)67330-0
- Hans, C.P., Weisenburger, D.D., Greiner, T.C., Gascoyne, R.D., Delabie, J., et al. (2004) Confirmation of the Molecular Classification of Diffuse Large B-Cell Lymphoma by Immunohistochemistry Using a Tissue Microarray. Blood, 103, 275-282. http://dx.doi.org/10.1182/blood-2003-05-1545
- Ngo, V.N., Young, R.M., Schmitz, R., Jhavar, S., Xiao, W., et al. (2011) Oncogenically Active MYD88 Mutations in human Lymphoma. Nature, 470, 115-119. http://dx.doi.org/10.1038/nature09671
- Bostwick, D.G., Iczkowski, K.A., Amin, M.B., Discigil, G. and Osborne, B. (1998) Malignant Lymphoma Involving the Prostate: Report of 62 Cases. Cancer, 83, 732-738. http://dx.doi.org/10.1002/(SICI)1097-0142(19980815)83:4<732::AID-CNCR15>3.0.CO;2-T
- Pham-Ledard, A., Cappellen, D., Martinez, F., Vergier, B., Beylot-Barry, M., et al. (2012) MYD88 Somatic Mutation Is a Genetic Feature of Primary Cutaneous Diffuse Large B-Cell Lymphoma, Leg Type. Journal of Investigative Dermatology, 132, 2118-2120. http://dx.doi.org/10.1038/jid.2012.102
- Treon, S.P., Xu, L., Yang, G., Zhou, Y., Liu, X., et al. (2012) MYD88 L265P Somatic Mutation in Waldenstrom’s Macroglobulinemia. The New England Journal of Medicine, 367, 826-833. http://dx.doi.org/10.1056/NEJMoa1200710
NOTES

*Corresponding author.

